Effective Modulation of Optical and Photoelectrical Properties of SnS2 Hexagonal Nanoflakes via Zn Incorporation
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of Sn1−xZnxS2 Nanoflakes
2.2. Characterization
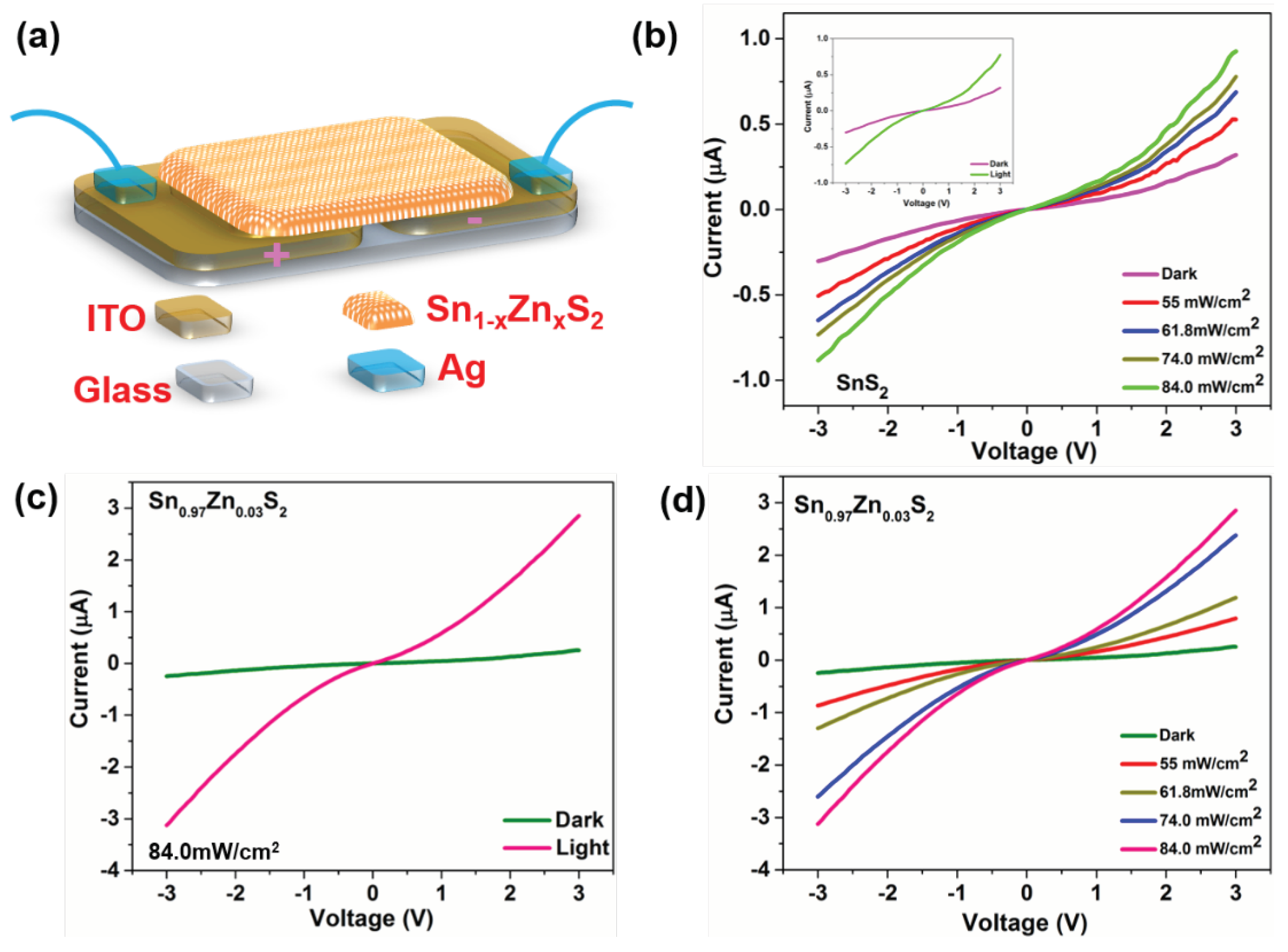
2.3. Device Fabrication
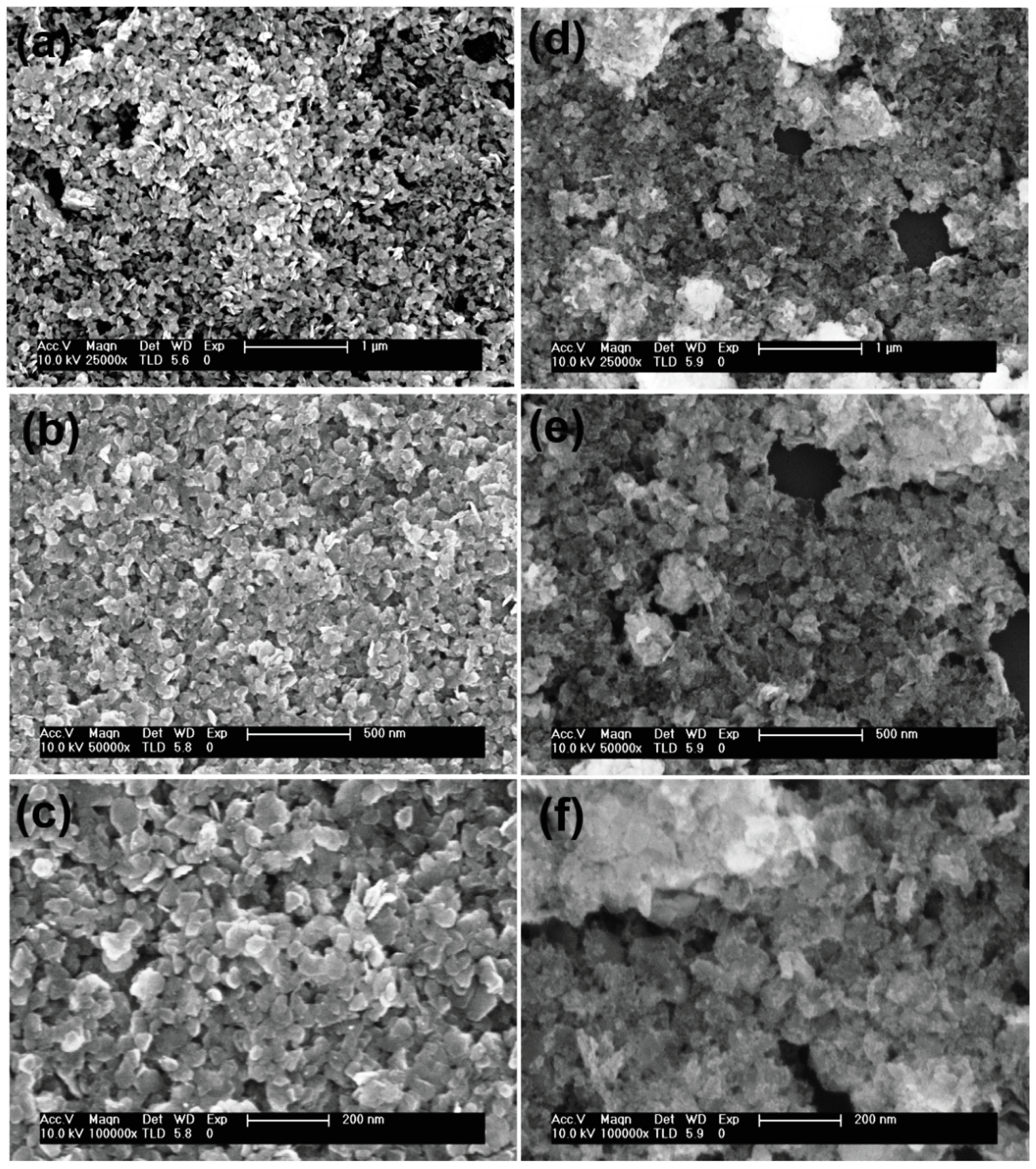
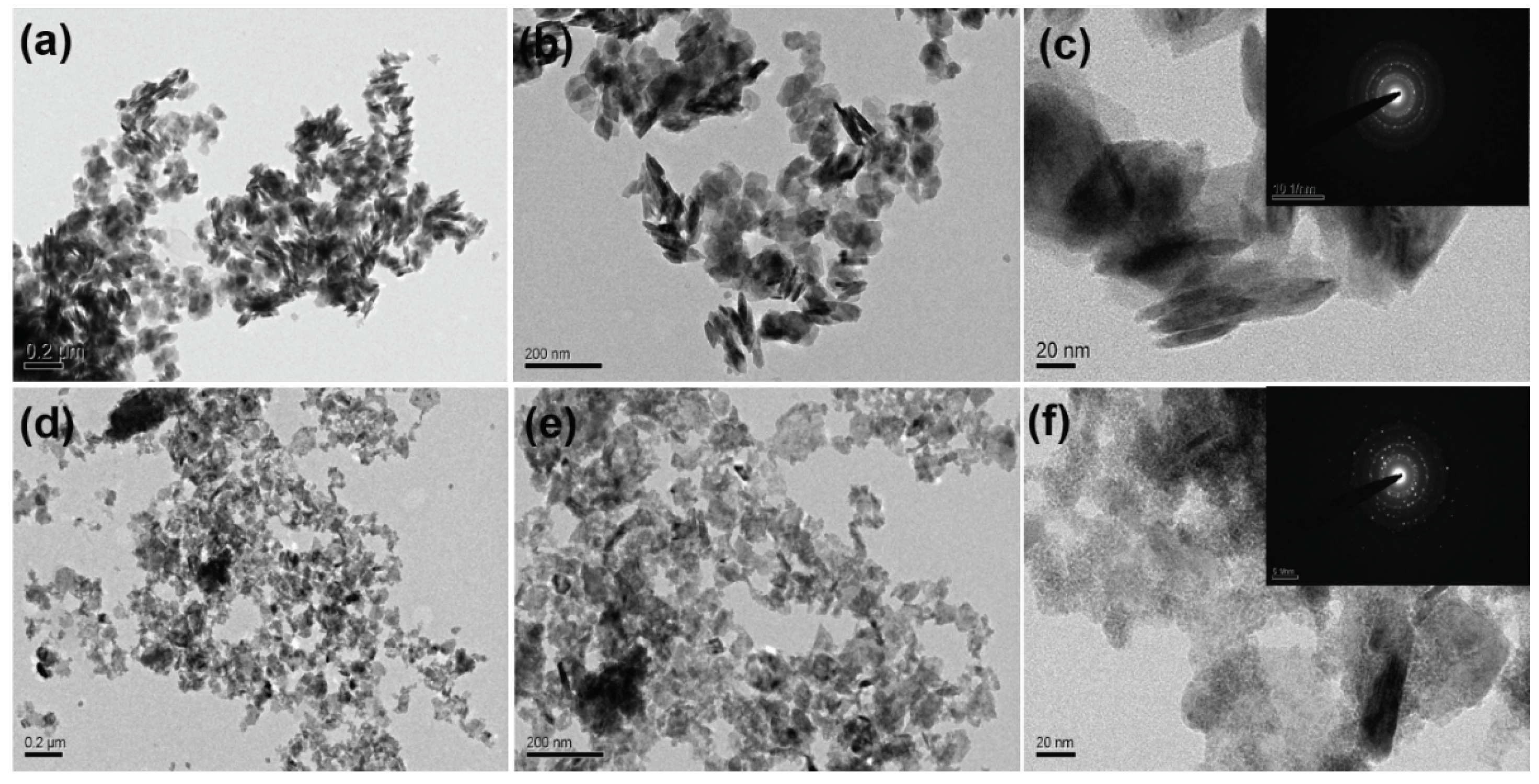
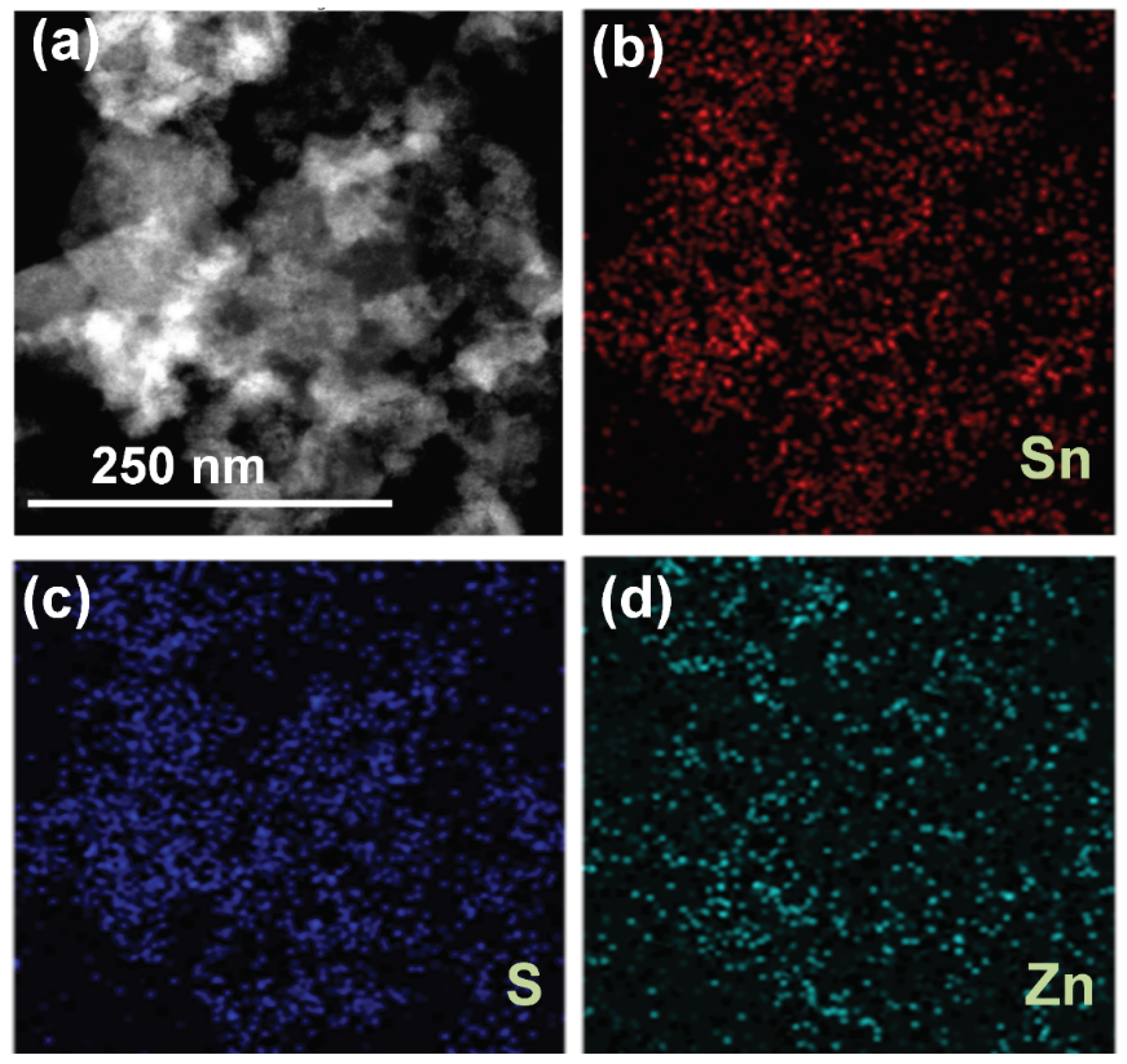
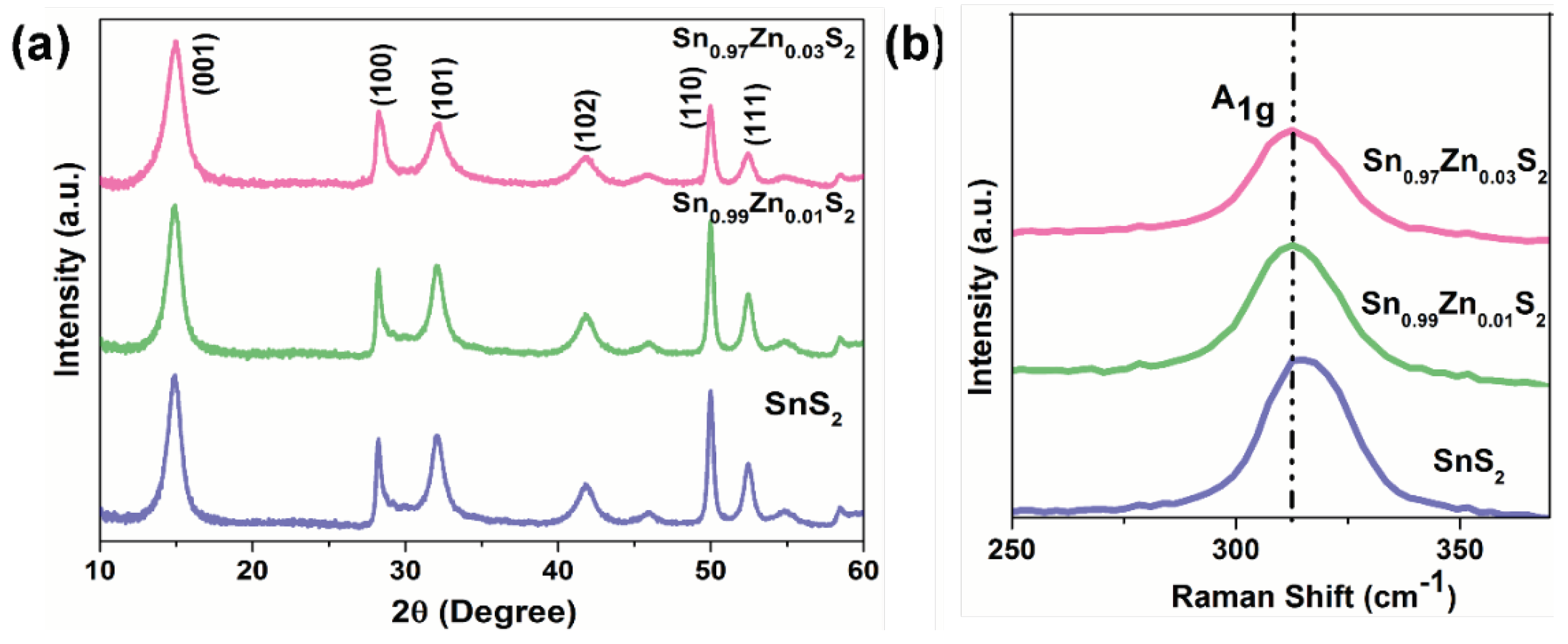
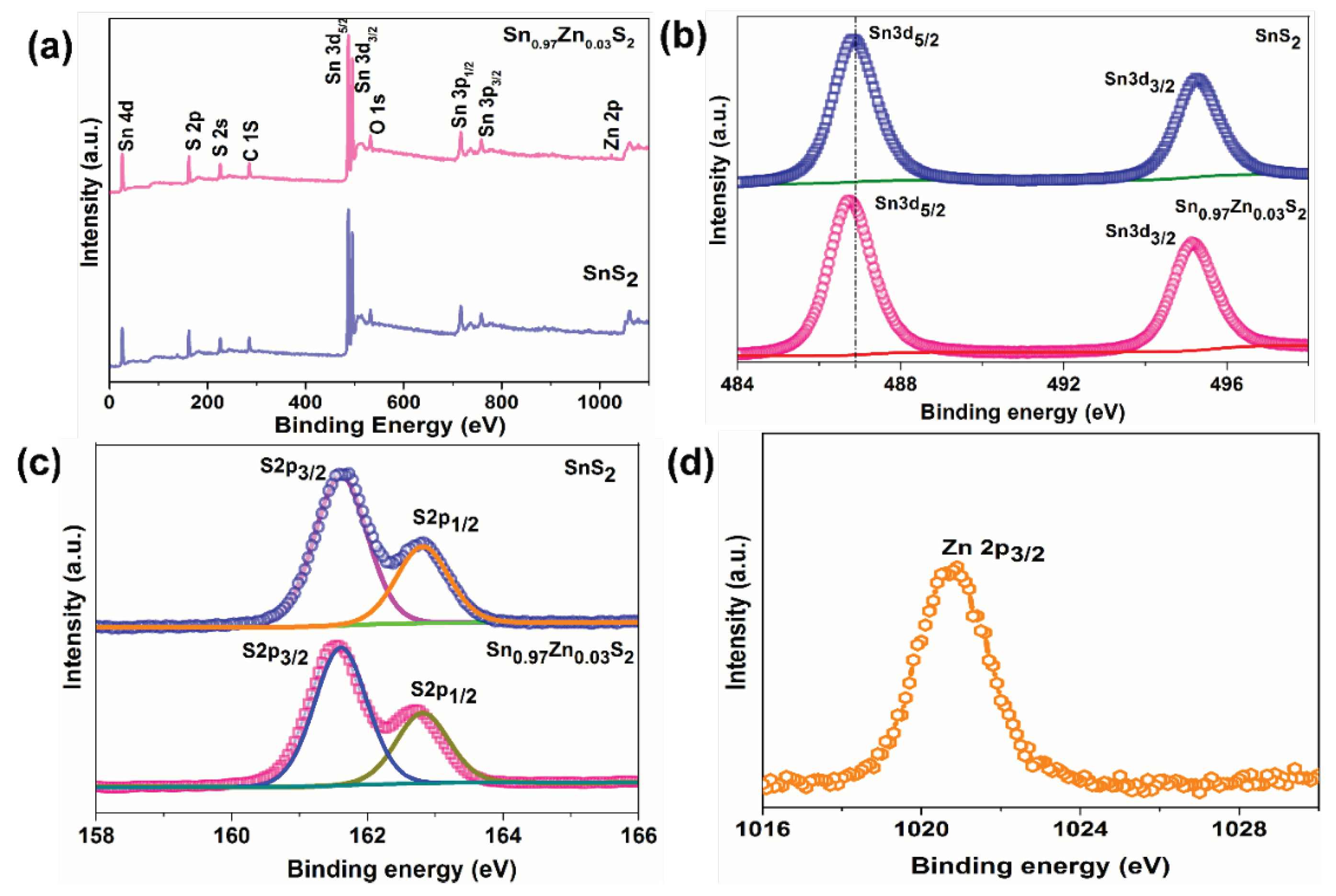
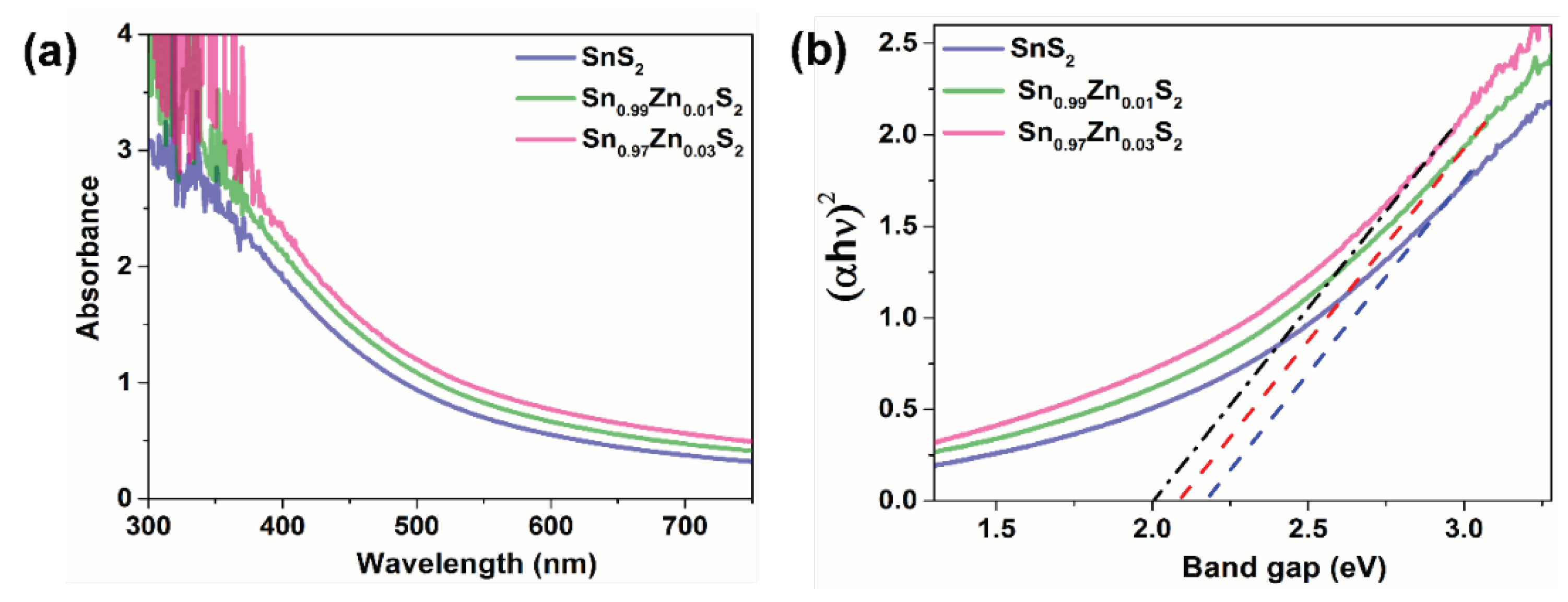
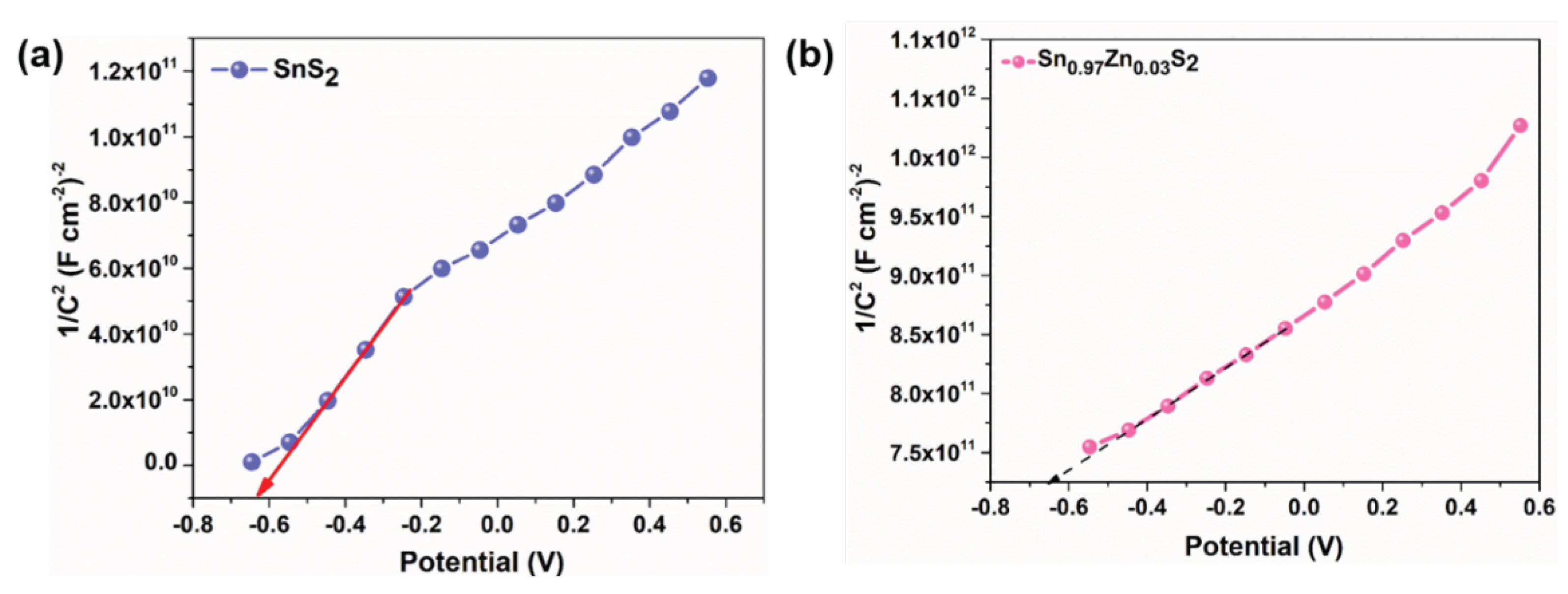
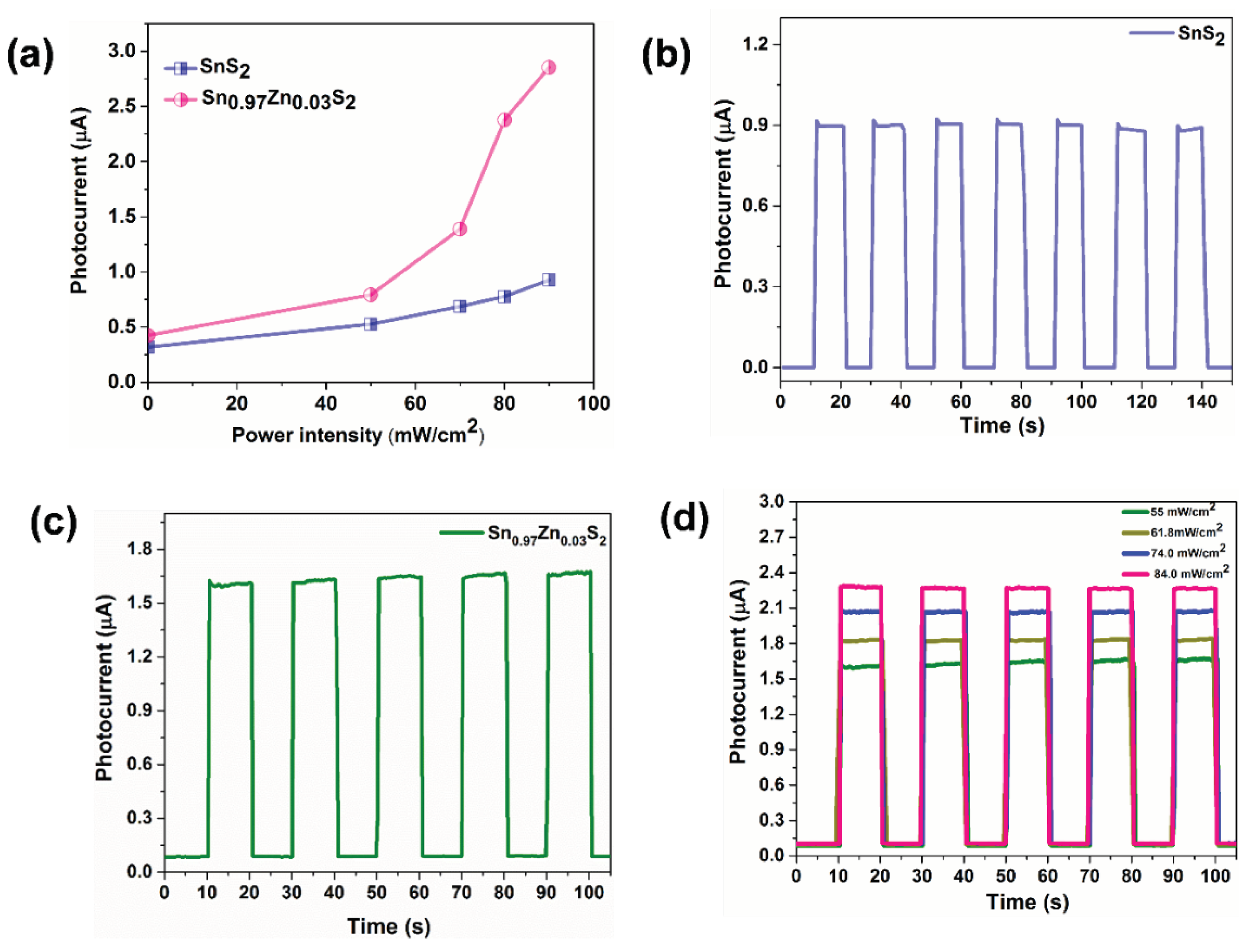
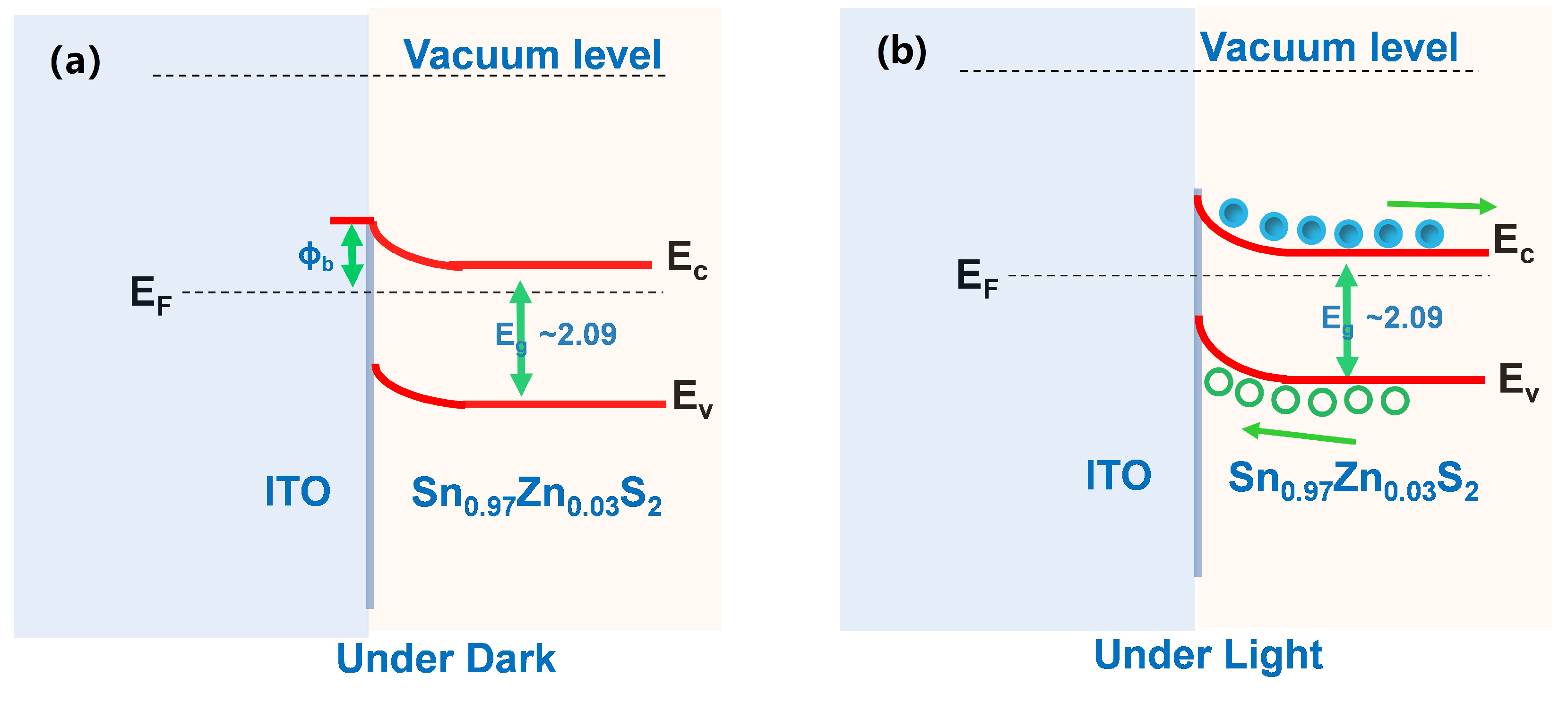
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Najmaei, S.; Liu, Z.; Zhou, W.; Zou, X.; Shi, G.; Lei, S.; Yakobson, B.I.; Idrobo, J.; Ajayan, P.M.; Lou, J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of two- dimensional layered transition metal dichalcogenides nanoflakes. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Van der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.-H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 15, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, W.; Gong, Q.; Liu, C.; Liu, Z.; Li, Y.; Dai, H. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef] [PubMed]
- Peimyoo, N.; Yang, W.; Shang, J.; Shen, X.; Wang, Y.; Yu, T. Chemically driven tunable light emission of charged and neutral excitons in monolayer WS2. ACS Nano 2014, 8, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Liu, B.; Ramakrishna Matte, H.S.S.; Dravid, V.P.; Rao, C.N.R. Hysteresis in single-layer MoS2 field effect transistors. ACS Nano 2012, 6, 5635–5641. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, H.; Hong, S.S.; Li, Y.; Cui, Y. Physical and chemical tuning of two-dimensional transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2664–2680. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Z.; Gao, S.; Cheng, H.; Liu, Q.; Piao, J.; Yao, T.; Wu, C.; Hu, S.; Wei, S.; et al. Fabrication of flexible and freestanding zinc chalcogenide single layers. Nat. Commun. 2012, 3, 1057–1063. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef]
- Yang, J.; Son, J.S.; Yu, J.H.; Joo, J.; Hyeon, T. Advances in the colloidal synthesis of two-dimensional semiconductor nanoribbons. Chem. Mater. 2013, 25, 1190–1198. [Google Scholar] [CrossRef]
- Huang, J.-K.; Pu, J.; Hsu, C.-L.; Chiu, M.-H.; Juang, Z.-Y.; Chang, Y.-H.; Chang, W.-H.; Iwasa, Y.; Takenobu, T.; Li, L.-J. Large area synthesis of highly crystalline WSe2 monolayers and device applications. ACS Nano 2014, 8, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanoflakes: High-yield preparation and device fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, D.; Wang, L.; Huang, B.; Huang, X.; Meng, X.-M. Large-scale growth of two-dimensional SnS2 crystals driven by screw dislocations and application to photodetectors. Adv. Funct. Mater. 2015, 25, 4255–4261. [Google Scholar] [CrossRef]
- Fu, X.; Ilanchezhiyan, P.; Mohan Kumar, G.; Cho, H.D.; Zhang, L.; Sattar Chan, A.; Lee, D.J.; Panin, G.N.; Kang, T.W. Tunable UV-visible absorption of SnS2 layered quantum dots produced by liquid phase exfoliation. Nanoscale 2017, 9, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, X.; Wang, W.; Wang, J. Flexible photodetector from ultraviolet to near infrared based on a SnS2 nanosheet microsphere film. J. Mater. Chem. C 2015, 3, 1347–1353. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, H.-X.; Xu, K.; Wang, Z.-X.; Wang, Q.-S.; Wang, F.-M.; Wang, F.; Zhan, X.-Y.; Li, S.-S.; Luo, J.-W.; et al. Highly sensitive and fast phototransistor based on large size CVD-grown SnS2 nanoflakes. Nanoscale 2015, 7, 14093–14099. [Google Scholar] [CrossRef]
- Mohan Kumar, G.; Fu, X.; Ilanchezhiyan, P.; Yuldashev, S.U.; Lee, D.J.; Cho, H.D.; Kang, T.W. Highly sensitive flexible photodetectors based on self-assembled tin monosulfide nanoflakes with graphene electrodes. ACS Appl. Mater. Interfaces 2017, 9, 32142–32150. [Google Scholar] [CrossRef]
- Mohan Kumar, G.; Xiao, F.; Ilanchezhiyan, P.; Yuldashev, S.U.; Kang, T.W. Enhanced photoelectrical performance of chemically processed SnS2 nanoplates. RSC Adv. 2016, 6, 99631–99637. [Google Scholar] [CrossRef]
- De, D.; Manongdo, J.; See, S.; Zhang, V.; Guloy, A.; Peng, H.B. High on/off ratio field effect transistors based on exfoliated crystalline SnS2 nano-membrane. Nanotechnology 2013, 24, 025202. [Google Scholar] [CrossRef]
- Ye, X.; Chen, J.; Engel, M.; Millan, J.A.; Li, W.; Qi, L.; Xing, G.; Collins, J.E.; Kagan, C.R.; Li, J.; et al. Competition of shape and interaction patchiness for self-assembling nanoplates. Nat. Chem. 2013, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Collins, J.E.; Kang, Y.; Chen, J.; Chen, D.T.; Yodh, A.G.; Murray, C.B. Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly. Proc. Natl. Acad. Sci. USA 2010, 107, 22430–22435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, T.N.; Cheng, G.H.; Li, T.X.; Wang, S.X.; Wei, W.; Zhou, X.H.; Yu, W.W.; Sun, Y.; Wang, P.; et al. Interlayer transition and infrared photodetection in atomically thin type-II MoTe2/MoS2 van der Waals heterostructures. ACS Nano 2016, 10, 3852–3858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, P.; Huang, L.; Xie, J.; Zhang, S.; Cao, G.; Zhao, X. Few-layered SnS2 on few-layered reduced graphene oxide as Na-Ion battery anode with ultralong cycle life and superior rate capability. Adv. Funct. Mater. 2015, 25, 481–489. [Google Scholar] [CrossRef]
- Tan, F.R.; Qu, S.C.; Wu, J.; Liu, K.; Zhou, S.Y.; Wang, Z.G. Preparation of SnS2 colloidal quantum dots and their application in organic/inorganic hybrid solar cells. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Hadjiev, V.G.; Loya, P.E.; Zhang, J.; Lei, S.; Maharjan, S.; Dong, P.; Ajayan, P.M.; Lou, J.; Peng, H. Chemical vapor deposition of thin crystals of layered semiconductor SnS2 for fast photodetection application. Nano Lett. 2015, 15, 506–513. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Gan, L.; Li, H.; Zhai, T. Large-size growth of ultrathin SnS2 nanoflakes and high performance for phototransistors. Adv. Funct. Mater. 2016, 26, 4405–4413. [Google Scholar] [CrossRef]
- Wei, R.; Hu, J.; Zhou, T.; Zhou, X.; Liu, J.; Li, J. Ultrathin SnS2 nanoflakes with exposed {001} facets and enhanced photocatalytic properties. Acta Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Xu, H.; Ji, S.; Wang, J.; Zhou, Y.; Hodgson, P.; Li, Y. Gram-scale and template- free synthesis of ultralong tin disulfide nanobelts and their lithium ion storage performances. J. Mater. Chem. A 2013, 1, 1117–1122. [Google Scholar] [CrossRef]
- Cui, X.; Xu, W.; Xie, Z.; Dorman, J.A.; Gutierrez-Wing, M.T.; Wang, Y. Effect of dopant concentration on visible light driven photocatalytic activity of Sn1−xAgxS2. Dalton Trans. 2016, 45, 16290–16297. [Google Scholar] [CrossRef] [PubMed]
- Wahnón, P.; Conesa, J.C.; Palacios, P.; Lucena, R.; Aguilera, I.; Seminovski, Y.; Fresno, F. V-doped SnS2: A new intermediate band material for a better use of the solar spectrum. Phys. Chem. Chem. Phys. 2011, 13, 20401–20407. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wang, D.; Zhao, W.; Gu, Y.; Bu, K.; Pan, J.; Qin, P.; Zhang, X.; Huang, F. Intermediate band material of titanium-doped tin disulfide for wide spectrum solar absorption. Inorg. Chem. 2018, 57, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xing, T.; Zhong, M.; Huang, L.; Lei, N.; Zhang, J.; Li, J.; Wei, Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8, 1958. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, G.; Fu, X.; Ilanchezhiyan, P.; Yuldashev, S.U.; Madhan Kumar, A.; Cho, H.D.; Kang, T.W. High performance photodiodes based on chemically processed Cu doped SnS2 nanoflakes. Appl. Surf. Sci. 2018, 455, 446–454. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, Z.; Miao, L.; Liu, X.; Li, B.; Tang, L.; Chen, K.; Liu, Y.; Li, J.; et al. Tunable Schottky barrier width and enormously enhanced photoresponsivity in Sb doped SnS2 monolayer. Nano Res. 2018, 12, 463–468. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.-Y.; Ma, F.-X.; Hu, S.-P.; Zhang, Y.-W.; Zhen, L. Monodisperse SnS2 nanoflakes for high-performance photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 22370–22377. [Google Scholar] [CrossRef]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-reduced graphene oxide composite—a high-capacity, high-rate, and long-cycle life sodium- ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef]
- Du, Y.; Yin, Z.; Rui, X.; Zeng, Z.; Wu, X.J.; Liu, J.; Zhu, Y.; Zhu, J.; Huang, X.; Yan, Q.; et al. A facile, relative green, and inexpensive synthetic approach toward large-scale production of SnS2 nanoplates for high-performance lithium-ion batteries. Nanoscale 2013, 5, 1456–1459. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, F.; Xia, Y.; Wang, H.; Kuang, X. Interlayer expansion of few layered Mo-doped SnS2 nanoflakes grown on carbon cloth with excellent lithium storage performance for lithium ion batteries. J. Mater. Chem. A 2017, 5, 4075–4083. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.L.; Kulka, A.; Trenczek-Zajac, A.; Xie, J.Y.; Chen, N.; Swierczek, K. Characterization of the physicochemical properties of novel SnS2 with cubic structure and diamond-like Sn sublattice. Acta Mater. 2015, 82, 212–223. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Kang, T.W. Electrochemical studies of spherically clustered MoS2 nanostructures for electrode applications. J. Alloys Compd. 2015, 634, 104–108. [Google Scholar] [CrossRef]
- Liu, X.; Bai, H. Hydrothermal synthesis of visible light active zinc-doped tin disulfide photocatalyst for the reduction of aqueous Cr(VI). Powder Technol. 2013, 237, 610–615. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Tang, J. Biomolecule-assisted fabrication of Copper doped SnS2 nanosheet–reduced graphene oxide junctions with enhanced visible-light photocatalytic activity. J. Mater. Chem. A 2014, 2, 1000–1005. [Google Scholar] [CrossRef]
- Yassin, O.A.; Abdelaziz, A.A.; Jaber, A.Y. Structural and optical characterization of V-and W-doped SnS2 thin films prepared by spray pyrolysis. Mater. Sci. Semicond. Process. 2015, 38, 81–86. [Google Scholar] [CrossRef]
- Patel, M.; Chavda, A.; Mukhopadhyay, I.; Kim, J.; Ray, A. Nanostructured SnS with inherent anisotropic optical properties for high photoactivity. Nanoscale 2016, 8, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, L.; Wu, Y.; Tian, Y. Zr-Doped β-In2S3 ultrathin nanoflakes as photoanodes: Enhanced visible-light-driven photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 2016, 4, 2606–2614. [Google Scholar] [CrossRef]
- Mohan Kumar, G.; Ilanchezhiyan, P.; Madhan Kumar, A.; Yuldashev, S.U.; Kang, T.W. Electrical property studies on chemically processed polypyrolle/aluminum doped ZnO based hybrid heterostructures. Chem. Phys. Lett. 2016, 649, 130–134. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan Kumar, G.; Ilanchezhiyan, P.; Cho, H.D.; Yuldashev, S.; Jeon, H.C.; Kim, D.Y.; Kang, T.W. Effective Modulation of Optical and Photoelectrical Properties of SnS2 Hexagonal Nanoflakes via Zn Incorporation. Nanomaterials 2019, 9, 924. https://doi.org/10.3390/nano9070924
Mohan Kumar G, Ilanchezhiyan P, Cho HD, Yuldashev S, Jeon HC, Kim DY, Kang TW. Effective Modulation of Optical and Photoelectrical Properties of SnS2 Hexagonal Nanoflakes via Zn Incorporation. Nanomaterials. 2019; 9(7):924. https://doi.org/10.3390/nano9070924
Chicago/Turabian StyleMohan Kumar, Ganesan, Pugazhendi Ilanchezhiyan, Hak Dong Cho, Shavkat Yuldashev, Hee Chang Jeon, Deuk Young Kim, and Tae Won Kang. 2019. "Effective Modulation of Optical and Photoelectrical Properties of SnS2 Hexagonal Nanoflakes via Zn Incorporation" Nanomaterials 9, no. 7: 924. https://doi.org/10.3390/nano9070924
APA StyleMohan Kumar, G., Ilanchezhiyan, P., Cho, H. D., Yuldashev, S., Jeon, H. C., Kim, D. Y., & Kang, T. W. (2019). Effective Modulation of Optical and Photoelectrical Properties of SnS2 Hexagonal Nanoflakes via Zn Incorporation. Nanomaterials, 9(7), 924. https://doi.org/10.3390/nano9070924




