Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties
Abstract
:1. Introduction
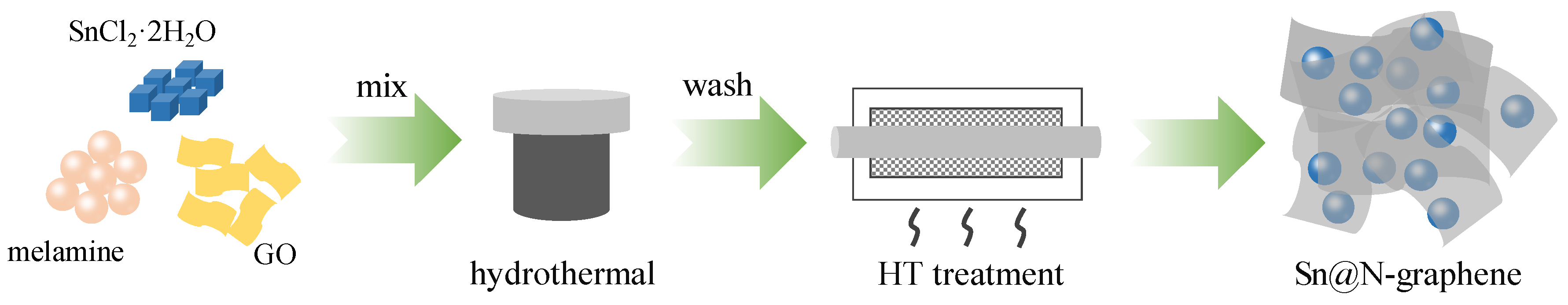
2. Materials and Methods
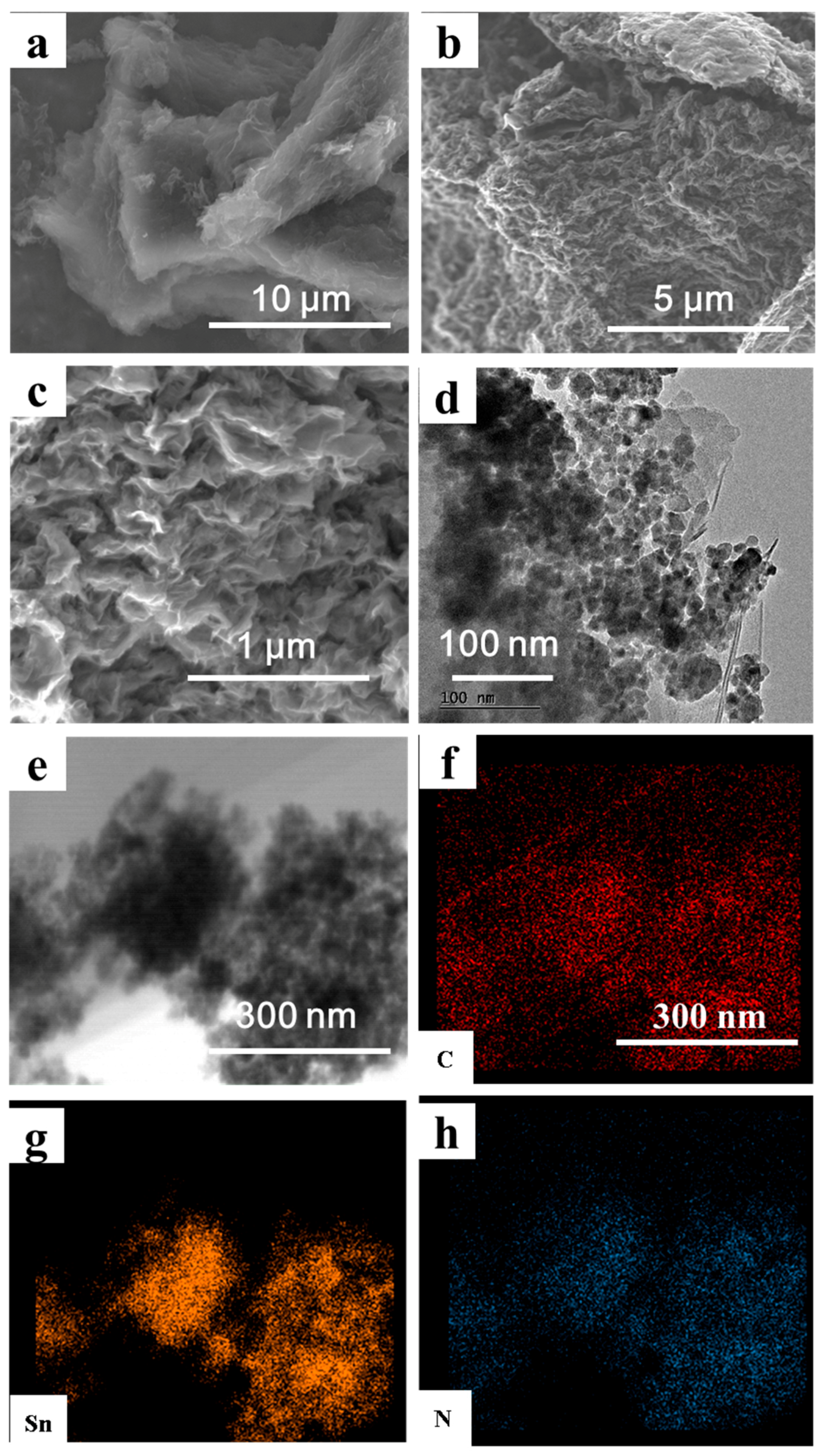
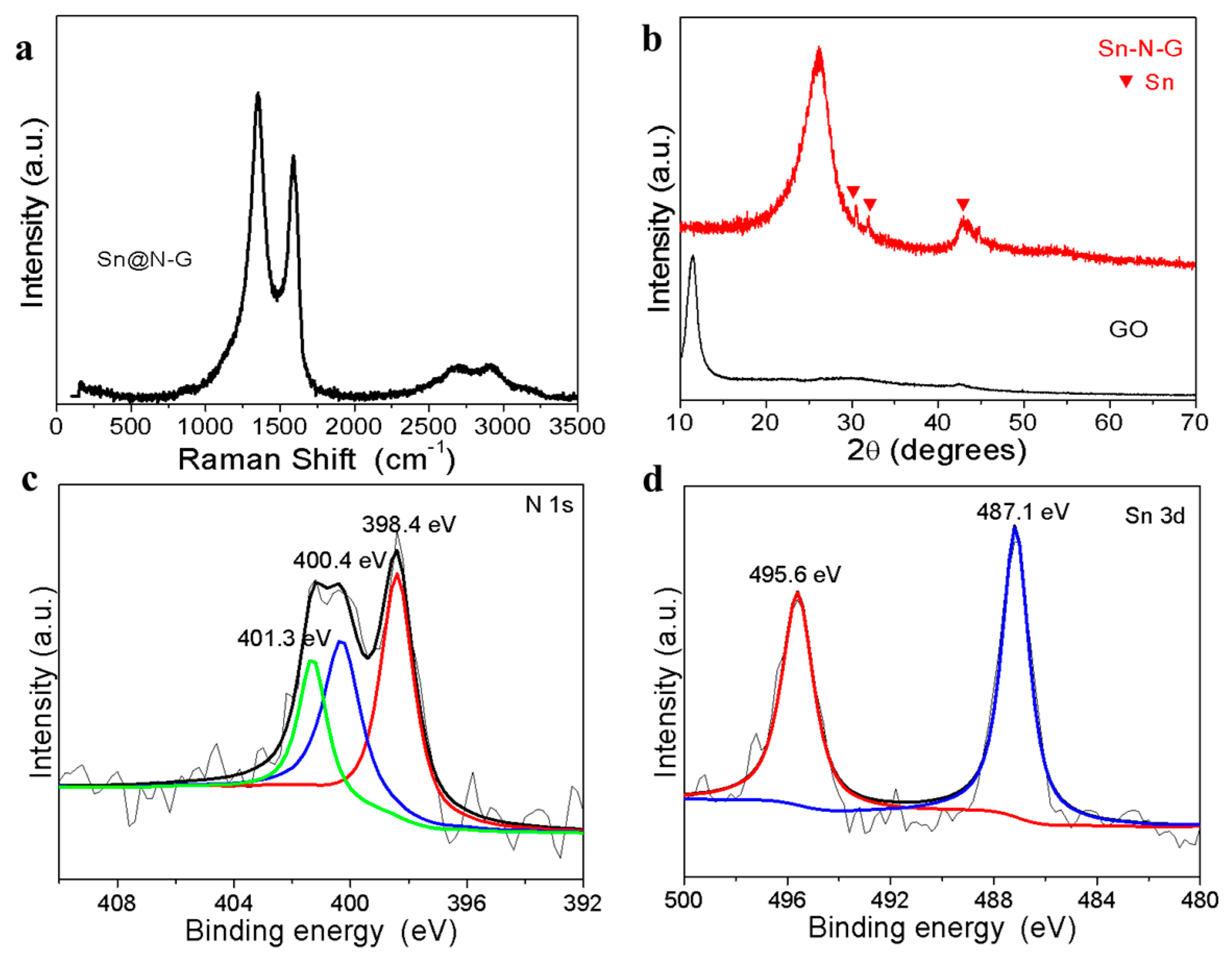
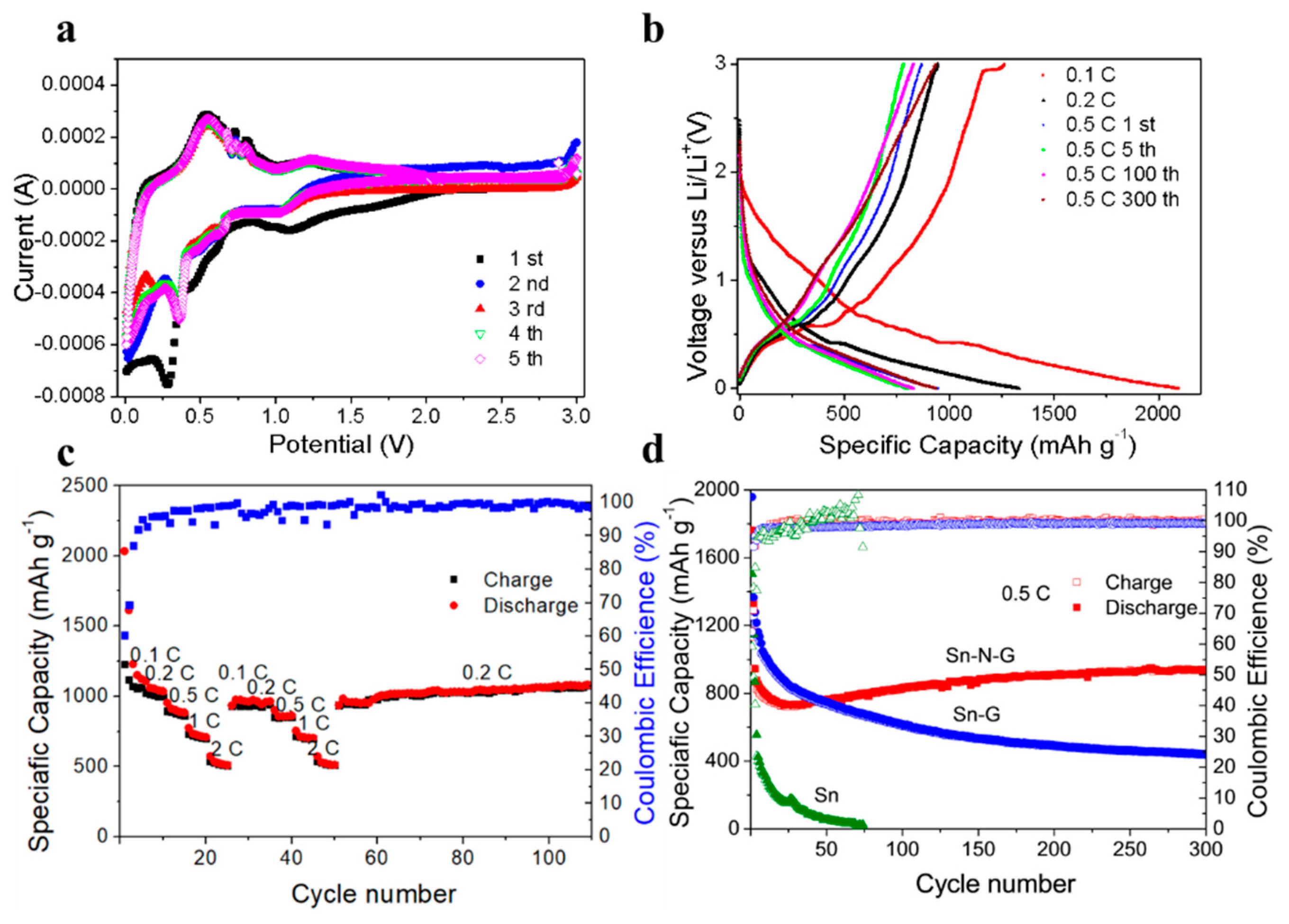
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Armand:, M.; Tarascon, J.M. Building Better Batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Cui, C.; Liu, X.; Wu, N.; Sun, Y. Facile synthesis of core/shell-structured Sn/onion-like carbon nanocapsules as Highperformance anode material for lithium-ion batteries. Mater. Lett. 2015, 143, 35–37. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Chui, Y.; Wu, Q.; Chen, X.; Zhang, W. Three-dimensional Sn-graphene anode for high-performance lithium-ion battery. Nanoscale 2013, 5, 10599–10604. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Zhu, Y.; Liu, Y.; Langrock, A.; Zachariah, M.R.; Wang, C. Uniform Nano-Sn/C Composite Anodes for Lithium Ion Batteries. Nano Lett. 2013, 13, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.; Cao, Z.; Wang, C.; Zheng, J.; Xu, X. Ultrafine Sn nanoparticles embedded in shell of N-doped hollow carbon spheres as high rate anode for lithium-ion batteries. Appl. Surf. Sci. 2017, 404, 342–349. [Google Scholar] [CrossRef]
- Deng, D.; Kim, M.G.; Lee, J.Y.; Cho, J. Green Energy Storage Materials: Nanostructured TiO2 and Sn-Based Anodes for Lithium-Ion Batteries. Energy Environ. Sci. 2009, 2, 818–837. [Google Scholar] [CrossRef]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.Z.; Li, J. Graphene networks anchored with Sn@ graphene as lithium ion battery anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Gu, L.; Wang, C. Three-Dimensional Porous Core-Shell Sn@ Carbon Composite Anodes for High-Performance Lithium-Ion Battery Applications. Adv. Energy Mater. 2012, 2, 238–244. [Google Scholar] [CrossRef]
- Derrien, G.; Hassoun, J.; Panero, S.; Scrosati, B. Nanostructured Sn–C composite as an advanced anode material in high-performance Lithium-ion batteries. Adv. Mater. 2007, 19, 2336–2340. [Google Scholar] [CrossRef]
- Ishihara, T.; Nakasu, M.; Yoshio, M.; Nishiguchi, H.; Takita, Y. Carbon nanotube coating silicon doped with Cr as a high capacity anode. J. Power Sources 2005, 146, 161–165. [Google Scholar] [CrossRef]
- Ardhi, R.E.A.; Liu, G.; Tran, M.X.; Hudaya, C.; Kim, J.Y.; Yu, H.; Lee, J.K. Self-Relaxant Superelastic Matrix Derived from C60 Incorporated Sn Nanoparticles for Ultra-High-Performance Li-Ion Batteries. ACS Nano 2018, 12, 5588–5604. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ Intercalation Pseudocapacitance in Graphene-Coupled Titanium Oxide Enabling Ultra-Fast Sodium Storage and Long-Term Cycling. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yi, Z.; Wang, C.; Wu, Y.; Wang, L. Controllable Fabrication of C/Sn and C/SnO/Sn Composites as Anode Materials for High-Performance Lithium-Ion Batteries. Chem. Eng. J. 2017, 330, 1035–1043. [Google Scholar] [CrossRef]
- Dou, Y.; Xu, J.; Ruan, B.; Liu, Q.; Pan, Y.; Sun, Z.; Dou, S.X. Atomic Layer-by-Layer Co3O4/Graphene Composite for High Performance Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501835. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, X.; Lian, P.; Yang, W.; Wang, H. Superior cycle performance of Sn@C/graphene nanocomposite as an anode material for lithium-ion batteries. J. Solid State Chem. 2011, 184, 1400–1404. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Yang, J.; Wang, J.; Geng, D.; Li, R.; Cai, M.; Sham, T.-K.; Sun, X. Hierarchical nanostructured core–shell Sn@C nanoparticles embedded in graphene nanosheets: Spectroscopic view and their application in lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 3535–3542. [Google Scholar] [CrossRef]
- Wen, Z.; Cui, S.; Kim, H.; Mao, S.; Yu, K.; Lu, G.; Pu, H.; Mao, O.; Chen, J. Binding Sn-based nanoparticles on graphene as the anode of rechargeable lithium-ion batteries. J. Mater. Chem. 2012, 22, 3300–3306. [Google Scholar] [CrossRef]
- Nithya, C.; Gopukumar, S. Reduced Graphite Oxide/Nano Sn: A Superior Composite Anode Material for Rechargeable Lithium-Ion Batteries. ChemSusChem 2013, 6, 898–904. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. Sn@CNT Nanostructures Rooted in Graphene with High and Fast Li-Storage Capacities. ACS Nano 2011, 5, 8108–8114. [Google Scholar] [CrossRef]
- Zhou, D.; Song, W.; Li, X.; Fan, L.; Deng, Y. Tin nanoparticles embedded in porous N-doped graphene-like carbon network as high-performance anode material for lithium-ion batteries. J. Alloys Compd. 2017, 699, 730–737. [Google Scholar] [CrossRef]
- Luo, B.; Wang, B.; Liang, M.; Ning, J.; Li, X.; Zhi, L. Reduced Graphene Oxide-Mediated Growth of Uniform Tin-Core/Carbon-Sheath Coaxial Nanocables with Enhanced Lithium Ion Storage Properties. Adv. Mater. 2012, 24, 1405–1409. [Google Scholar] [CrossRef]
- Ji, L.; Tan, Z.; Kuykendall, T.; An, E.J.; Fu, Y.; Battaglia, V.; Zhang, Y. Multilayer nanoassembly of Sn-nanopillar arrays sandwiched between graphene layers for high-capacity lithium storage. Energy Environ. Sci. 2011, 4, 3611–3616. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.-M.; Wang, Z.-H.; Shao, Q.-G.; Li, X.; Yuan, L.-X.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H. Nitrogen-Doped Porous Carbon Nanofiber Webs as Anodes for Lithium Ion Batteries with a Superhigh Capacity and Rate Capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior Cycle Stability of Nitrogen-Doped Graphene Nanosheets as Anodes for Lithium Ion Batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Nair, A.K.; Elizabeth, I.; Thomas, G.S.S.; Kalarikkal, K.M.S.N. Nitrogen Doped Graphene-Silver Nanowire Hybrids: An Excellent Anode Material for Lithium Ion Batteries. Appl. Surf. Sci. 2018, 2, 1119–1129. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Liu, Z.; Zheng, X.; Zheng, J.; Li, X. Ultrafine Sn nanocrystals in a hierarchically porous N-doped carbon for lithium ion batteries. Nano Res. 2017, 10, 1950–1958. [Google Scholar] [CrossRef]
- Liu, C.-J.; Huang, H.; Cao, G.-Z.; Xue, F.-H.; Paredes Camacho, R.A.; Camacho, P.; Dong, X.L. Dong Enhanced Electrochemical Stability of Sn-Carbon Nanotube Nanocapsules as Lithium-Ion Battery Anode. Electrochim. Acta 2014, 144, 376–382. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Du, J.; Jin, Q.; Zhang, T.; Cheng, F.; Chen, J. Ultrasmall Sn Nanoparticles Embedded in Nitrogen-Doped Porous Carbon as High-Performance Anode for Lithium-Ion Batteries. Nano Lett. 2013, 14, 153–157. [Google Scholar] [CrossRef]
- Huang, X.; Cui, S.; Chang, J.; Hallac, P.B.; Fell, C.R.; Luo, Y.; Metz, B.; Jiang, J.; Hurley, P.T.; Chen, J. A Hierarchical Tin/Carbon Composite as an Anode for Lithium-Ion Batteries with a Long Cycle Life. Angew. Chem. 2015, 127, 1510–1513. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Yu, J.; Sun, Y. Ultra small Sn Nanoparticles Embedded in N-doped Carbon Nanospheres as Long Cycle Life Anode for Lithium Ion Batteries. Mater. Lett. 2018, 223, 203–206. [Google Scholar] [CrossRef]
- Chen, C.; Peng, L.; Li, Y.; Zhang, L.; Xiang, J.; Hu, P.; Cheng, S.; Huang, Y.; Xie, J. Granadilla-Inspired Structure Design for Conversion/Alloy-Reaction Electrode with Integrated Lithium Storage Behaviors. ACS Appl. Mater. Interfaces 2017, 9, 15470–15476. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Huang, Y.; Wu, S.; Gao, Z.; Liu, H.; Hu, P.; Qie, L. Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties. Nanomaterials 2019, 9, 1084. https://doi.org/10.3390/nano9081084
Sun Q, Huang Y, Wu S, Gao Z, Liu H, Hu P, Qie L. Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties. Nanomaterials. 2019; 9(8):1084. https://doi.org/10.3390/nano9081084
Chicago/Turabian StyleSun, Quan, Ying Huang, Shi Wu, Zhonghui Gao, Hang Liu, Pei Hu, and Long Qie. 2019. "Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties" Nanomaterials 9, no. 8: 1084. https://doi.org/10.3390/nano9081084
APA StyleSun, Q., Huang, Y., Wu, S., Gao, Z., Liu, H., Hu, P., & Qie, L. (2019). Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties. Nanomaterials, 9(8), 1084. https://doi.org/10.3390/nano9081084



