Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates
Abstract
1. Introduction
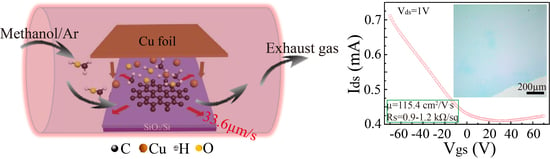
2. Experimental Details
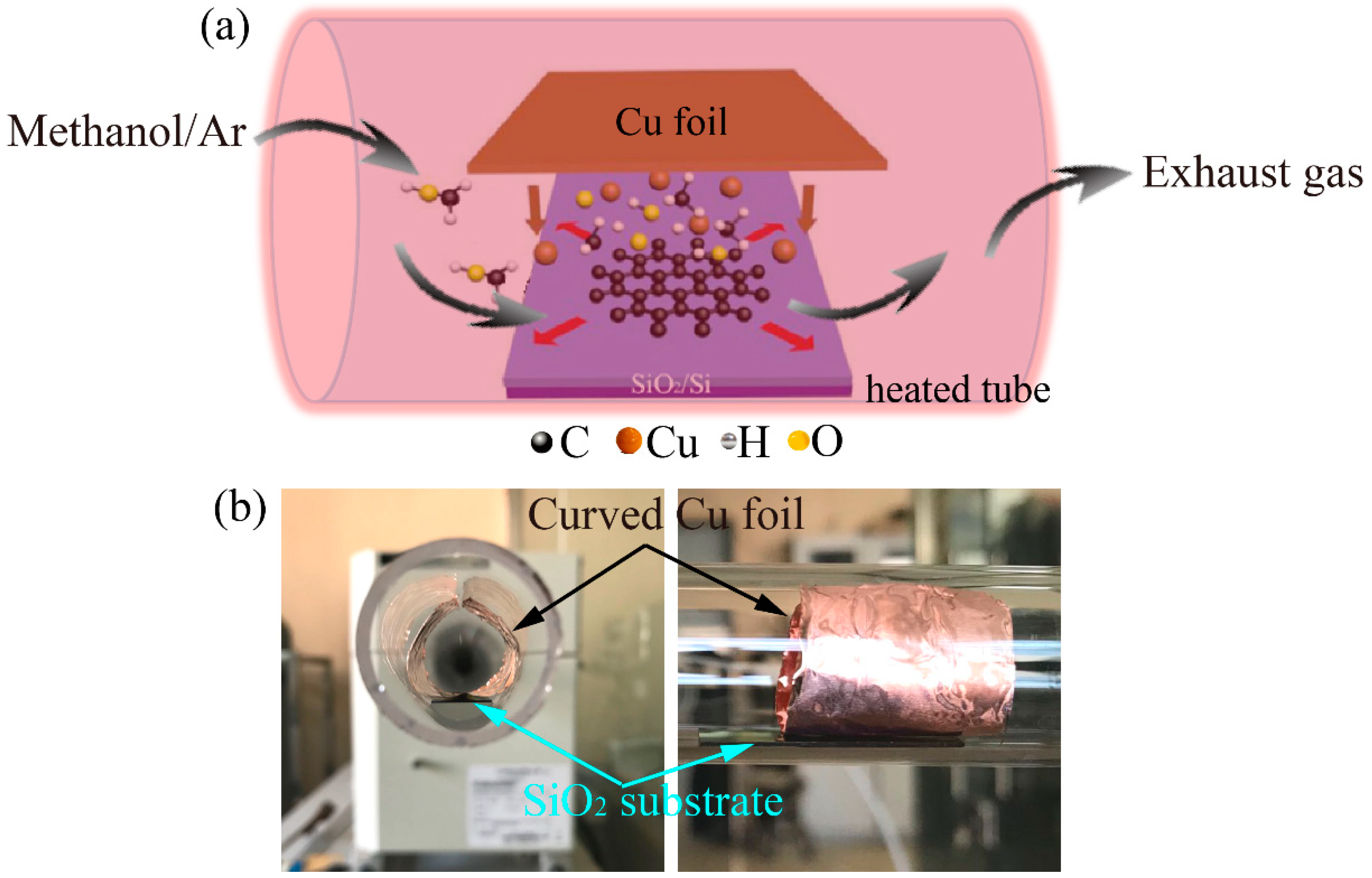
2.1. Direct Growth of Graphene on the SiO2/Si Substrate
2.2. Characterization
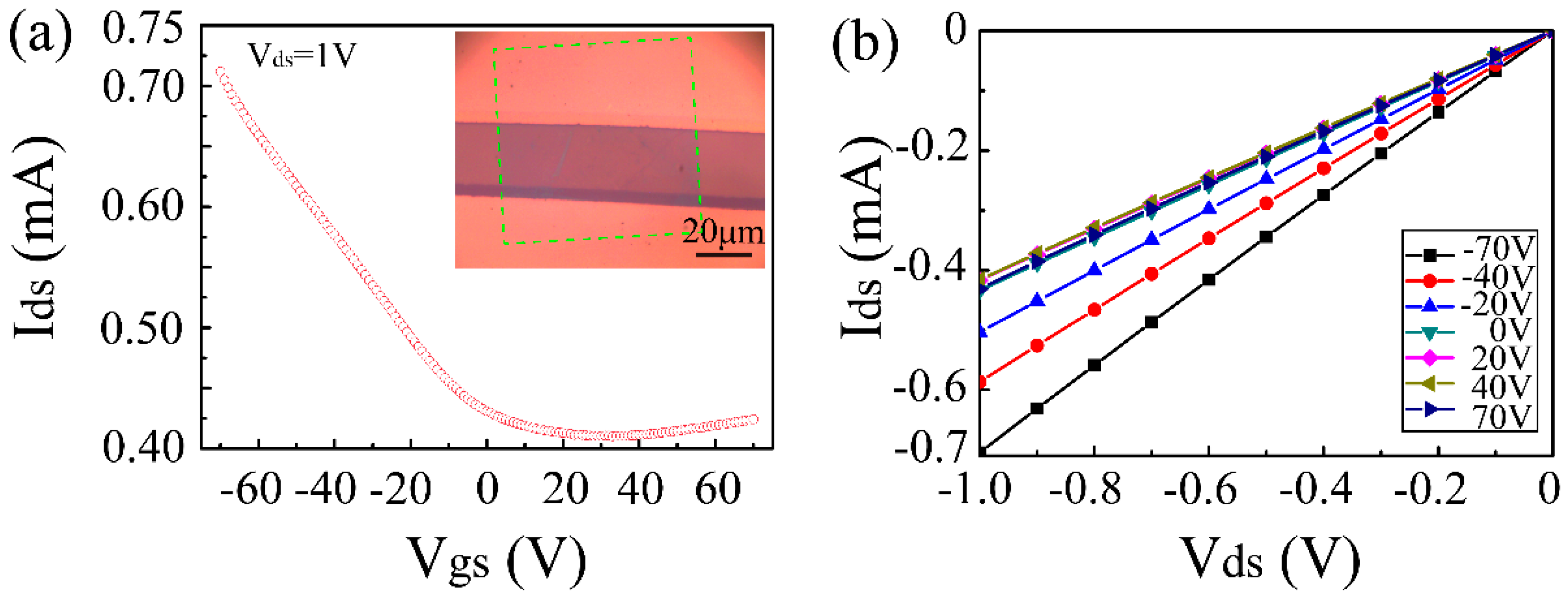
2.3. Fabrication of Field Effect Transistors for Electrical Measurements
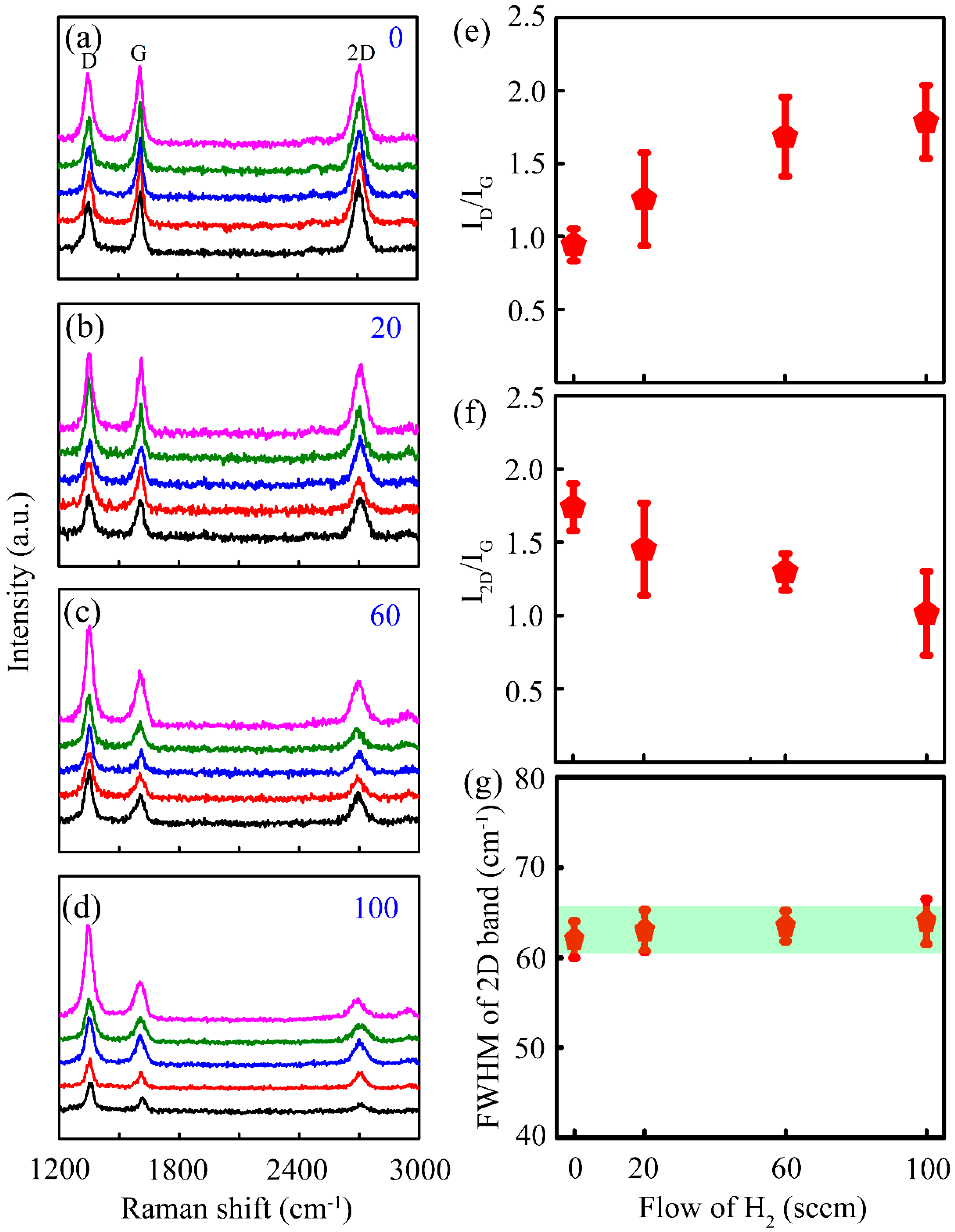
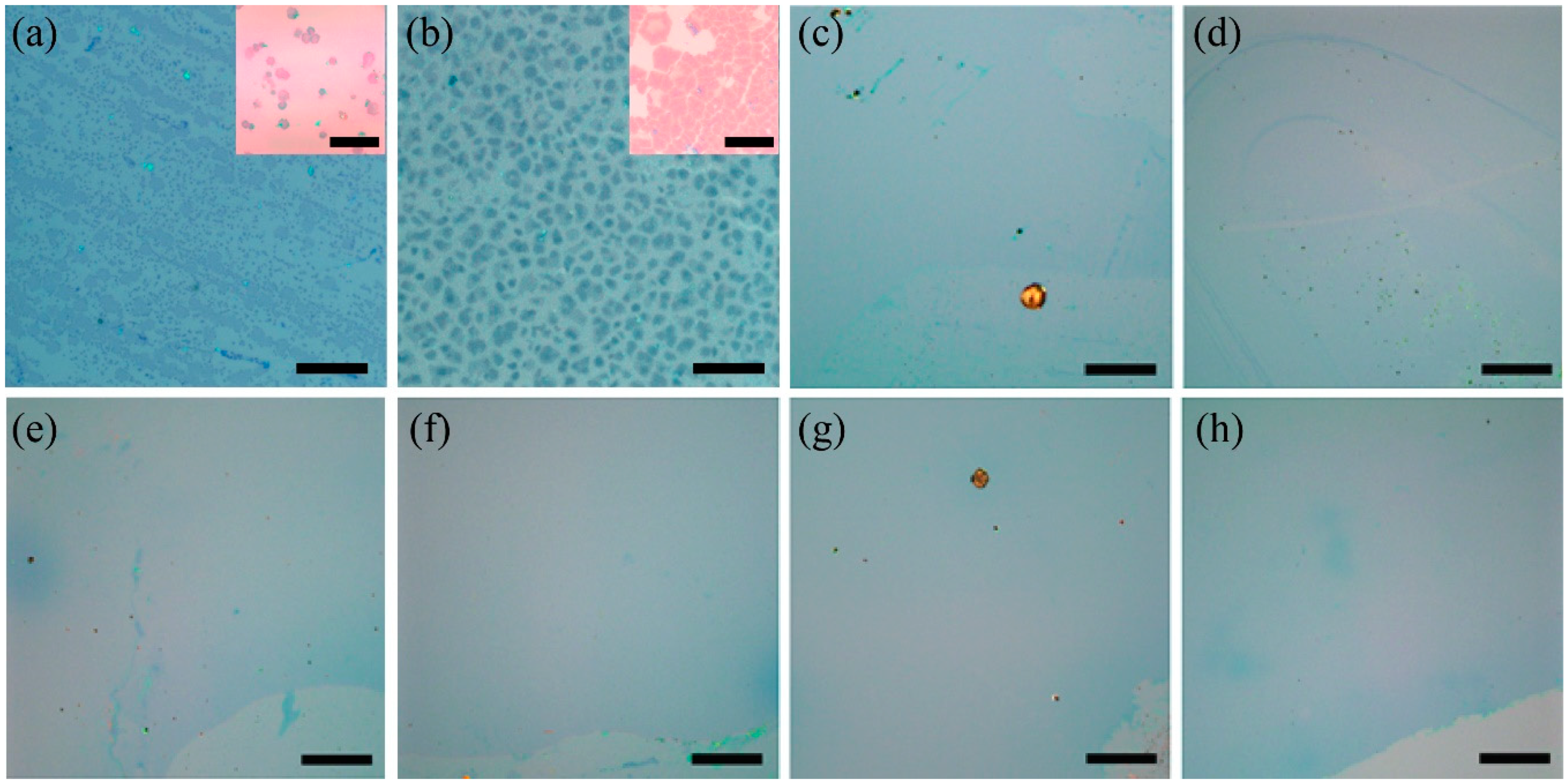
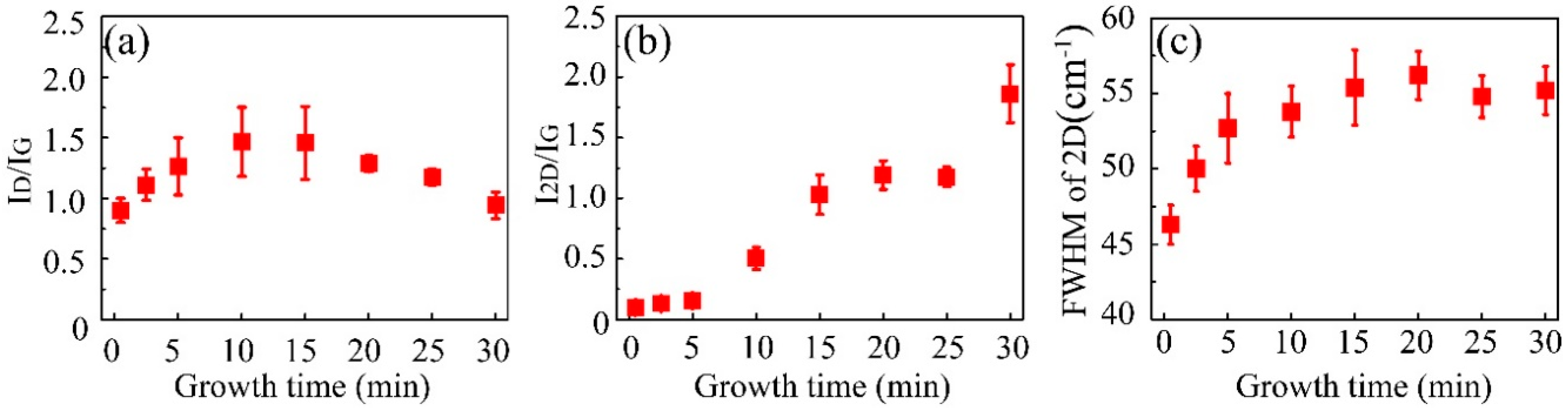
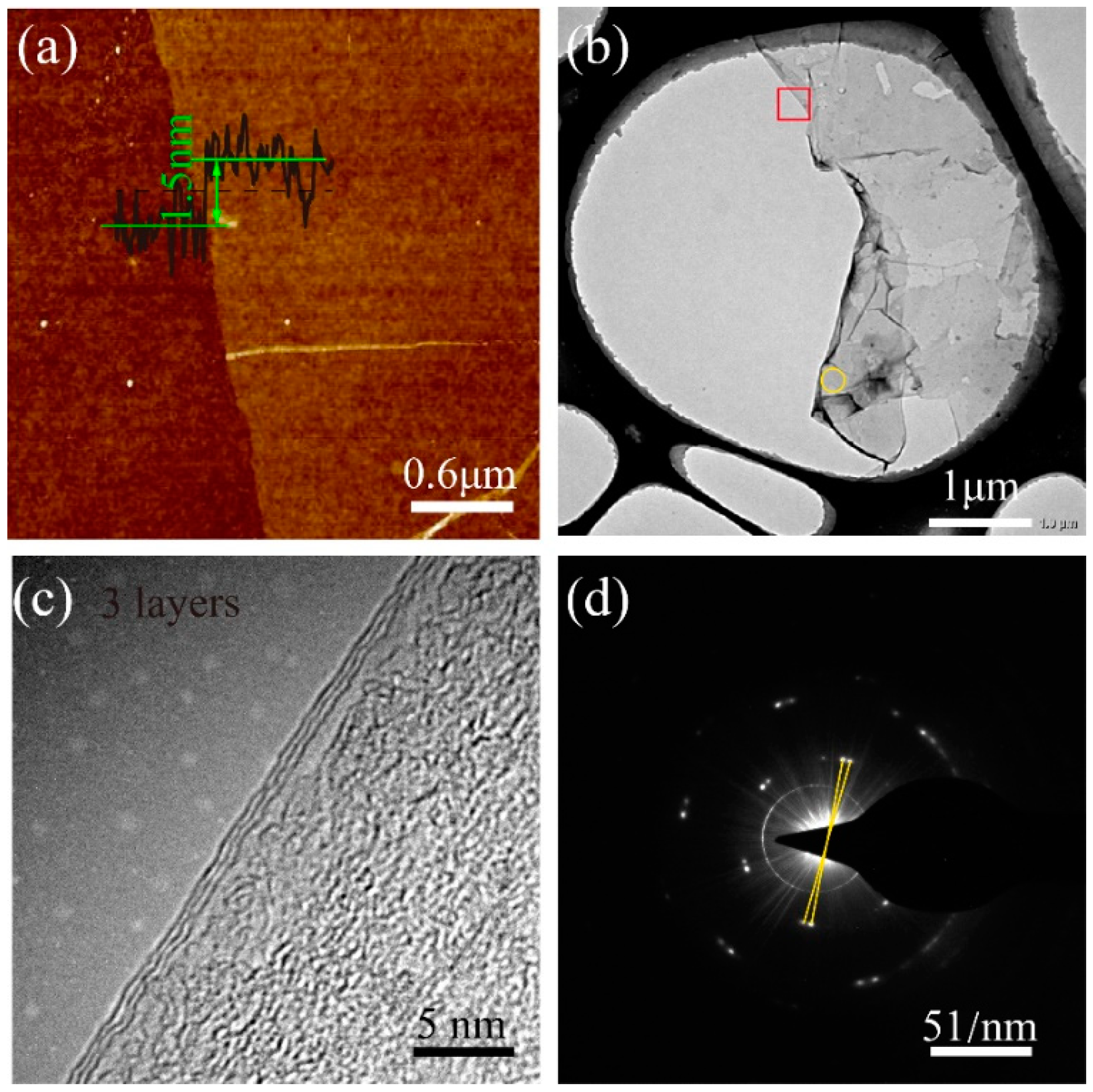
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.; Farmer, D.B.; Xia, F.; Avouris, P. Graphene electronics: Materials, devices, and circuits. Proc. IEEE 2013, 101, 1620–1637. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, X.-L.; Gai, J.-G. Progress and challenges in transfer of large-area graphene films. Adv. Sci. 2016, 3, 1500343. [Google Scholar] [CrossRef]
- Chen, X.; Wu, B.; Liu, Y. Direct preparation of high quality graphene on dielectric substrates. Chem. Soc. Rev. 2016, 45, 2057–2074. [Google Scholar] [CrossRef]
- Ning, J.; Wang, D.; Chai, Y.; Feng, X.; Mu, M.; Guo, L.; Zhang, J.; Hao, Y. Review on mechanism of directly fabricating wafer-scale graphene on dielectric substrates by chemical vapor deposition. Nanotechnology 2017, 28, 284001. [Google Scholar] [CrossRef]
- Pham, V.P.; Jang, H.-S.; Whang, D.; Choi, J.-Y. Direct growth of graphene on rigid and flexible substrates: Progress, applications, and challenges. Chem. Soc. Rev. 2017, 46, 6276–6300. [Google Scholar] [CrossRef]
- Chen, J.; Wen, Y.; Guo, Y.; Wu, B.; Huang, L.; Xue, Y.; Geng, D.; Wang, D.; Yu, G.; Liu, Y. Oxygen-aided synthesis of polycrystalline graphene on silicon dioxide substrates. J. Am. Chem. Soc. 2011, 133, 17548–17551. [Google Scholar] [CrossRef]
- Kim, H.; Song, I.; Park, C.; Son, M.; Hong, M.; Kim, Y.; Kim, J.S.; Shin, H.-J.; Baik, J.; Choi, H.C. Copper-vapor-assisted chemical vapor deposition for high-quality and metal-free single-layer graphene on amorphous SiO2 substrate. ACS Nano 2013, 7, 6575–6582. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Lu, C.-C.; Akiyama-Hasegawa, K.; Lin, Y.-C.; Yeh, C.-H.; Suenaga, K.; Chiu, P.-W. Remote catalyzation for direct formation of graphene layers on oxides. Nano Lett. 2012, 12, 1379–1384. [Google Scholar] [CrossRef]
- Bi, H.; Sun, S.; Huang, F.; Xie, X.; Jiang, M. Direct growth of few-layer graphene films on SiO2 substrates, their photovoltaic applications. J. Mater. Chem. 2012, 22, 411–416. [Google Scholar] [CrossRef]
- Wei, D.; Lu, Y.; Han, C.; Niu, T.; Chen, W.; Wee, A.T.S. Critical crystal growth of graphene on dielectric substrates at low temperature for electronic devices. Angew. Chem. Int. Ed. 2013, 52, 14121–14126. [Google Scholar] [CrossRef]
- Wei, D.; Peng, L.; Li, M.; Mao, H.; Niu, T.; Han, C.; Chen, W.; Wee, A.T.S. Low temperature critical growth of high quality nitrogen doped graphene on dielectrics by plasma-enhanced chemical vapor deposition. ACS Nano 2015, 9, 164–171. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Qiu, L.; Zhuang, J.; Zhang, L.; Wang, H.; Liao, C.; Song, H.; Qiao, R.; Gao, P.; et al. Ultrafast growth of single-crystal graphene assisted by a continuous oxygen supply. Nat. Nanotechnol. 2016, 11, 930–935. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Xin, J.; Yakobson, B.I.; Ding, F. Role of Hydrogen in Graphene Chemical Vapor Deposition Growth on a Copper Surface. J. Am. Chem. Soc. 2014, 136, 3040–3047. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Regmi, M.; Fulvio, P.; Dai, S.; Datskos, P.; Eres, G.; Smirnov, S. Role of hydrogen in chemical vapor deposition growth of large single–crystal graphene. ACS Nano 2011, 5, 6069–6076. [Google Scholar] [CrossRef]
- Jung, D.H.; Kang, C.; Kim, M.; Cheong, H.; Lee, H.; Lee, J.S. Effects of hydrogen partial pressure in the annealing process on graphene growth. J. Phys. Chem. C 2014, 118, 3574–3580. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, J.; Li, X.; Wang, B.; Hao, L.; Liang, M.; Jin, M.; Zhi, L. Hydrogen-induced effects on the CVD growth of high-quality graphene structure. Nanoscale 2013, 5, 8363–8366. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, B.; Wei, Z.; Luo, Z.; Wei, D.; Xi, Y.; Zhang, Y.; Liu, Y. Roles of H2 in annealing and growth times of graphene CVD synthesis over copper foil. J. Mater. Chem. A 2014, 2, 16208–16216. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, J.; Qiu, Y.; Yang, J.; Hu, P.A. Fast growth of graphene on SiO2/Si substrates by atmospheric pressure chemical vapor deposition with floating metal catalysts. Sci. China Chem. 2016, 59, 707–712. [Google Scholar] [CrossRef]
- Oshima, H.; Suzuki, Y.; Shimazu, T.; Maruyama, S. Novel and simple synthesis method for submillimeter long vertically aligned single-walled carbon nanotubes by no-flow alcohol catalytic chemical vapor deposition. Jpn. J. Appl. Phys. 2008, 47, 1982–1984. [Google Scholar] [CrossRef]
- Haw, J.F.; Song, W.; Marcus, D.M.; Nicholas, J.B. The mechanism of methanol to hydrocarbon catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Gadipelli, S.; Calizo, I.; Ford, J.; Cheng, G.; Hight Walker, A.R.; Yildirim, T. A highly practical route for large-area, single layer graphene from liquid carbon sources such as benzene and methanol. J. Mater. Chem. 2011, 21, 16057–16065. [Google Scholar] [CrossRef]
- Kaiser, P.; Unde, R.B.; Kern, C.; Jess, A. Production of liquid hydrocarbons with CO2 as carbon source based on reverse water-gas shift and Fischer-Tropsch synthesis. Chem. Ing. Tech. 2013, 85, 489–499. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, P.; Wang, X.; Wang, Z.; Liu, D.; Yang, B.; Cao, W. CVD growth of large area and uniform graphene on tilted copper foil for high performance flexible transparent conductive film. J. Mater. Chem. 2012, 22, 18283–18290. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kim, P.; Zhang, L.; Zhou, C. Anisotropic hydrogen etching of chemical vapor deposited graphene. ACS Nano 2012, 6, 126–132. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T.L. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Zhao, P.; Kumamoto, A.; Kim, S.; Chen, X.; Hou, B.; Chiashi, S.; Einarsson, E.; Ikuhara, Y.; Maruyama, S. Self-limiting chemical vapor deposition growth of monolayer graphene from ethanol. J. Phys. Chem. C 2013, 117, 10755–10763. [Google Scholar] [CrossRef]
- Tamboli, S.H.; Kim, B.S.; Choi, G.; Lee, H.; Lee, D.; Patil, U.M.; Lim, J.; Kulkarni, S.B.; Jun, S.C.; Cho, H.H. Post-heating effects on the physical and electrochemical capacitive properties of reduced graphene oxide paper. J. Mater. Chem. A 2014, 2, 5077–5082. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene–based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, P.; Zhang, R.; Wang, X.; Yang, B.; Cao, W.; Li, Y.; He, X.; Wang, Z.; O’Neill, W. Soft-lithographic processed soluble micropatterns of reduced graphene oxide for wafer-scale thin film transistors and gas sensors. J. Mater. Chem. 2012, 22, 714–718. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Priydarshi, M.K.; Chen, Z.; Bachmatiuk, A.; Zou, Z.; Chen, Z.; Song, X.; Gao, Y.; Rümmeli, M.H.; et al. Direct chemical vapor deposition-derived graphene glasses targeting wide ranged applications. Nano Lett. 2015, 15, 5846–5854. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Phys. Rev. B 2008, 77, 235430. [Google Scholar] [CrossRef]
- Shin, H.J.; Choi, W.M.; Yoon, S.M.; Han, G.H.; Woo, Y.S.; Kim, E.S.; Chae, S.J.; Li, X.S.; Benayad, A.; Loc, D.D.; et al. Transfer-free growth of few-layer graphene by self-assembled monolayers. Adv. Mater. 2011, 23, 4392–4397. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, S.J.; Jung, M.H.; Choi, K.Y.; Bae, S.; Lee, S.-K.; Lee, Y.; Shin, D.; Lee, B.; Shin, H. Low-temperature growth and direct transfer of graphene-graphitic carbon films on flexible plastic substrate. Nanotechnology 2012, 23, 344016. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, G.; Wu, X.; D’Arsiè, L.; Robertson, J. Metal-catalyst-free growth of graphene on insulating substrates by ammonia–assisted microwave plasma-enhanced chemical vapor deposition. RSC Adv. 2017, 7, 33185–33193. [Google Scholar] [CrossRef]
- Murakami, K.; Tanaka, S.; Hirukawa, A.; Hiyama, T.; Kuwajima, T.; Kano, E.; Takeguchi, M.; Fujita, J.-I. Direct synthesis of large area graphene on insulating substrate by gallium vapor-assisted chemical vapor deposition. Appl. Phys. Lett. 2015, 106, 093112. [Google Scholar] [CrossRef]
- Kwak, J.; Chu, J.H.; Choi, J.-K.; Park, S.-D.; Go, H.; Kim, S.Y.; Park, K.; Kim, S.-D.; Kim, Y.-W.; Yoon, E.; et al. Near room-temperature synthesis of transfer-free graphene films. Nat. Commun. 2012, 3, 645. [Google Scholar] [CrossRef]
- Yan, Z.; Peng, Z.; Sun, Z.; Yao, J.; Zhu, Y.; Liu, Z.; Ajayan, P.M.; Tour, J.M. Growth of bilayer graphene on insulating substrates. ACS Nano 2011, 5, 8187–8192. [Google Scholar] [CrossRef]
- Kato, T.; Hatakeyama, R. Direct Growth of doping-density-controlled hexagonal graphene on SiO2 substrate by rapid-heating plasma CVD. ACS Nano 2012, 6, 8508–8515. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Liu, B.; Gao, L.; Pei, S.; Wu, Z.-S.; Zhao, J.; Cheng, H.-M. Bulk growth of mono- to few-layer graphene on nickel particles by chemical vapor deposition from methane. Carbon 2010, 48, 3543–3550. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrate by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Wu, W.; Yu, Q.; Peng, P.; Liu, Z.; Bao, J.; Pei, S.-S. Control of thickness uniformity and grain size in graphene films for transparent conductive electrode. Nanotechnology 2012, 23, 035603. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R.; Kholmanov, I.; Wu, Y.; Li, H.; Ji, H.; Ruoff, R.S. Low-temperature chemical vapor deposition growth of graphene from toluene on electropolished copper foil. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Y.; Singer, D.W.; Berck, M.E.; Somers, L.A.; Goldsmith, B.R.; Johnson, A.T. Effect of substrate roughness and feedback concentration on growth of wafer-scale graphene at atmospheric pressure. Chem. Mater. 2011, 23, 1441–1447. [Google Scholar] [CrossRef]
| Substrate | Hole Mobility (cm2/V·s) | Sheet Resistance (kΩ/sq) | Ref. |
|---|---|---|---|
| SiO2 | 115.4 | 0.9–1.2 | This work |
| SiO2 | 1.2–19.1 | - | [21] |
| Al2O3 | 16 | 3.8–6.6 | [41] |
| quartz | 3.8 | - | [42] |
| SiO2 | 70 | 32.7 | [43] |
| SiO2 | 120 | 2 | [44] |
| SiO2 | 43–580 | 20–50 | [45] |
| Ni | - | 6.6–8.5 | [46] |
| Ni | 10–2000 | 1–2.6 | [47] |
| Cu | - | 1.3 | [48] |
| Cu | 190 | ~8.02 | [49] |
| Cu | 20–200 | - | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wei, S.; Ge, C.; Zhao, C.; Guo, B.; Zhang, J.; Zhao, J. Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates. Nanomaterials 2019, 9, 964. https://doi.org/10.3390/nano9070964
Zhou L, Wei S, Ge C, Zhao C, Guo B, Zhang J, Zhao J. Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates. Nanomaterials. 2019; 9(7):964. https://doi.org/10.3390/nano9070964
Chicago/Turabian StyleZhou, Lijie, Shuai Wei, Chuanyang Ge, Chao Zhao, Bin Guo, Jia Zhang, and Jie Zhao. 2019. "Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates" Nanomaterials 9, no. 7: 964. https://doi.org/10.3390/nano9070964
APA StyleZhou, L., Wei, S., Ge, C., Zhao, C., Guo, B., Zhang, J., & Zhao, J. (2019). Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates. Nanomaterials, 9(7), 964. https://doi.org/10.3390/nano9070964