Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Lignin (A-EHL)
2.3. Grafting of β-CD (CD-EHL)
2.4. Preparation of CD-EHL Hollow Nanoparticles (CD-LHNPs)
2.5. Drug Loading
2.6. In Vitro Release Studies
2.7. In Vitro Cytotoxicity Studies
3. Results and Discussion
3.1. Characterization of β-Cyclodextrin-Modified EHL
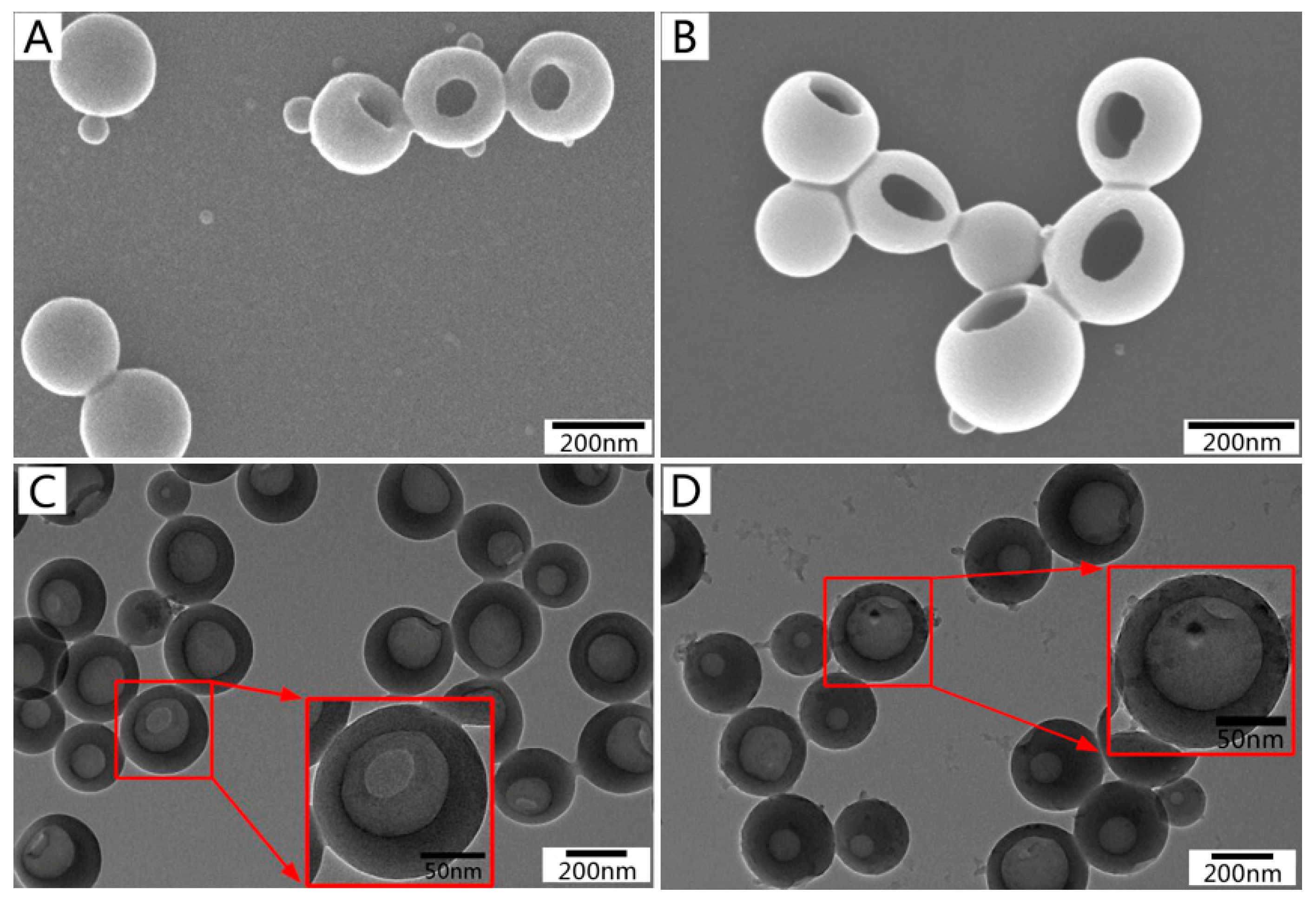
3.2. Morphology, Size, and Chemical Characterization of β-CD-Modified LHNPs
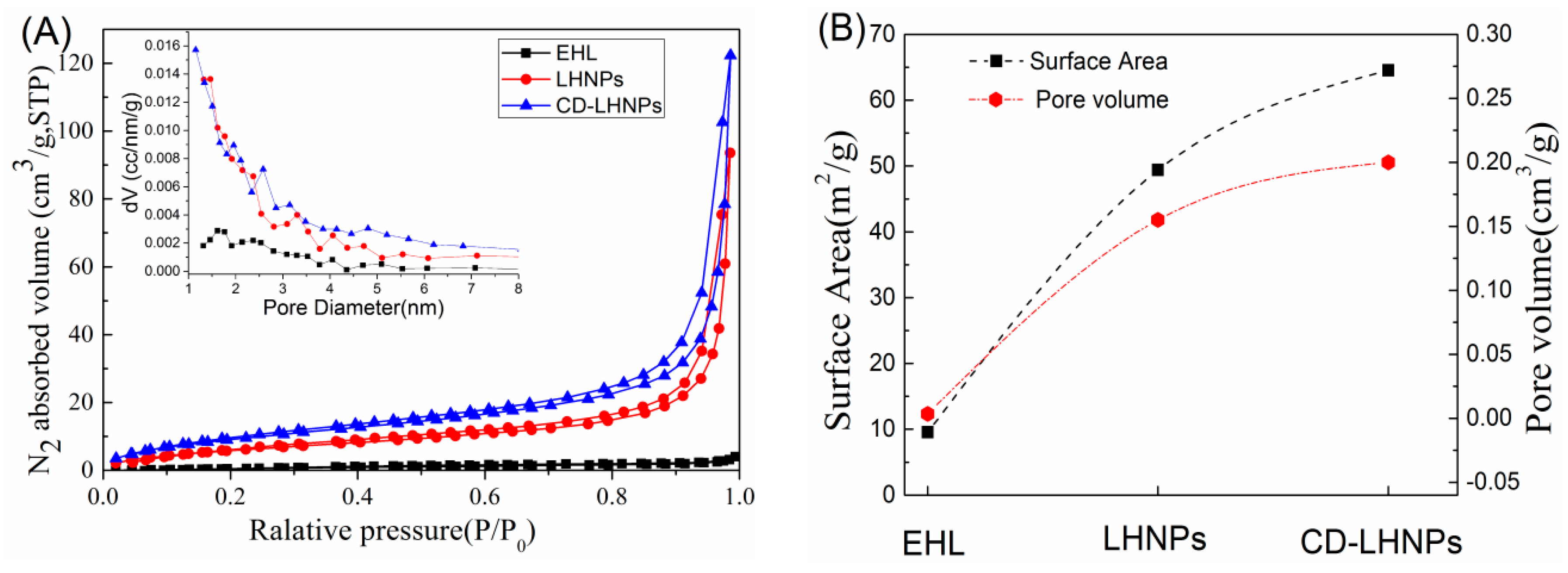
3.3. Space Structure Performance Characteristics of LHNPs
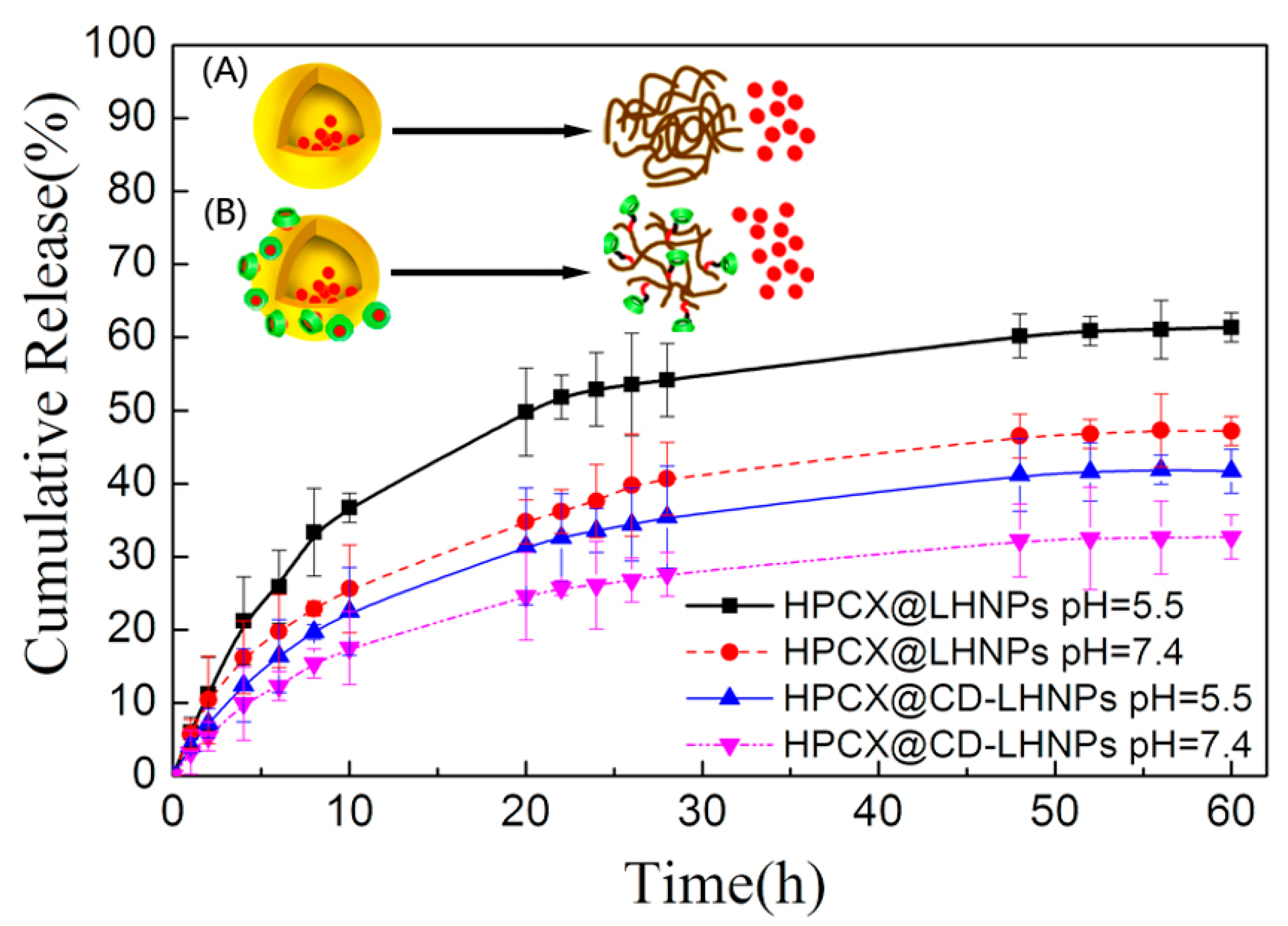
3.4. Encapsulation and Sustained-Release Capacity of CD-LHNPs
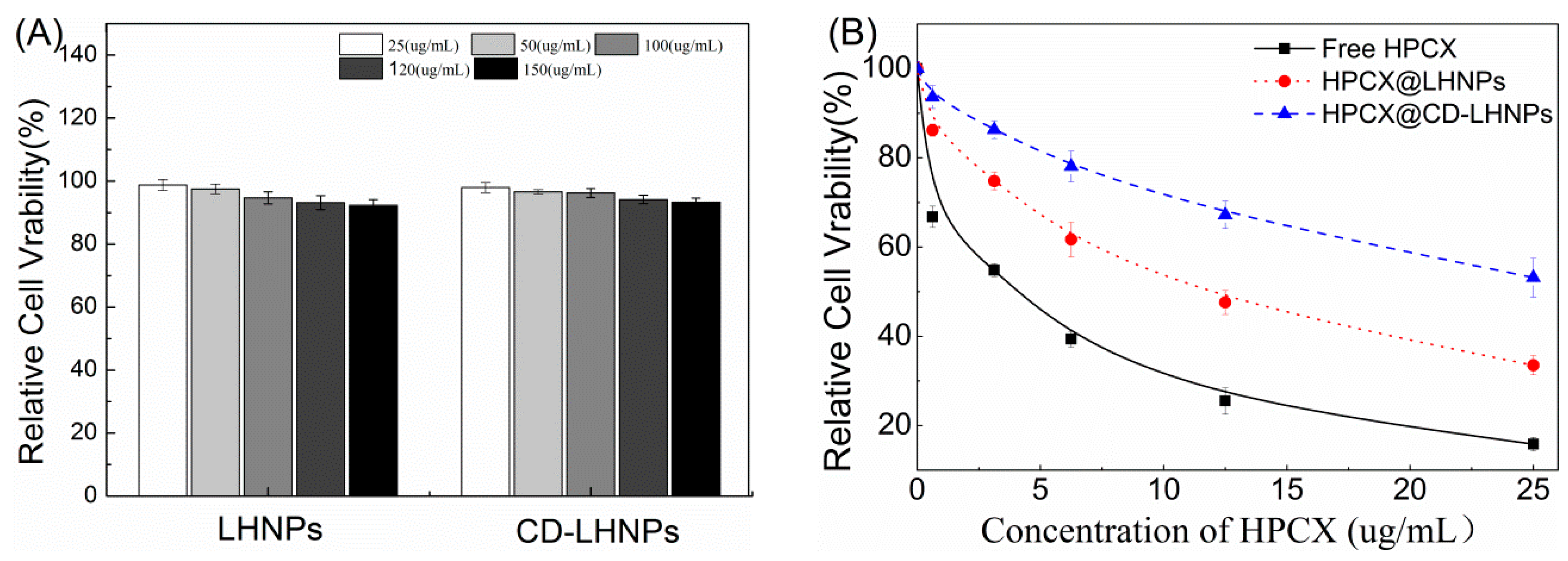
3.5. In Vitro Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, Z.; Li, F.; Wen, J.L.; Wang, X.; Sun, R.C. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Mariarosaria, T.; Francesca, C.; Pasquale, M.; Flavia, C.; Federica, M.; Claudia, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar]
- Li, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Klett, A.S.; Thies, M.; Zheng, Y. Inhibitory Effects of Lignin on Enzymatic Hydrolysis: The Role of Lignin Chemistry and Molecular Weight. Renew. Energy 2018, 123, 664–674. [Google Scholar] [CrossRef]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef]
- Shaffer, S.M.; Dunagin, M.C.; Torborg, S.R.; Torre, E.A.; Emert, B.; Krepler, C.; Beqiri, M.; Sproesser, K.; Brafford, P.A.; Xiao, M. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017, 546, 431. [Google Scholar] [CrossRef]
- Kamran, M.; Long, Z.J.; Xu, D.; Lv, S.S.; Liu, B.; Wang, C.L.; Xu, J.; Lam, E.W.; Liu, Q. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis 2017, 6, e298. [Google Scholar] [CrossRef] [PubMed]
- Marzi, L.; Agama, K.; Murai, J.; Difilippantonio, S.; James, A.; Peer, C.J.; Figg, W.D.; Beck, D.; Elsayed, M.; Cushman, M. Novel fluoroindenoisoquinoline non-camptothecin topoisomerase I inhibitors. Mol. Cancer Ther. 2018, 17, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef]
- Tardy, B.L.; Richardson, J.J.; Guo, J.; Lehtonen, J.; Rojas, O.J. Lignin Nano- and Microparticles as Template for Nanostructured Materials: Formation of Hollow Metal-Phenolic Capsules. Green Chem. 2018, 20, 1335–1344. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.; Nikitin, M. Nanoparticle-based drug delivery: Via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2018, 11, 1636–1646. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Caruso, F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Zhu, W.; Rui, L.; Si, C. Lignin-Containing Self-Nanoemulsifying Drug Delivery System for Enhance Stability and Oral Absorption of trans-Resveratrol. Part. Part. Syst. Charact. 2018, 35, 1700447. [Google Scholar]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-responsive Lignin Based Nanocapsules for Controlled Release of Hydrophobic Molecules. ACS Sustain. Chem. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-Based Microsphere: Preparation and Performance on Encapsulating the Pesticide Avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.; Zou, Z.; Si, C. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Marcelo, G.; López-González, M.; Trabado, I.; Rodrigo, M.M.; Valiente, M.; Mendicuti, F. Lignin inspired PEG hydrogels for drug delivery. Mater. Today Commun. 2016, 7, 73–80. [Google Scholar] [CrossRef]
- Cheng, J.G.; Zhang, Y.M.; Yu, L. Supramolecular Assembly of Thiolated Cyclodextrin and Ferrocene Derivative for Controlled Drug Delivery. Chemnanomat 2018, 4, 758–763. [Google Scholar] [CrossRef]
- Sonia, M.; Cristina, M.; Kostas, K.; Maurizio, P.; Ester, V. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar]
- Qiu, H.B.; Yang, S.; Han, Y.; Shen, X.S.; Fan, D.; Li, G.; Chu, F. Improvement of the Performance of Plantation Wood by Grafting Water-Soluble Vinyl Monomers onto Cell Walls. ACS Sustain. Chem. Eng. 2018, 6, 14450–14459. [Google Scholar] [CrossRef]
- Lu, B.; Wei, L.; Meng, G.; Hou, J.; Liu, Z.; Guo, X. Synthesis of Self-assemble pH-responsive Cyclodextrin Block Copolymer for Sustained Anticancer Drug Delivery. Chin. J. Poly. Sci. 2017, 35, 924–938. [Google Scholar] [CrossRef]
- Liu, M.; Lv, P.; Liao, R.; Zhao, Y.; Bo, Y. Synthesis, characterization and biological activity of Rhein-cyclodextrin conjugate. J. Mol. Struct. 2017, 1128, 239–244. [Google Scholar] [CrossRef]
- Xiong, F.; Chu, F.; Li, G.; Wang, S.; Qin, T.; Han, Y.; Chen, Y. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Casas, A.; Alonso, M.V.; Oliet, M.; Rojo, E.; Rodríguez, F. FTIR analysis of lignin regenerated from Pinus radiata and Eucalyptus globulus woods dissolved in imidazolium-based ionic liquids. J. Chem. Technol. Biotechnol. 2012, 87, 472–480. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Li, S.; Zhuang, X.; Xu, Y.; Wang, C.; Chu, F. Preparation and properties of UV-absorbent lignin graft copolymer films from lignocellulosic butanol residue. Ind. Crops Prod. 2014, 52, 633–641. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Li, G.; Qin, T.; Wang, S.; Chu, F. Synthesis and characterization of renewable woody nanoparticles fluorescently labeled by pyrene. Ind. Crops Prod. 2016, 83, 663–669. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature;carbonization reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Li, Z.; Bo, Z.; Jia, S.; Ma, M.; Hao, J. Novel supramolecular organogel based on β-cyclodextrin as a green drug carrier for enhancing anticancer effects. J. Mol. Liquids 2017, 250, 19–25. [Google Scholar] [CrossRef]
- Hong, N.; Yuan, L.; Qiu, X. A highly efficient dispersant from black liquor for carbendazim suspension concentrate: Preparation, self-assembly behavior and investigation of dispersion mechanism. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Hong, N.; Wei, Y.; Xue, Y.; Zeng, W.; Huang, J.; Xie, W.; Qiu, X.; Yuan, L. A novel and highly efficient polymerization of sulfomethylated alkaline lignins via alkyl chain cross-linking method. Holzforschung 2016, 70, 297–304. [Google Scholar] [CrossRef]
- Yong, Q.; Deng, Y.; Qiu, X.; Hao, L.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar]
- Lunde, P.J.; Kester, F.L. Chemical and physical gas adsorption in finite multimolecular layers. Chem. Eng. Sci. 1975, 30, 1497–1505. [Google Scholar] [CrossRef]



| Samples | Surface Elemental Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C% | O% | N% | O/C Ratio | C–C | C–N | C–O | C=O | |
| LHNPs | 82.96 | 15.9 | 1.13 | 0.19 | 68. 82 | 0 | 29.55 | 1.64 |
| CD-LHNPs | 78.62 | 18.7 | 2.69 | 0.23 | 43. 93 | 20.96 | 31.23 | 3.88 |
| Sample | LHNPs | CD-LHNPs | ||
|---|---|---|---|---|
| m0/m | EE (%) | DL (%) | EE (%) | DL (%) |
| 10% | 58.6 ± 5 | 5.54 ± 0.3 | 82.4 ± 3 | 7.61 ± 1 |
| 20% | 59.4 ± 7 | 10.62 ± 0.7 | 78.5 ± 5 | 13.57 ± 2 |
| 40% | 51.1 ± 7 | 16.97 ± 0.9 | 70.6 ± 9 | 22.02 ± 2 |
| 60% | 40.5 ± 11 | 19.55 ± 0.7 | 54.8 ± 9 | 24.76 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Chu, F. Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin. Nanomaterials 2019, 9, 997. https://doi.org/10.3390/nano9070997
Zhou Y, Han Y, Li G, Yang S, Chu F. Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin. Nanomaterials. 2019; 9(7):997. https://doi.org/10.3390/nano9070997
Chicago/Turabian StyleZhou, Yu, Yanming Han, Gaiyun Li, Sheng Yang, and Fuxiang Chu. 2019. "Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin" Nanomaterials 9, no. 7: 997. https://doi.org/10.3390/nano9070997





