Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
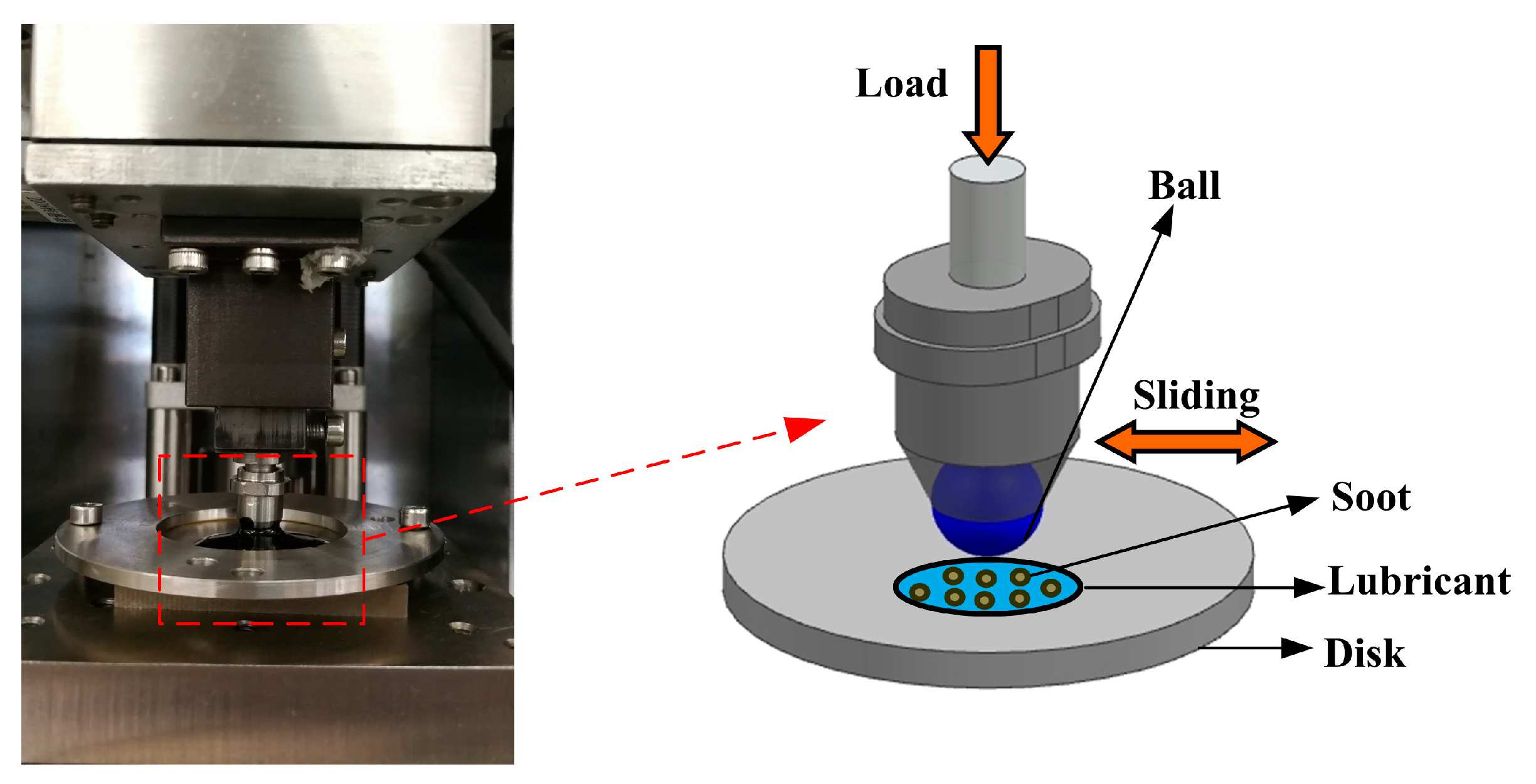
2.2. Tribological Testing and Characterization
3. Results and Discussion
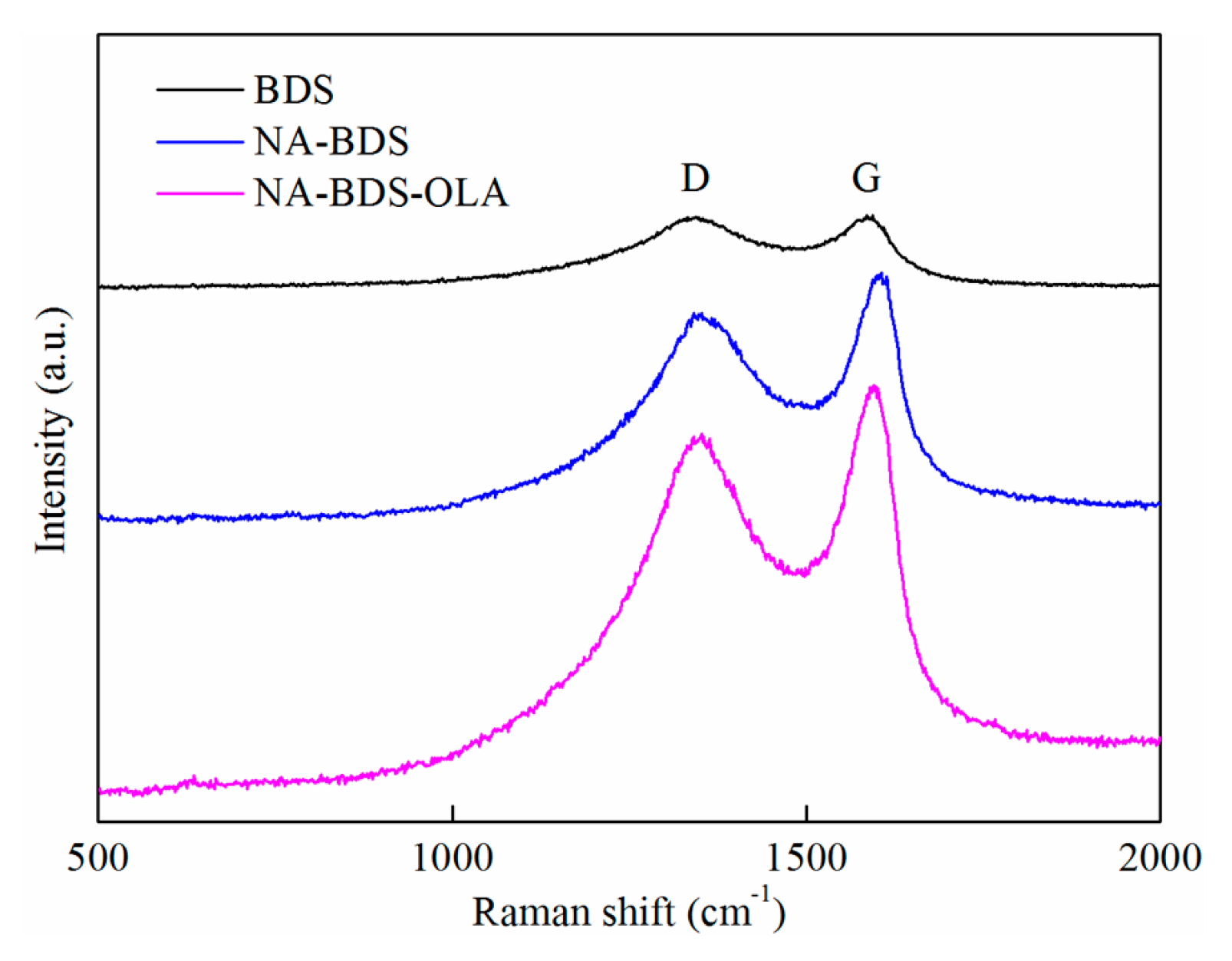
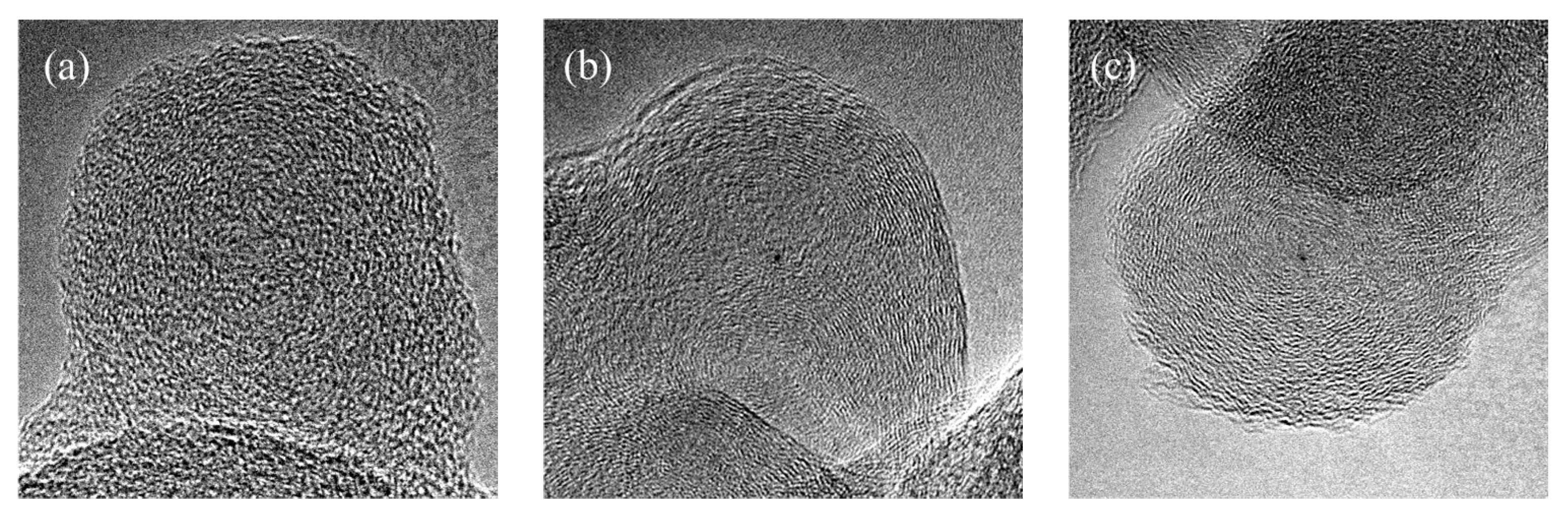
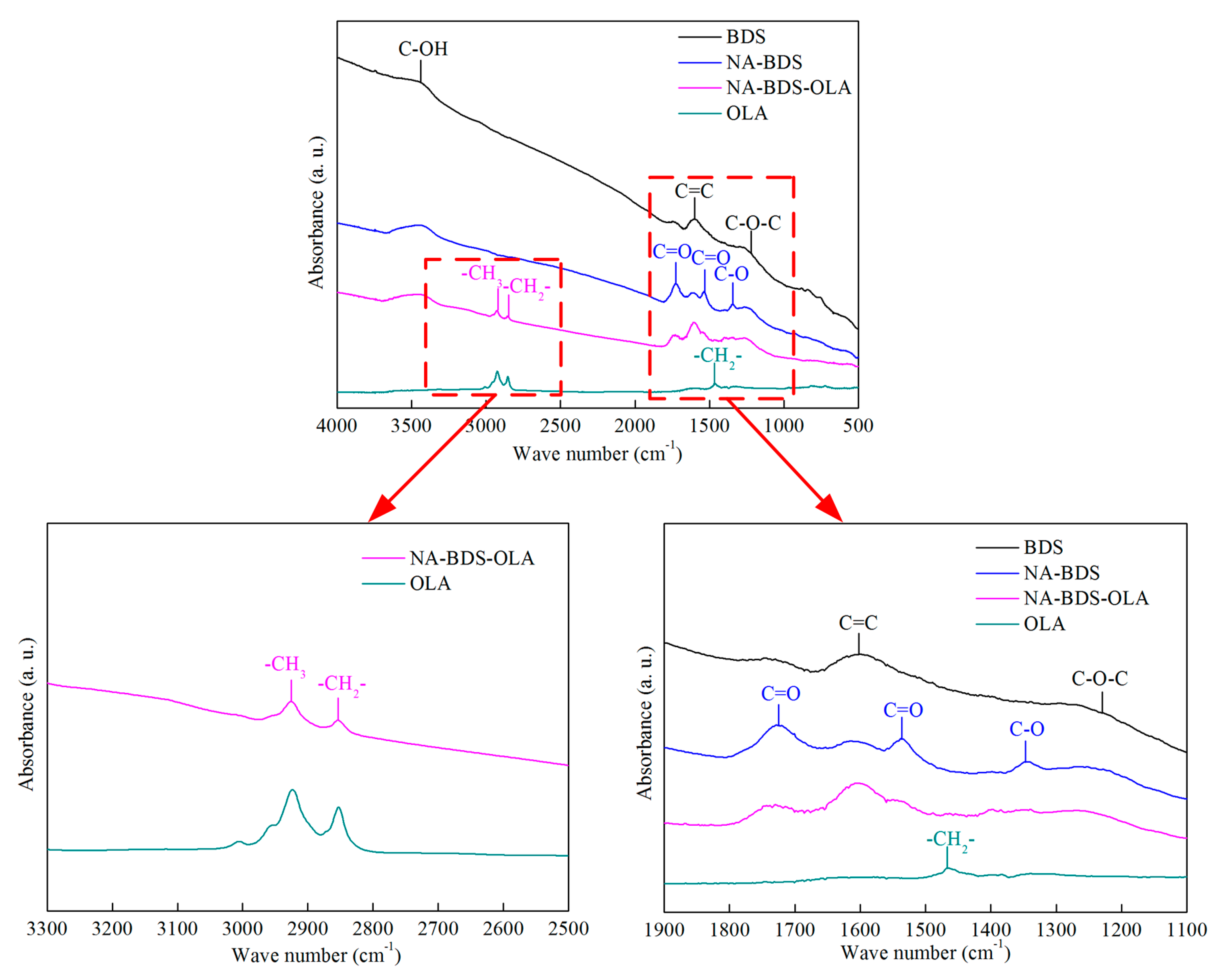
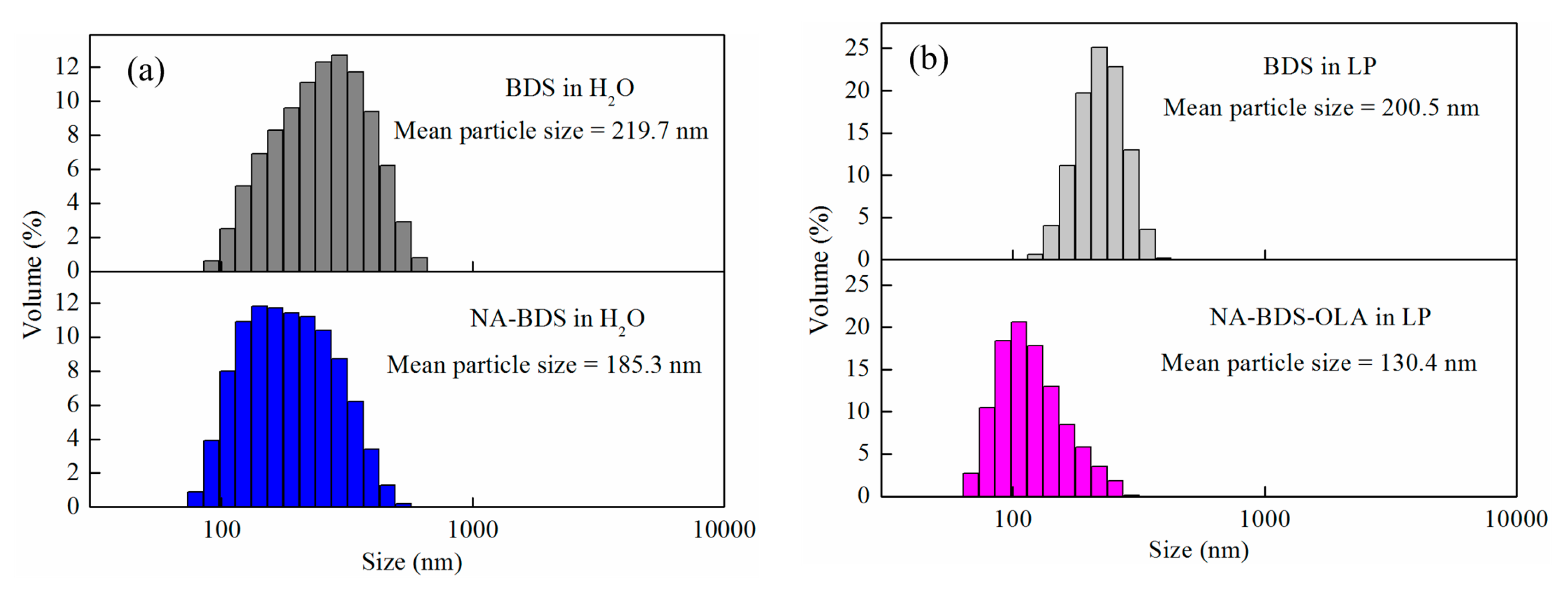
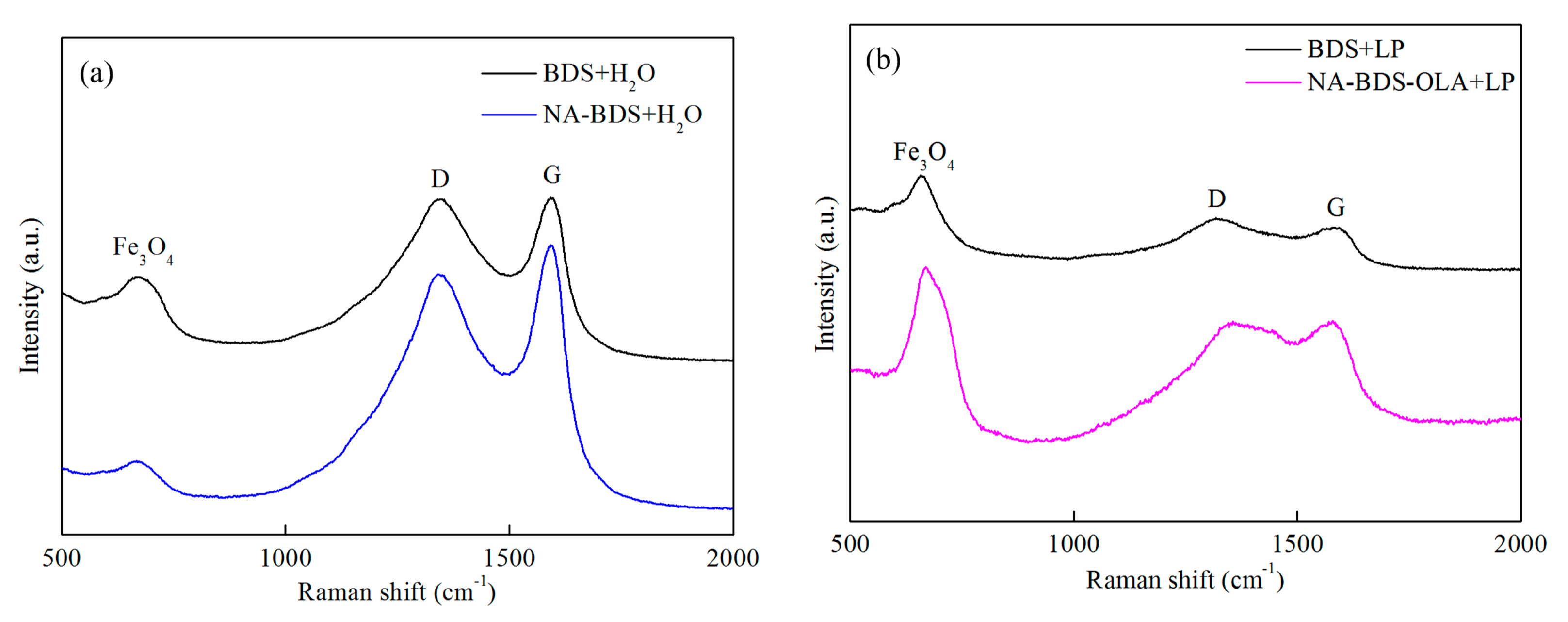
3.1. Characterization
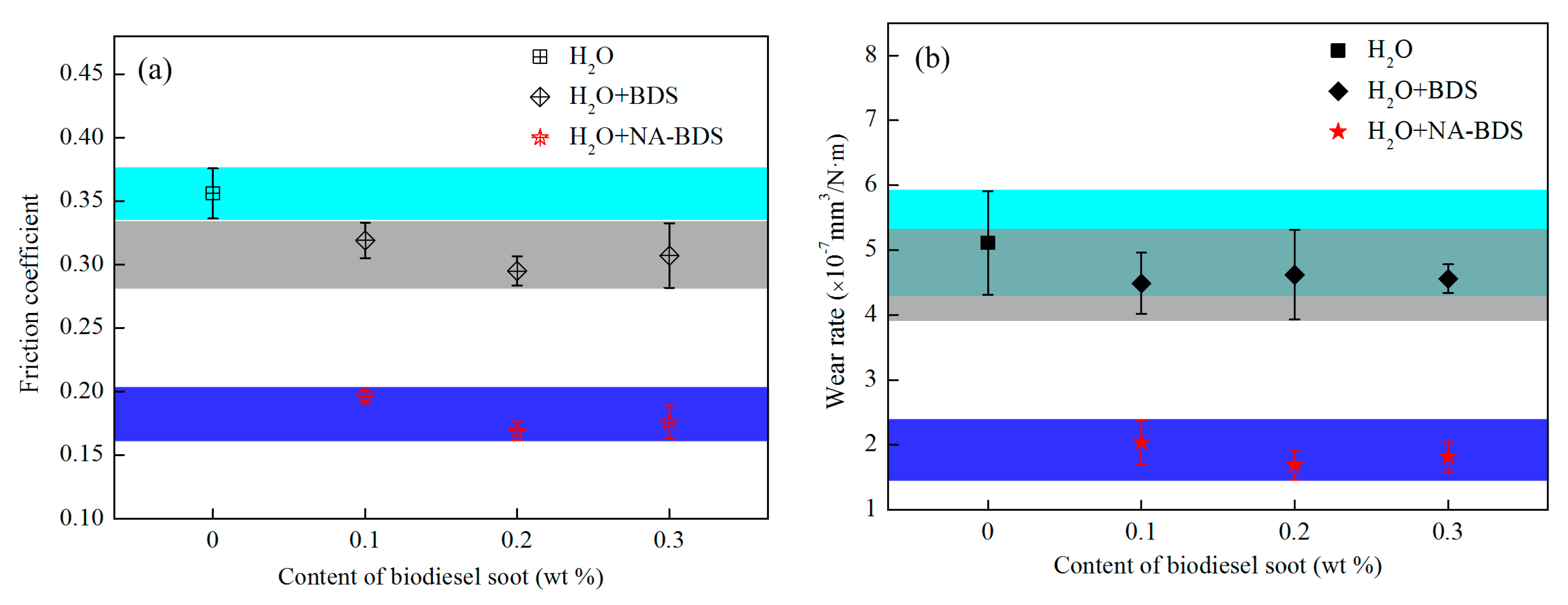
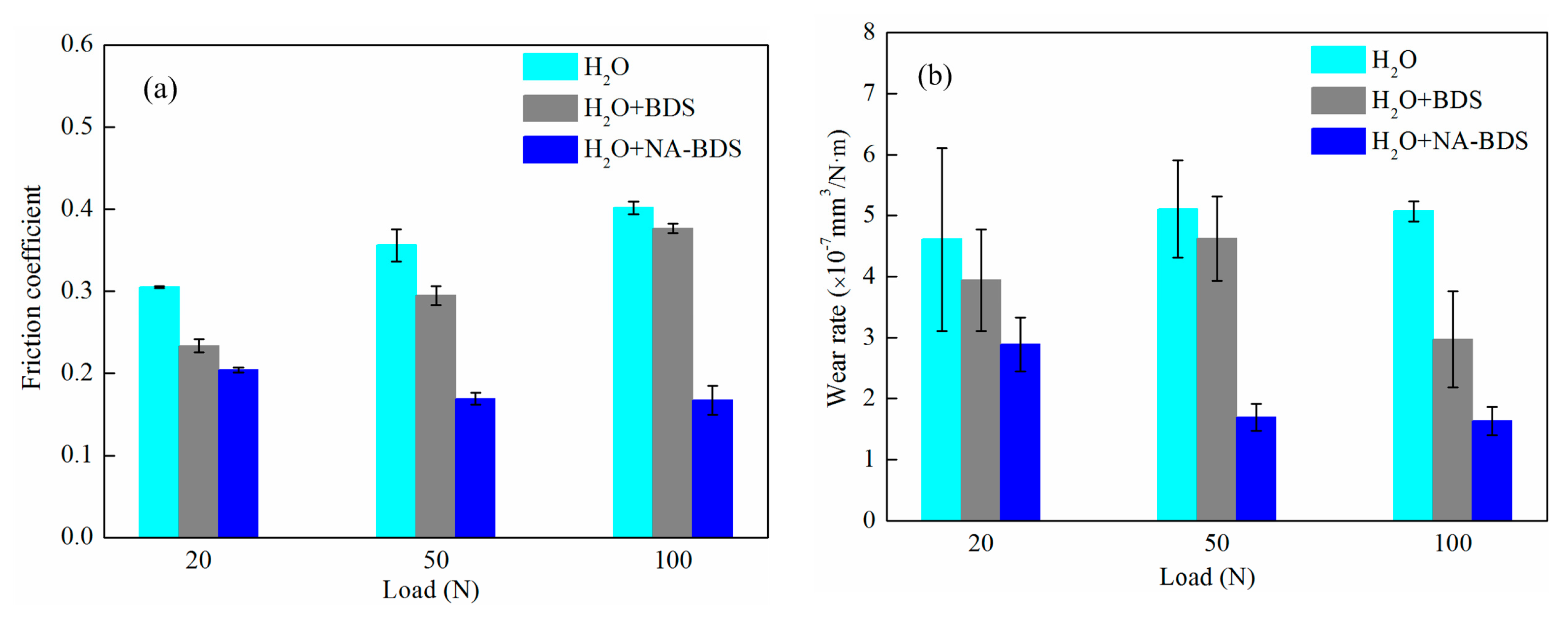
3.2. Tribological Performance of Soot Samples in H2O
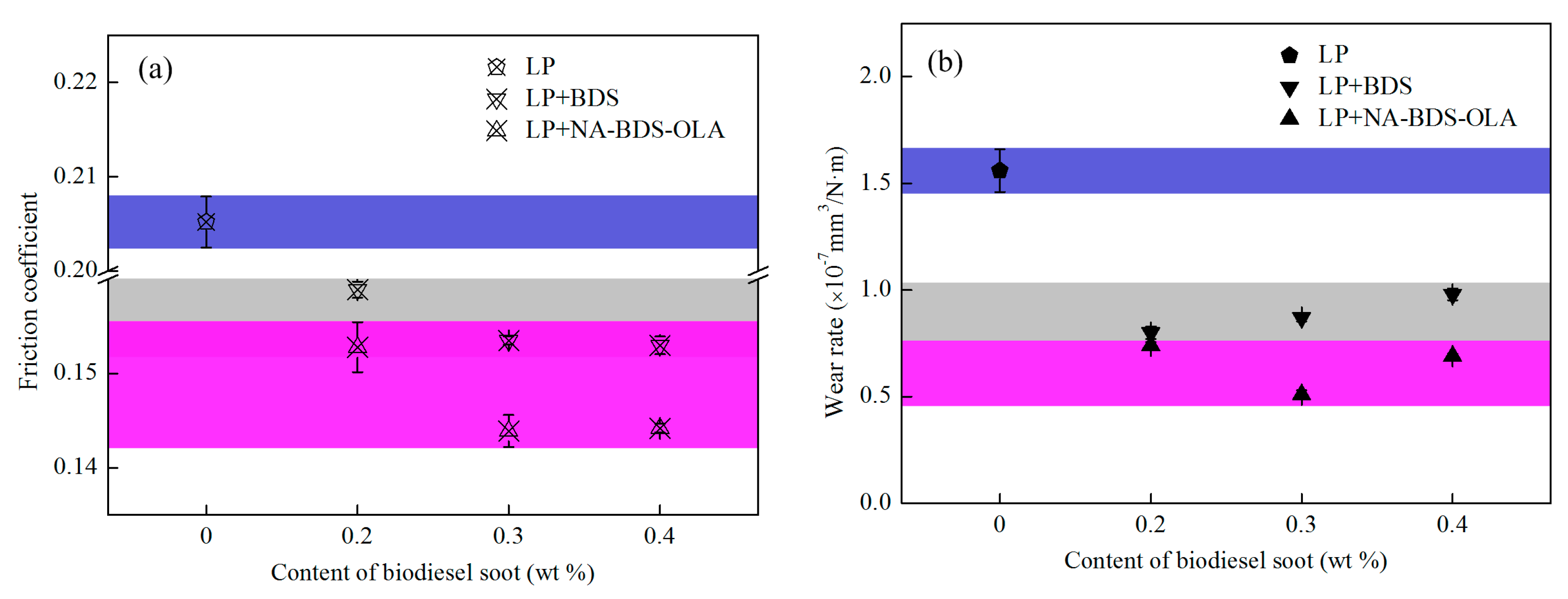
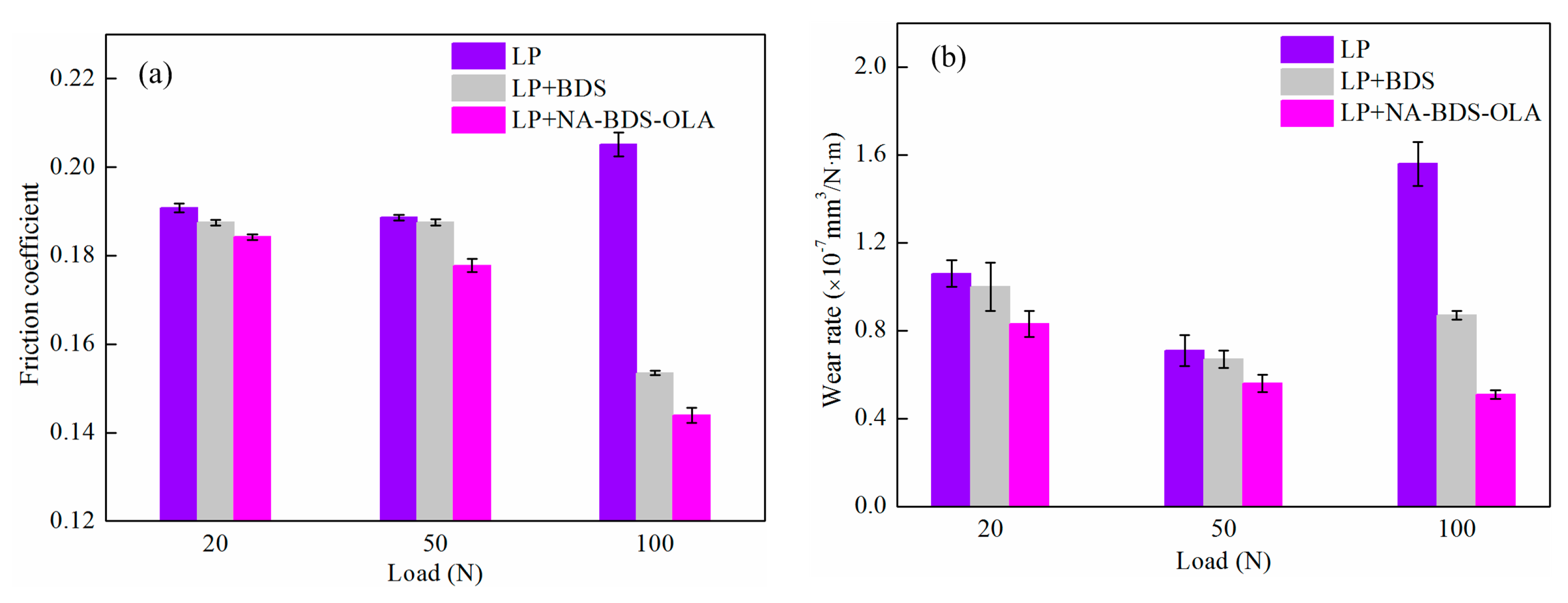
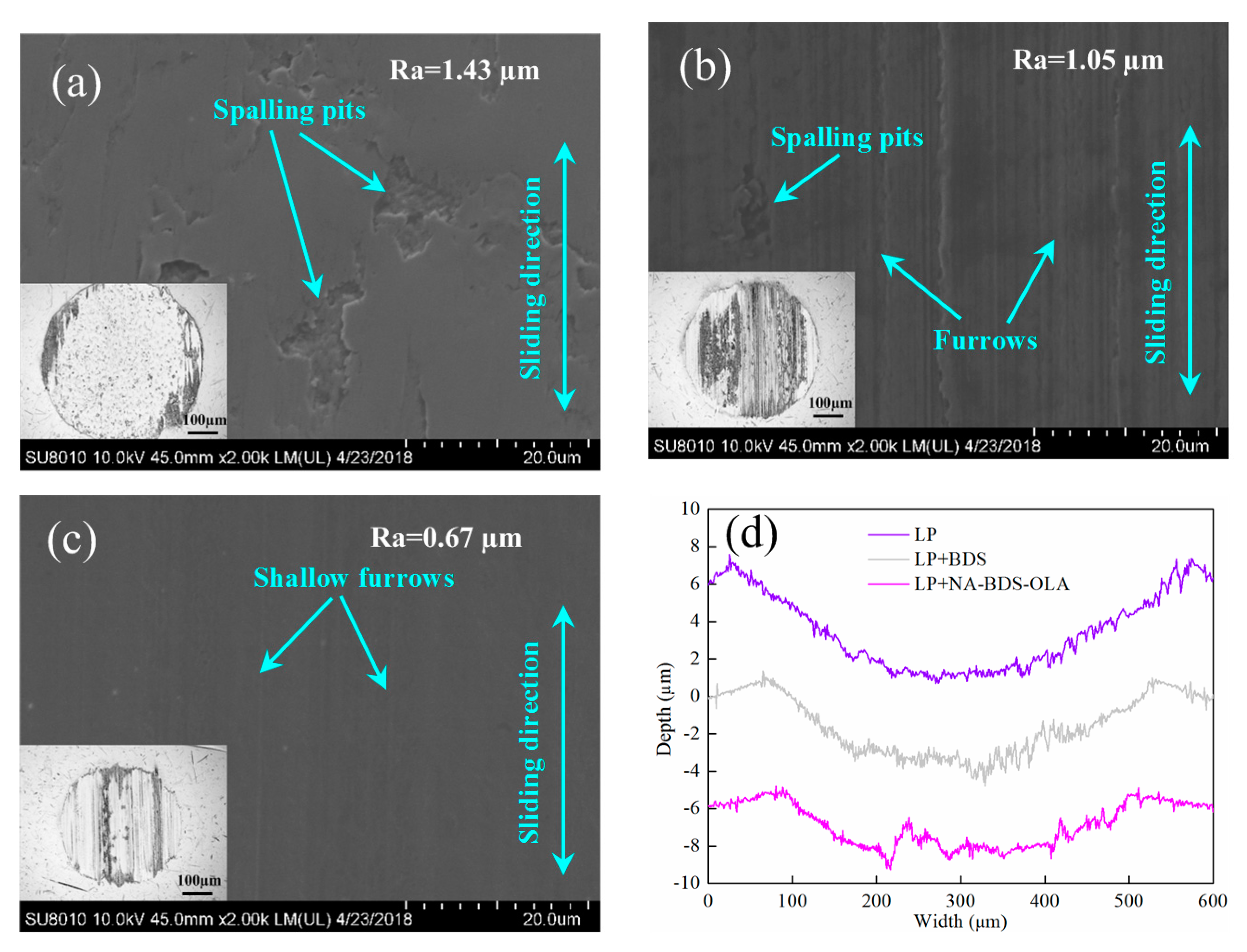
3.3. Tribological Performance of Soot Samples in LP
3.4. Lubrication Mechanisms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palash, S.M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Rizwanul Fattah, I.M.; Sanjid, A. Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers. Manag. 2015, 91, 149–157. [Google Scholar] [CrossRef]
- Singh, V.; Bux, F.; Sharma, Y.C. A low cost one pot synthesis of biodiesel from waste frying oil (WFO) using a novel material, β-potassium dizirconate (β-K2Zr2O5). Appl. Energy 2016, 172, 23–33. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K.; Oßwald, P.; Cool, T.A.; Kasper, T.; Hansen, N.; Qi, F.; Westbrook, C.K.; Westmoreland, P.R. Biofuel combustion chemistry: From ethanol to biodiesel. Angew. Chem. Int. Ed. 2010, 49, 3572–3597. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.F.; Huang, J.H.; Huang, D.Y. Experimental study of the effects of vegetable oil methyl ester on DI diesel engine performance characteristics and pollutant emissions. Fuel 2009, 88, 1779–1785. [Google Scholar] [CrossRef]
- Hu, E.Z.; Hu, X.G.; Liu, T.X.; Song, R.H.; Dearn, K.D.; Xu, H.M. Role of TiF3 catalyst in the tribological properties of biofuel soot-contaminated liquid paraffin. Tribol. Int. 2014, 77, 122–131. [Google Scholar] [CrossRef]
- Salehi, F.M.; Khaemba, D.N.; Morina, A.; Neville, A. Corrosive–abrasive wear induced by soot in boundary lubrication regime. Tribol. Lett. 2016, 63, 19. [Google Scholar] [CrossRef]
- Green, D.A.; Lewis, R.; Dwyer-Joyce, R.S. Wear effects and mechanisms of soot-contaminated automotive lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 159–169. [Google Scholar] [CrossRef]
- Guo, M.F.; Cai, Z.B.; Zhang, Z.C.; Zhu, M.H. Characterization and lubrication performance of diesel soot nanoparticles as oil lubricant additives. RSC Adv. 2015, 5, 101965–101974. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Vacher, B.; Ohmae, N.; Martin, J.M.; Epicier, T. Anti-wear and friction reducing mechanisms of carbon nano-onions as lubricant additives. Tribol. Lett. 2008, 30, 69–80. [Google Scholar] [CrossRef]
- Hirata, A.; Igarashi, M.; Kaito, T. Study on solid lubricant properties of carbon onions produced by heat treatment of diamond clusters or particles. Tribol. Int. 2004, 37, 899–905. [Google Scholar] [CrossRef]
- Jung, Y.; Hwang, J.; Bae, C. Assessment of particulate matter in exhaust gas for biodiesel and diesel under conventional and low temperature combustion in a compression ignition engine. Fuel 2016, 165, 413–424. [Google Scholar] [CrossRef]
- Savic, N.; Rahman, M.M.; Miljevic, B.; Saathoff, H.; Naumann, K.H.; Leisner, T.; Riches, J.; Gupta, B.; Motta, N.; Ristovski, Z.D. Influence of biodiesel fuel composition on the morphology and microstructure of particles emitted from diesel engines. Carbon 2016, 104, 179–189. [Google Scholar] [CrossRef]
- Growney, D.J.; Mykhaylyk, O.O.; Middlemiss, L.; Fielding, L.A.; Derry, M.J.; Aragrag, N.; Lamb, G.D.; Armes, S.P. Is carbon black a suitable model colloidal substrate for diesel soot. Langmuir 2015, 31, 10358–10369. [Google Scholar] [CrossRef] [PubMed]
- Kontou, A.; Southby, M.; Spikes, H.A. Effect of steel hardness on soot wear. Wear 2017, 390, 236–245. [Google Scholar] [CrossRef]
- Hu, E.; Dearn, K.D.; Yang, B.X.; Song, R.H.; Xu, Y.F.; Hu, X.G. Tribofilm formation and characterization of lubricating oils with biofuel soot and inorganic fluorides. Tribol. Int. 2017, 107, 163–172. [Google Scholar] [CrossRef]
- Clague, A.D.H.; Donnet, J.B.; Wang, T.K.; Peng, J.C.M. A comparison of diesel engine soot with carbon black. Carbon 1999, 37, 1553–1565. [Google Scholar] [CrossRef]
- Ferraro, G.; Fratini, E.; Rausa, R.; Fiaschi, P.; Baglioni, P. Multiscale characterization of some commercial carbon blacks and diesel engine soot. Energy Fuels 2016, 30, 9859–9866. [Google Scholar] [CrossRef]
- Li, C.; Song, H.; Zhang, J.; Wu, B.; Zhang, Q.Q.; Zhuang, Y.; Hu, X.G. Novel approach for improved tribological behavior of biodiesel soot in liquid paraffin. China Pet. Process. Petrochem. Technol. 2019, 21, 62–70. [Google Scholar]
- Hu, E.Z.; Hu, X.G.; Liu, T.X.; Liu, Y.M.; Song, R.H.; Chen, Y.Z. Investigation of morphology, structure and composition of biomass-oil soot particles. Appl. Surf. Sci. 2013, 270, 596–603. [Google Scholar] [CrossRef]
- Li, C.; Wei, D.Z.; Zhuang, Y.; Song, R.H.; Hu, X.G. Effect of biodiesel soot on tribological behavior of liquid paraffin. China Pet. Process. Petrochem. Technol. 2018, 20, 106–113. [Google Scholar]
- Boehman, A.L.; Song, J.; Alam, M. Impact of biodiesel blending on diesel soot and the regeneration of particulate filters. Energy Fuels 2005, 19, 1857–1864. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Rao, L.; Kim, D.; Kook, S. The influence of a large methyl ester on in-flame soot particle structures in a small-bore diesel engine. Fuel 2017, 194, 423–435. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Mitra, T. A radical approach to soot formation. Science 2018, 361, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Mutyala, K.C.; Wu, Y.A.; Erdemir, A.; Sumant, A.V. Graphene-MoS2 ensembles to reduce friction and wear in DLC-Steel contacts. Carbon 2019, 146, 524–527. [Google Scholar] [CrossRef]
- Wang, W.Z.; Huang, P. Study on the lubrication state of frictional pairs with different surface roughness based on Stribeck curves. Tribol. 2004, 24, 254–257. [Google Scholar]
- Xu, Y.F.; Liu, Z.C.; Dearn, K.D.; Dong, Y.H.; You, T.; Hu, X.G. Thermo-tribological behaviour of microgels for improved aqueous lubrication for steel/UHMWPE contact. Tribol. Int. 2019, 130, 63–73. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yu, J.Y.; Dong, Y.H.; You, T.; Hu, X.G. Boundary lubricating properties of black phosphorus nanosheets in polyalphaolefin oil. J. Tribol. 2019, 141, 072101. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Freeman, L.; Fleischmann, S.; Lasserre, F.; Fainman, Y.; Talke, F.E. Tip-enhanced Raman spectroscopy studies of nanodiamonds and carbon onions. Carbon 2018, 132, 495–502. [Google Scholar] [CrossRef]
- Du, S.N.; Sun, J.L.; Wu, P. Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 2018, 140, 338–351. [Google Scholar] [CrossRef]
- Wei, J.X.; Cai, M.R.; Zhou, F.; Liu, W.M. Candle soot as particular lubricant additives. Tribol. Lett. 2014, 53, 521–531. [Google Scholar] [CrossRef]
- Klokkenburg, M.; Hilhorst, J.; Erne, B.H. Surface analysis of magnetite nanoparticles in cyclohexane solutions of oleic acid and oleylamine. Vib. Spectro. 2007, 43, 243–248. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Xu, Y.; Xiang, X.Z.; Zheng, G.L.; Zeng, X.Q.; Li, Z.P.; Ren, T.H.; Zhang, Y.D. Tribological performances of highly dispersed graphene oxide derivatives in vegetable oil. Tribol. Int. 2018, 126, 39–48. [Google Scholar] [CrossRef]
- Xiao, H.P.; Liu, S.H.; Wang, D.G.; Chen, Y. Abrasion–corrosion behaviors of steel–steel contact in seawater containing abrasive particles. Tribol. Trans. 2018, 61, 12–18. [Google Scholar] [CrossRef]
- Wei, J.X.; Yao, M.H.; Cai, M.R.; Zhou, F. Tribological properties of cetyltrimethyl ammonium bromide modified candle soot as effective lubricant additive in oil. Tribology 2014, 34, 428–436. [Google Scholar]
- Fan, X.Q.; Li, W.; Fu, H.M.; Zhu, M.H.; Wang, L.P.; Cai, Z.B.; Liu, J.H.; Li, H. Probing the function of solid nanoparticle structure under boundary lubrication. ACS Sustain. Chem. Eng. 2017, 5, 4223–4233. [Google Scholar] [CrossRef]
- Liu, Y.R.; Hu, K.H.; Hu, E.Z.; Guo, J.H.; Han, C.L.; Hu, X.G. Double hollow MoS2 nano-spheres: Synthesis, tribological properties, and functional conversion from lubrication to photocatalysis. Appl. Surf. Sci. 2017, 392, 1144–1152. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Etacheri, V.; Dysart, A.D.; Stacke, L.E.; Pol, V.G.; Sadeghi, F. Ultrasmooth submicrometer carbon spheres as lubricant additives for friction and wear reduction. ACS Sustain. Chem. Eng. 2015, 7, 5514–5521. [Google Scholar] [CrossRef]
- Penchaliah, R.; Harvey, T.J.; Wood, R.J.K.; Nelson, K.; Powrie, H.E.G. The effects of diesel contaminants on tribological performance on sliding steel on steel contacts. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2011, 225, 779–797. [Google Scholar] [CrossRef]
- Huang, G.W.; Yu, Q.L.; Ma, Z.F.; Cai, M.R.; Zhou, F.; Liu, W.M. Fluorinated candle soot as the lubricant additive of perfluoropolyether. Tribol. Lett. 2017, 65, 28. [Google Scholar] [CrossRef]
- Cao, Z.F.; Xia, Y.Q. Study on the preparation and tribological properties of fly ash as lubricant additive for steel/steel pair. Tribol. Lett. 2017, 65, 104. [Google Scholar] [CrossRef]
- Knauer, M.; Carrara, M.; Rothe, D.; Niessner, R.; Ivleva, N.P. Changes in structure and reactivity of soot during oxidation and gasification by oxygen, studied by micro-Raman spectroscopy and temperature programmed oxidation. Aerosol Sci. Technol. 2009, 43, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.A.; Meng, X.D.; Li, Y.; Zhao, J.P. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Zin, V.; Agresti, F.; Barison, S.; Litti, L.; Fedele, L.; Meneghetti, M.; Fabrizio, M. Effect of external magnetic field on tribological properties of goethite (a-FeOOH) based nanofluids. Tribol. Int. 2018, 127, 341–350. [Google Scholar] [CrossRef]
- Shi, B.; Guo, J.H.; Cao, X.A.; Hu, E.Z.; Hu, K.H. Effects of carbon soot from the combustion of diesel fuels on the tribological properties of lubricating oil and diesel fuels. Ind. Lubr. Tribol. 2018, 70, 532–537. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cai, Z.B.; Peng, J.F.; Zhu, M.H. Comparison of the tribology performance of nano-diesel soot and graphite particles as lubricant additives. J. Phys. D Appl. Phys. 2015, 49, 045304. [Google Scholar] [CrossRef]
- Ma, T.B.; Wang, L.F.; Hu, Y.Z.; Li, X.; Wang, H. A shear localization mechanism for lubricity of amorphous carbon materials. Sci. Rep. 2014, 4, 3662. [Google Scholar] [CrossRef]

| Property | Value | Methods |
|---|---|---|
| Density at 20 °C, kg·m−3 | 875 | ASTM D4052 |
| Flash point (closed up), °C | 168 | ASTM D93 |
| Acid value, mgKOH·g−1 | 0.045 | ASTM D664 |
| Kinematic viscosity at 40 °C, mm2·s−1 | 4.5 | ASTM D445 |
| Water content, % | Trace | ASTM D1744 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, M.; Wang, X.; Feng, W.; Zhang, Q.; Wu, B.; Hu, X. Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives. Nanomaterials 2019, 9, 1115. https://doi.org/10.3390/nano9081115
Li C, Li M, Wang X, Feng W, Zhang Q, Wu B, Hu X. Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives. Nanomaterials. 2019; 9(8):1115. https://doi.org/10.3390/nano9081115
Chicago/Turabian StyleLi, Chuan, Mingling Li, Xinyun Wang, Weimin Feng, Qiangqiang Zhang, Bo Wu, and Xianguo Hu. 2019. "Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives" Nanomaterials 9, no. 8: 1115. https://doi.org/10.3390/nano9081115
APA StyleLi, C., Li, M., Wang, X., Feng, W., Zhang, Q., Wu, B., & Hu, X. (2019). Novel Carbon Nanoparticles Derived from Biodiesel Soot as Lubricant Additives. Nanomaterials, 9(8), 1115. https://doi.org/10.3390/nano9081115






