Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of ZnO Hybrid Nanostructures
2.3. Preparation of ZnO Electrodes and Dye-Sensitized Solar Cells (DSCs)
2.4. Material Characterization and Photoelectrochemical Measurements
3. Results and Discussion
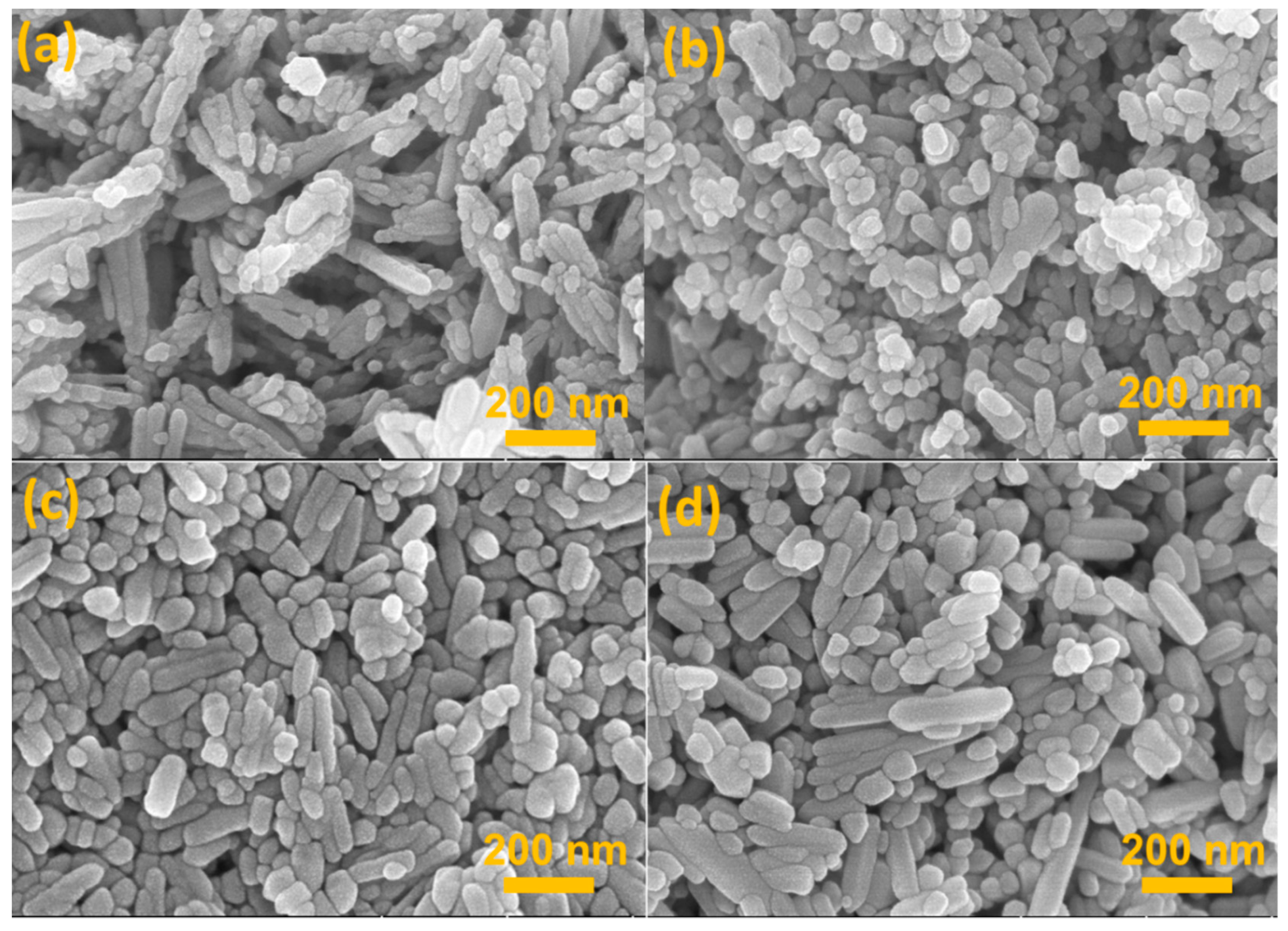
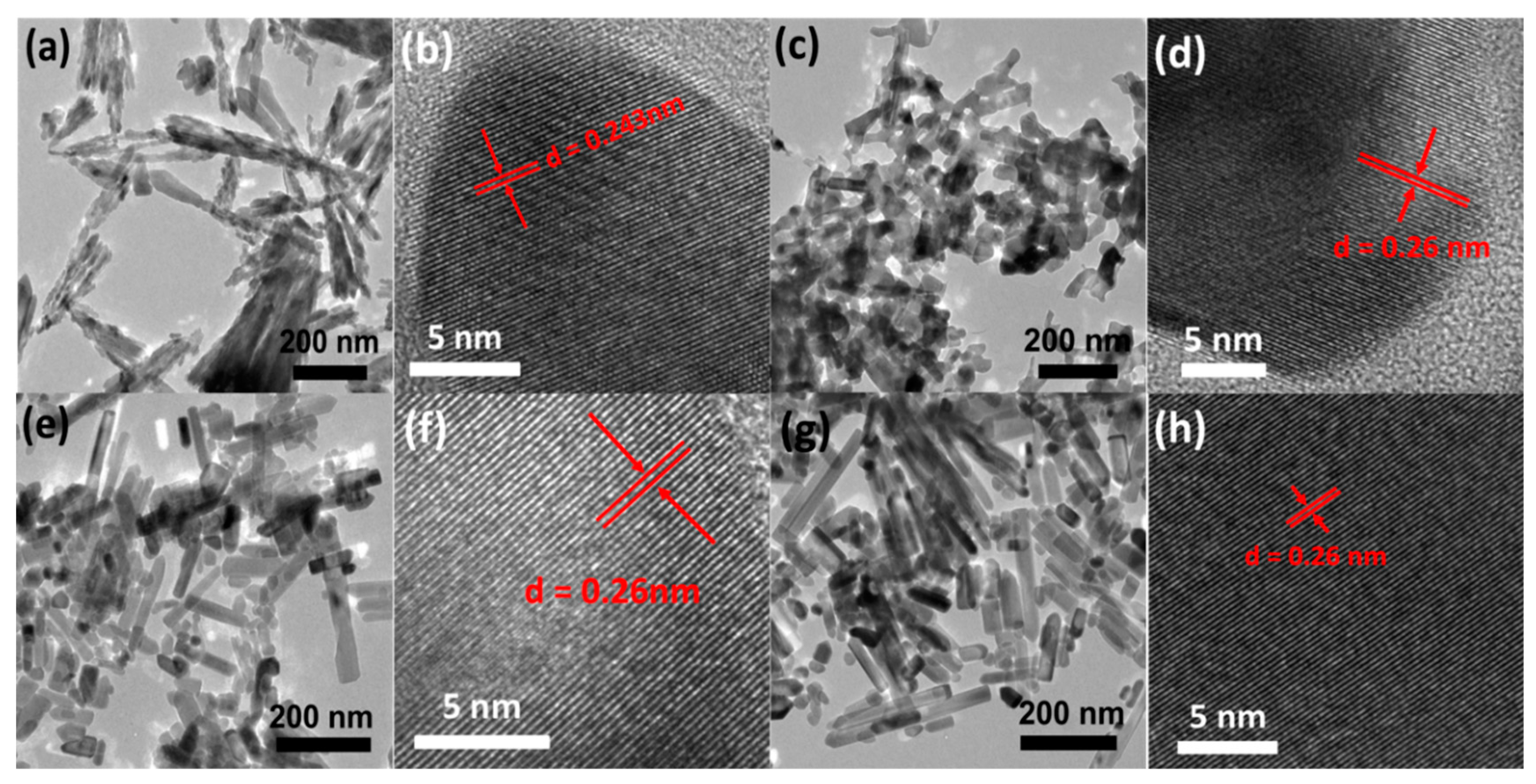
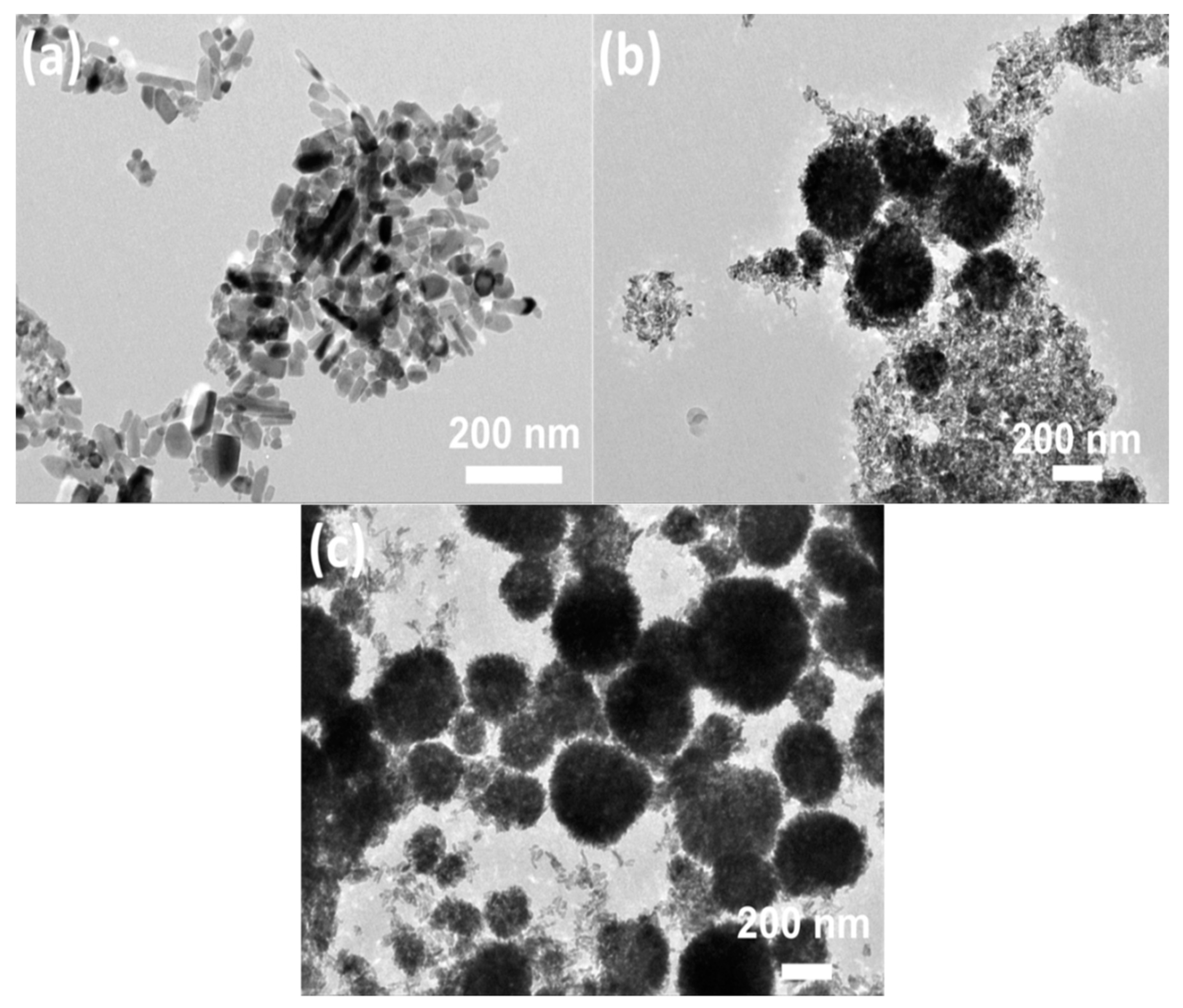
3.1. Morphological Characterization
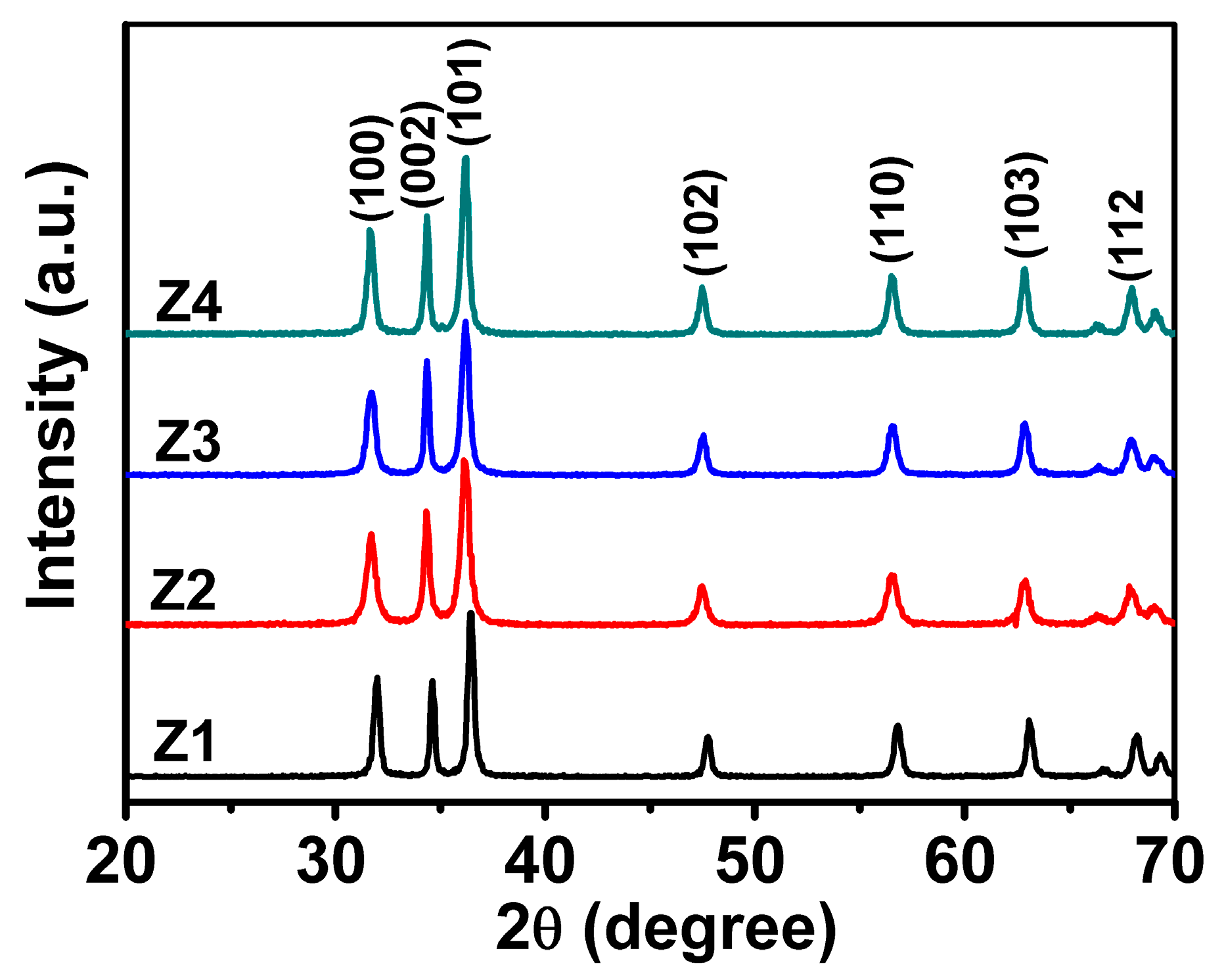
3.2. X-ray Diffraction (XRD) Analysis
3.3. Photovoltaic Performance Test
3.4. Dye Absorption and Diffuse Reflectivity
3.5. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.6. Incident Photon-to-Current Conversion Efficiency (IPCE) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.Y.; Giordano, F.; Liska, P.; Hua, J.L.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372. [Google Scholar] [CrossRef]
- Wu, G.H.; Kong, F.T.; Zhang, Y.H.; Zhang, X.X.; Li, J.Z.; Chen, W.C.; Liu, W.Q.; Ding, Y.; Zhang, C.N.; Zhang, B.; et al. Multiple-Anchoring Triphenylamine Dyes for Dye-Sensitized Solar Cell Application. J. Phys. Chem. C 2014, 118, 8756–8765. [Google Scholar] [CrossRef]
- Wang, D.T.; Liu, S.H.; Shao, M.F.; Zhao, J.H.; Gu, Y.K.; Li, Q.Y.; Zhang, X.X.; Zhao, J.S.; Fang, Y.Z. Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance. Materials 2018, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.W.; Yin, J.; Nie, Z.H.; Yang, Z.J.; Wang, J.H.; Liu, X.; Jin, C.Z.; Zhang, X.X.; Ma, T.L. Earth-Abundant and Nano-Micro Composite Catalysts of Fe3O4@reduced Graphene Oxide for Green and Economical Mesoscopic Photovoltaic Devices with High Effciencies up to 9%. J. Mater. Chem. A 2016, 4, 67–73. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, H.W.; Liu, Z.C.; Nie, Z.H.; Li, Y.H.; Qi, X.; Chen. B., L.; Zhang, Y.T.; Zhang, X.X. Indium- and Platinum-Free Counter Electrode for Green Mesoscopic Photovoltaics through Graphene Electrode and Graphene Composite Catalysts: Interfacial Compatibility. ACS Appl. Mater. Interfaces 2016, 8, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, B.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6, 242. [Google Scholar] [CrossRef]
- Wang, D.T.; Wang, W.X.; Ma, X.Y.; Zhang, C.; Zhao, J.S.; Zhang, X.X. Comparative Study on the Influence of TiO2 Precursors on ZnO-Based Dye-Sensitized Solar Cells. Ind. Eng. Chem. Res. 2015, 54, 12639–12645. [Google Scholar] [CrossRef]
- Wang, D.T.; Liu, S.H.; Shao, M.F.; Li, Q.Y.; Gu, Y.K.; Zhao, J.H.; Zhang, X.X.; Zhao, J.S.; Fang, Y.Z. Aqueous Solution-Processed Multifunctional SnO2 Aggregates for Highly Efficient Dye-Sensitized Solar Cells. Ind. Eng. Chem. Res. 2018, 57, 7136–7145. [Google Scholar] [CrossRef]
- Chen, J.J.; Lu, L.Y.; Wang, W.Y. Zn2SnO4 Nanowires as Photoanode for Dye-Sensitized Solar Cells and the Improvement on Open-Circuit Voltage. J. Phys. Chem. C 2012, 116, 10841–10847. [Google Scholar] [CrossRef]
- Chu, L.; Liu, W.; Yu, A.A.; Qin, Z.F.; Hu, R.Y.; Shu, H.Z.; Luo, Q.P.; Min, Y.G.; Yang, J.P.; Li, X.A. Effect of TiO2 Modification on Urchin-like Orthorhombic Nb2O5 Nanospheres as Photoelectrodes in Dye-Sensitized Solar Cells. Sol. Energy 2017, 153, 584–589. [Google Scholar] [CrossRef]
- Lu, X.H.; Zheng, Y.Z.; Bi, S.Q.; Wang, Y.; Tao, X.; Dai, L.M.; Chen, J.F. Multidimensional ZnO Architecture for Dye-Sensitized Solar Cells with High-Efficiency up to 7.35%. Adv. Energy Mater. 2014, 4, 1301802. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Zhai, S.Y.; Chen, G.F.; Zhang, K.; Yue, Q.L.; Wang, L.; Jia, J.B. Effects of Morphology of Nanostructured ZnO on Direct Electrochemistry and Biosensing Properties of Glucose Oxidase. J. Electroanal. Chem. 2011, 656, 198–205. [Google Scholar] [CrossRef]
- Wang, L.; Yue, Q.L.; Li, H.B.; Xu, S.L.; Liu, J.F. Study on Electrochemiluminescence Spectra of ZnO Flakes. Phys. Chem. Chem. Phys. 2013, 15, 9058–9061. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.P.; Gao, Z.Y.; Xu, F.; Chang, J.L.; Tao, W.G.; He, J.J.; Gao, S.Y.; Jiang, K. Hierarchical ZnO Aggregates Assembled by Orderly Aligned Nanorods for Dye-Sensitized Solar Cells. CrystEngComm 2013, 15, 1210–1217. [Google Scholar] [CrossRef]
- Memarian, N.; Concina, I.; Braga, A.; Rozati, S.M.; Vomiero, A.; Sberveglieri, G. Hierarchically Assembled ZnO Nanocrystallites for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2011, 123, 12321–12325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
- Sang, D.D.; Li, H.D.; Wang, Q.L. Aligned ZnO Nanorod Arrays Growth on GaN QDs for Excellent Optoelectronic Applications. Nanotechnology 2016, 27, 072501. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Liu, J.F.; Lin, H.M.; Chang, H.T. Synthesis of Copper Nanowire Decorated Reduced Graphene Oxide for Electro-Oxidation of Methanol. J. Mater. Chem. A 2013, 1, 5973–5981. [Google Scholar] [CrossRef]
- Galoppini, E.; Rochford, J.; Chen, H.H.; Saraf, G.; Lu, Y.C.; Hagfeldt, A.; Boschloo, G. Fast Electron Transport in Metal Organic Vapor Deposition Grown Dye-Sensitized ZnO Nanorod Solar Cells. J. Phys. Chem. B 2006, 110, 16159–16161. [Google Scholar] [CrossRef]
- Karam, C.; Habchi, R.; Tingry, S.; Miele, P.; Bechelany, M. Design of Multilayers of Urchin-Like ZnO Nanowires Coated with TiO2 Nanostructures for Dye-Sensitized Solar Cells. ACS Appl. Nano Mater. 2018, 1, 3705–3714. [Google Scholar] [CrossRef]
- Karam, C.; Guerra-Nuñez, C.; Habchi, R.; Herro, Z.; Abboud, N.; Khoury, A.; Tingry, S.; Miele, P.; Utke, I.; Bechelany, M. Urchin-Inspired ZnO-TiO2 Core-Shell as Building Blocks for Dye sensitized Solar Cells. Mater. Des. 2017, 126, 314–321. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Ding, H.Y.; Liu, Y.; Tao, X.; Cao, G.Z.; Chen, J.F. In Situ Hydrothermal Growth of Hierarchical ZnO Nanourchin for High-Efficiency Dye-Sensitized Solar Cells. J. Power Sources 2014, 254, 153–160. [Google Scholar] [CrossRef]
- Roy, A.; Mukhop, S. Polyaniline-Layered Rutile TiO2 Nanorods as Alternative Photoanode in Dye-Sensitized Solar Cells. ACS Omega 2019, 4, 1130–1138. [Google Scholar] [CrossRef]
- Kim, H.J.; Yong, K. A Highly Efficient Light Capturing 2D (Nanosheet)–1D (Nanorod) Combined Hierarchical ZnO Nanostructure for Efficient Quantum Dot Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2013, 15, 2109–2116. [Google Scholar] [CrossRef]
- Gao, R.; Cui, Y.X.; Liu, X.J.; Wang, L.D.; Cao, G.Z. A ZnO Nanorod/Nanoparticle Hierarchical Structure Synthesized through a Facile In Situ Method for Dye-Sensitized Solar Cells. J. Mater. Chem. A 2014, 2, 4765–4770. [Google Scholar] [CrossRef]
- Guillén, E.; Azaceta, E.; Vega-Poot, A.; Idígoras, J.; Echeberría, J.; Anta, J.A.; Tena-Zaera, R. ZnO/ZnO Core−Shell Nanowire Array Electrodes: Blocking of Recombination and Impressive Enhancement of Photovoltage in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 13365–13373. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yin, Y.T. Hierarchically Assembled ZnO Nanoparticles on High Diffusion Coefficient ZnO Nanowire Arrays for High Efficiency Dye-Sensitized Solar Cells. Nanoscale 2013, 5, 1777–1780. [Google Scholar] [CrossRef]
- Yodyingyong, S.; Zhang, Q.F.; Park, K.; Dandeneau, C.S.; Zhou, X.Y.; Triampo, D.; Cao, G.G. ZnO Nanoparticles and Nanowire Array Hybrid Photoanodes for Dye-Sensitized Solar Cells. Appl. Phys. Lett. 2010, 96, 073115. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G.Z. Aggregation of ZnO Nanocrystallites for High Conversion Efficiency in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2008, 120, 2402–2406. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, Q.L.; Wan, Q.; Dai, G.Z.; Zhou, C.J.; Zou, B.S. Controllable ZnO Architectures by Ethanolamine-Assisted Hydrothermal Reaction for Enhanced Photocatalytic Activity. J. Phys. Chem. C 2011, 115, 2769–2775. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G.Z. Polydisperse Aggregates of ZnO Nanocrystallites: A Method for Energy-Conversion-Efficiency Enhancement in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2008, 18, 1654–1660. [Google Scholar] [CrossRef]
- Weintraub, B.; Zhou, Z.Z.; Li, Y.H.; Deng, Y.L. Solution Synthesis of One-Dimensional ZnO Nanomaterials and Their Applications. Nanoscale 2010, 2, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, R.; Feng, L.; Liu, J.F.; Gao, X.X.; Wang, W.J. Study on the Interface Electronic States of Chemically Modified ZnO Nanowires. RSC Adv. 2015, 5, 98130–98135. [Google Scholar] [CrossRef]
- Pu, X.P.; Zhang, D.F.; Jia, L.P.; Su, C.H. Synthesis of Zinc Oxide Nanostructures with Controlled Morphologies Using a Simple Sonochemical Method. J. Am. Ceram. Soc. 2007, 90, 4076–4078. [Google Scholar] [CrossRef]
- Elias, J.; Utke, I.; Yoon, S.; Bechelany, M.; Weidenkaff, A.; Michler, J.; Philippe, L. Electrochemical Growth of ZnO Nanowires on Atomic Layer Deposition Coated Polystyrene Sphere Templates. Electrochim. Acta 2013, 110, 387–392. [Google Scholar] [CrossRef]
- Maiti, U.N.; Maiti, S.; Chattopadhyay, K.K. An Ambient Condition, One Pot Route for Large Scale Production of Ultrafine (<15 nm) ZnO Nanowires from Commercial Zinc Exhibiting Excellent Recyclable Catalytic Performance: Approach Extendable to CuO Nanostructures. CrystEngComm 2012, 14, 640–647. [Google Scholar] [CrossRef]
- Cahen, D.; Hodes, G.; Grätzel, M.; Guillemoles, J.F.; Riess, I. Nature of Photovoltaic Action in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2000, 104, 2053–2059. [Google Scholar] [CrossRef]
- Guo, D.P.; Xiao, S.Q.; Fan, K.; Yu, J.G. Hierarchical TiO2 Submicrorods Improve the Photovoltaic Performance of Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2017, 5, 1315–1321. [Google Scholar] [CrossRef]
- Qiu, J.H.; Guo, M.; Wang, X.D. Electrodeposition of Hierarchical ZnO Nanorod-Nanosheet Structures and Their Applications in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 2358–2367. [Google Scholar] [CrossRef]
- Yang, L.J.; Leung, W.W.F. Application of a Bilayer TiO2 Nanofiber Photoanode for Optimization of Dye-Sensitized Solar Cells. Adv. Mater. 2011, 23, 4559–4562. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of Parameters of Electron Transport in Dye-Sensitized Solar Cells Using Electrochemical Impedance Spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Zheng, Y.Z.; Zhao, J.X.; Chen, J.F.; Tao, X. Aqueous Chemical Synthesis of Large-Scale ZnO Aggregates with High-Efficient Light-Scattering and Application in Dye-Sensitized Solar Cells. Electrochim. Acta 2013, 90, 649–655. [Google Scholar] [CrossRef]
- Gao, C.; Peng, Y.Q.; Hu, L.H.; Mo, L.; Zhang, X.X.; Hayat, T.; Alsaedid, A.; Dai, S.Y. A Comparative Study of the Density of Surface States in Solid and Hollow TiO2 Microspheres. Inorg. Chem. Front. 2018, 5, 2284–2290. [Google Scholar] [CrossRef]
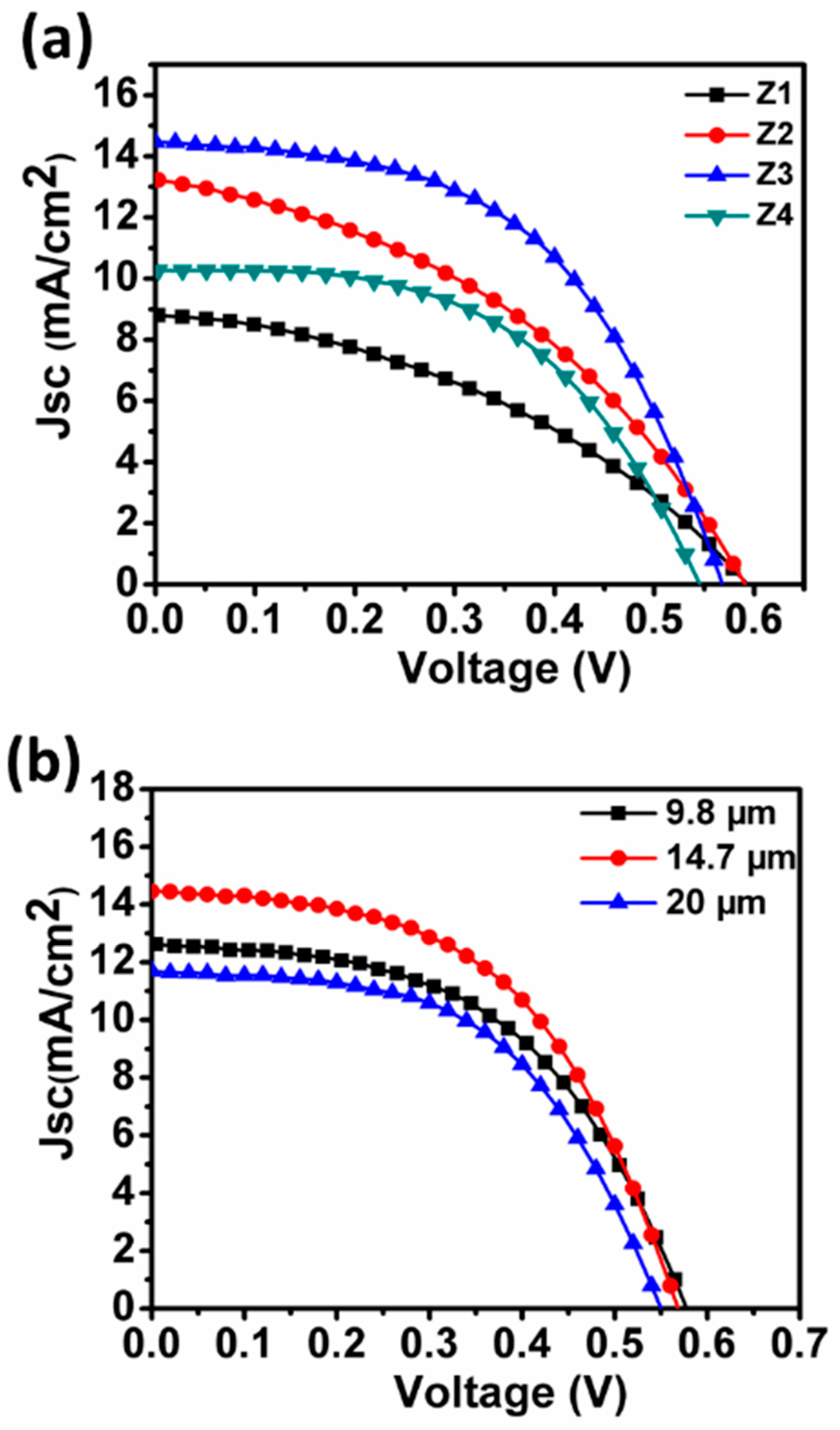
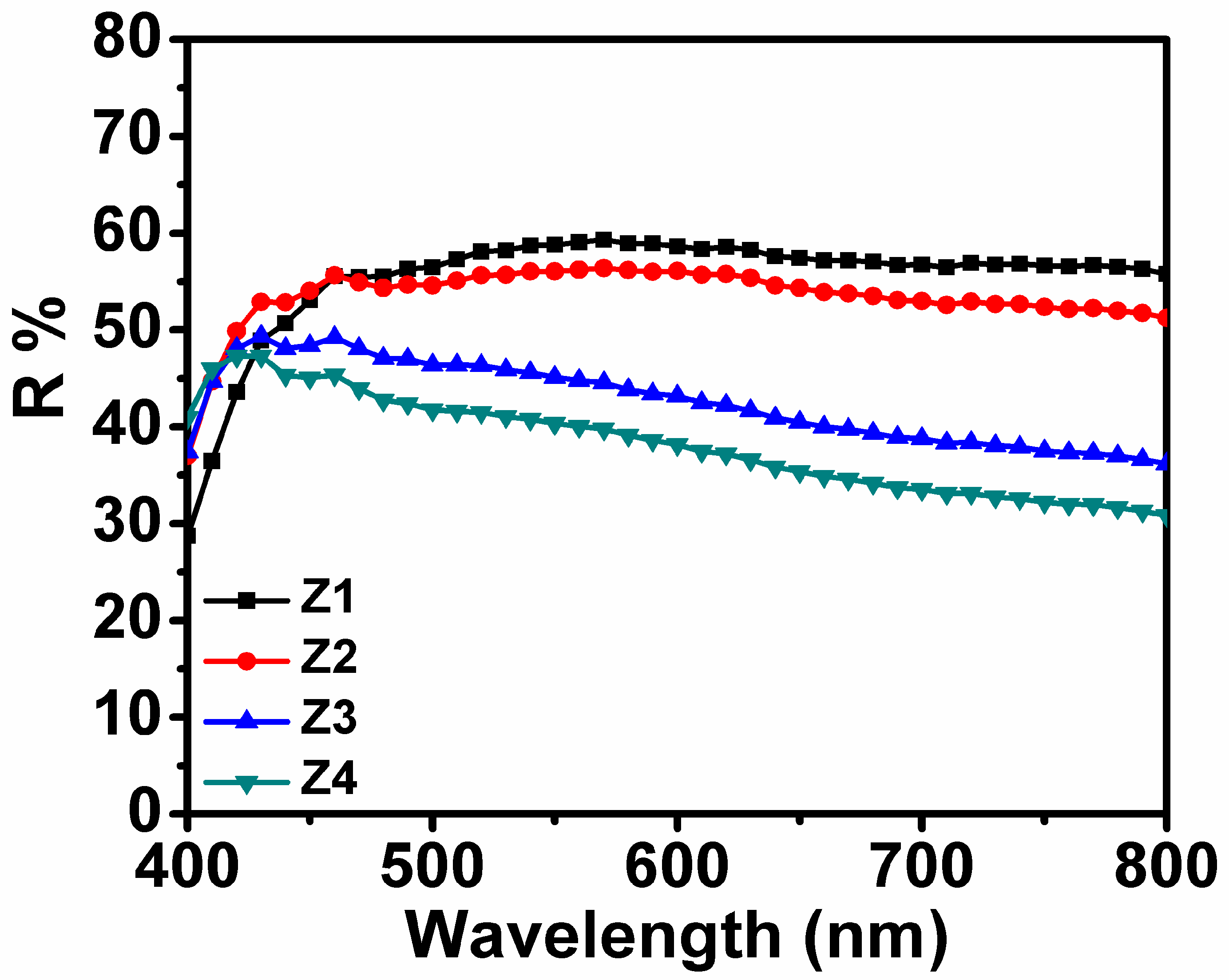
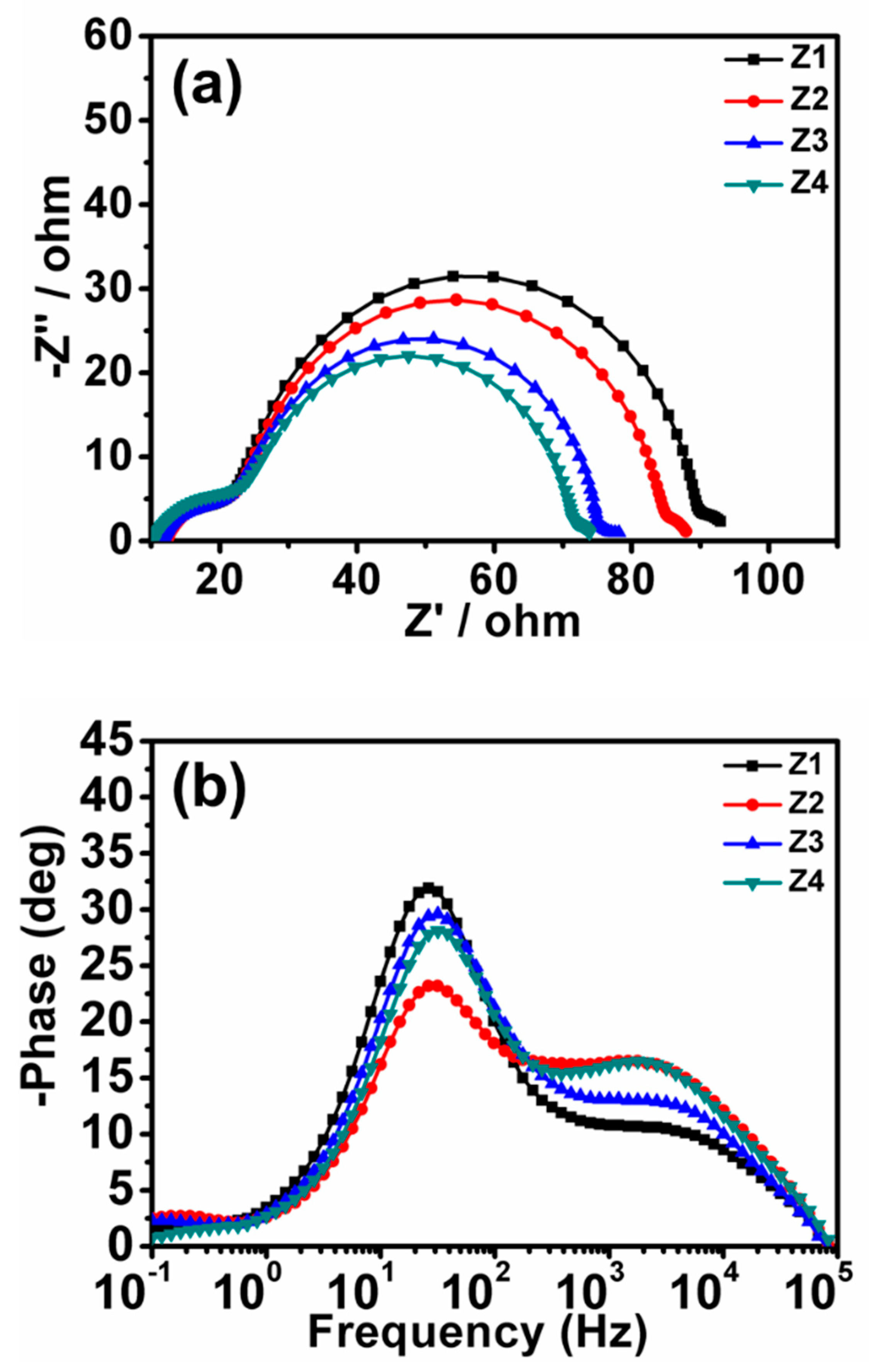
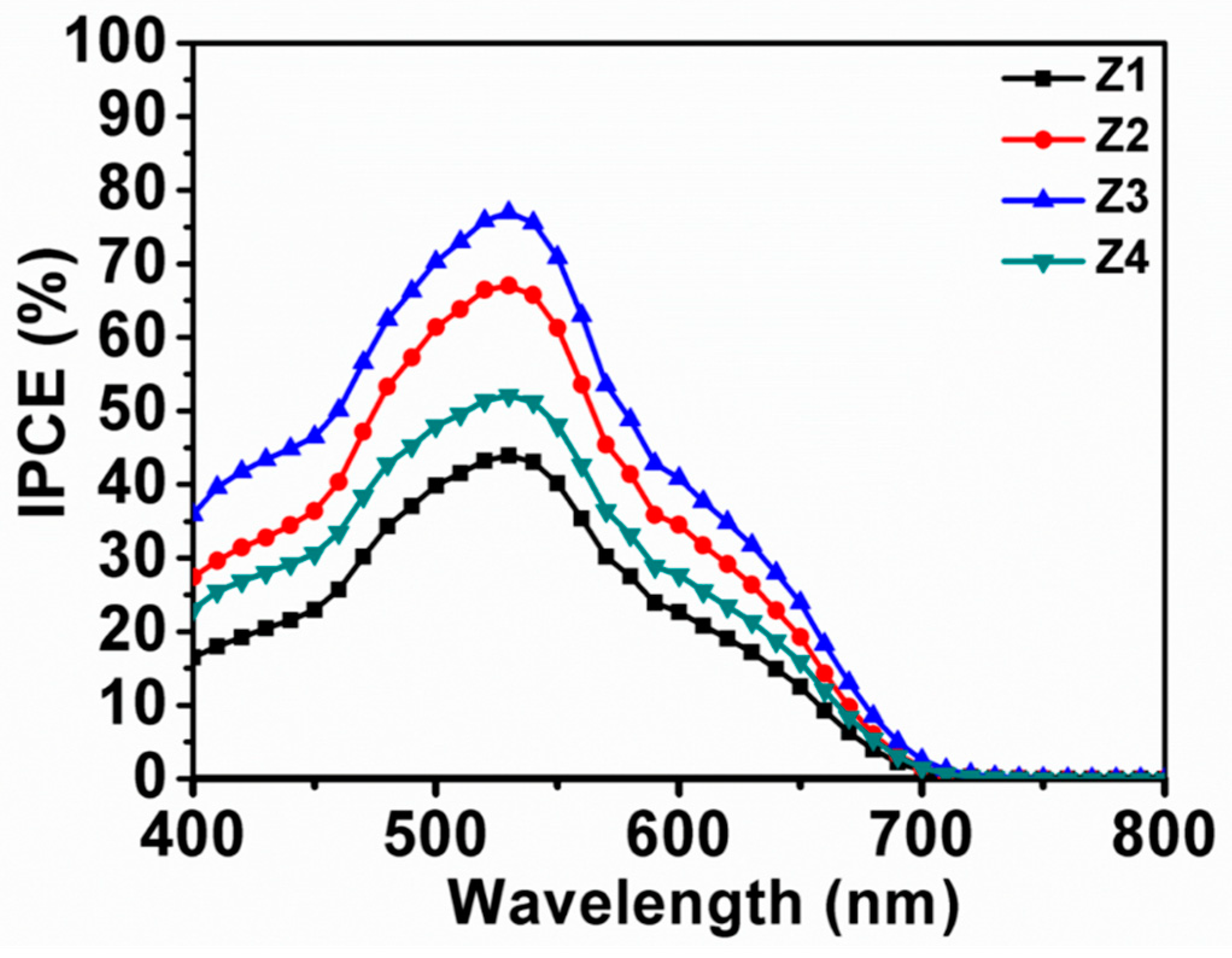
| Sample | Jsc (mA/cm²) | Voc (V) | FF | PCE | Adsorbed Dye (×10−7 mol·cm−2) | SBET (m2/g) | Crystal Size (nm) |
|---|---|---|---|---|---|---|---|
| Z1 | 8.72 | 0.593 | 0.400 | 2.07 | 0.87 | 9.8 | 21.5 |
| Z2 | 13.30 | 0.591 | 0.405 | 3.18 | 1.35 | 15.4 | 19.8 |
| Z3 | 14.50 | 0.567 | 0.523 | 4.30 | 2.15 | 24.4 | 20.6 |
| Z4 | 10.28 | 0.545 | 0.556 | 3.12 | 1.41 | 16.6 | 22.8. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, Y.; Su, M.; Xu, T.; Yang, H.; Bi, S.; Zhang, X.; Fang, Y.; Zhao, J. Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 931. https://doi.org/10.3390/nano9070931
Wang D, Zhang Y, Su M, Xu T, Yang H, Bi S, Zhang X, Fang Y, Zhao J. Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells. Nanomaterials. 2019; 9(7):931. https://doi.org/10.3390/nano9070931
Chicago/Turabian StyleWang, Dongting, Yuting Zhang, Meng Su, Ting Xu, Haizhou Yang, Shiqing Bi, Xianxi Zhang, Yuzhen Fang, and Jinsheng Zhao. 2019. "Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells" Nanomaterials 9, no. 7: 931. https://doi.org/10.3390/nano9070931
APA StyleWang, D., Zhang, Y., Su, M., Xu, T., Yang, H., Bi, S., Zhang, X., Fang, Y., & Zhao, J. (2019). Design of Morphology-Controllable ZnO Nanorods/Nanopariticles Composite for Enhanced Performance of Dye-Sensitized Solar Cells. Nanomaterials, 9(7), 931. https://doi.org/10.3390/nano9070931




