Large-Scale Fabrication of Nanostructure on Bio-Metallic Substrate for Surface Enhanced Raman and Fluorescence Scattering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characterization
2.3. Reflectance and Absorption Spectroscopy
2.4. Raman and Fluorescence Spectroscopy
3. Results and Discussion
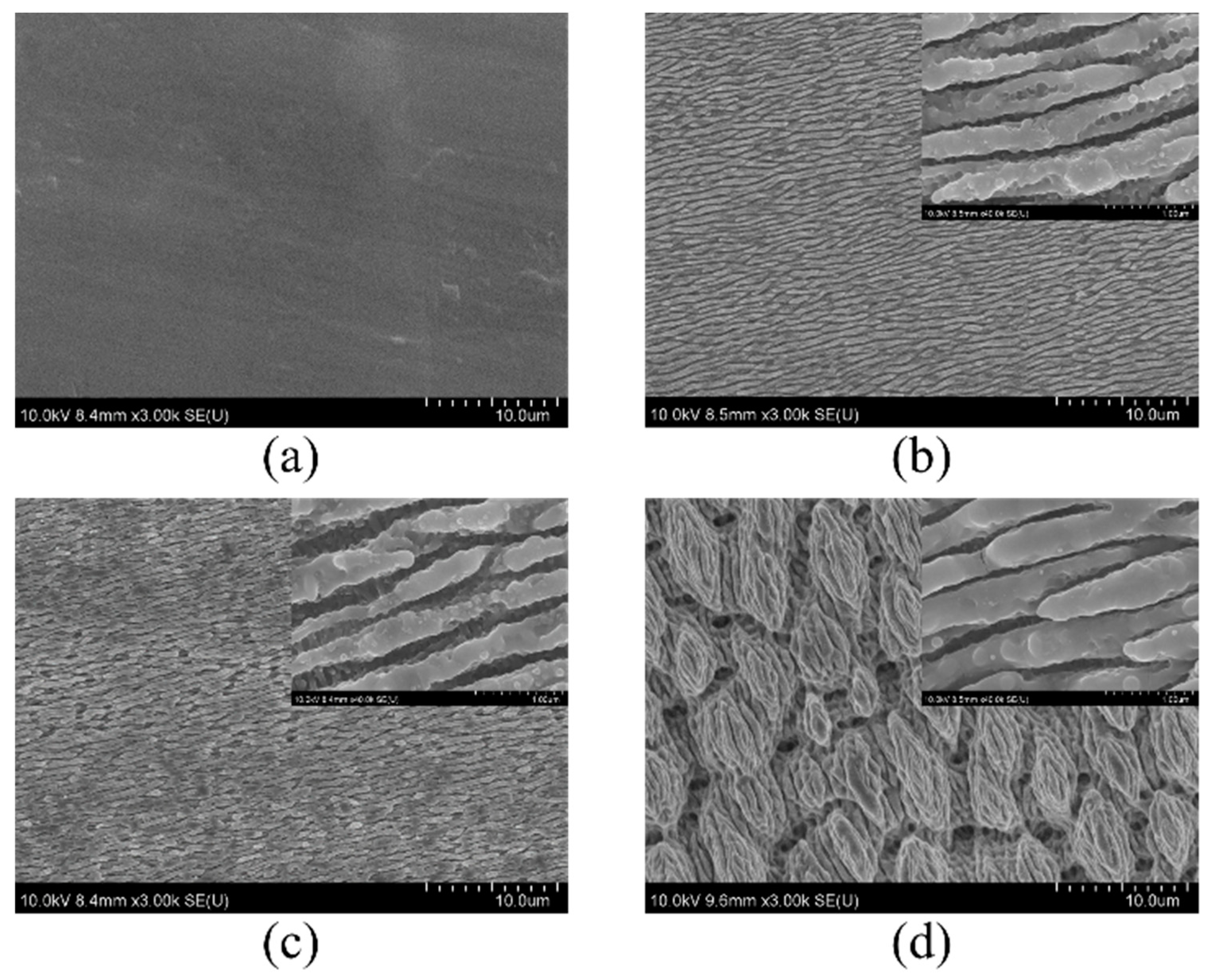
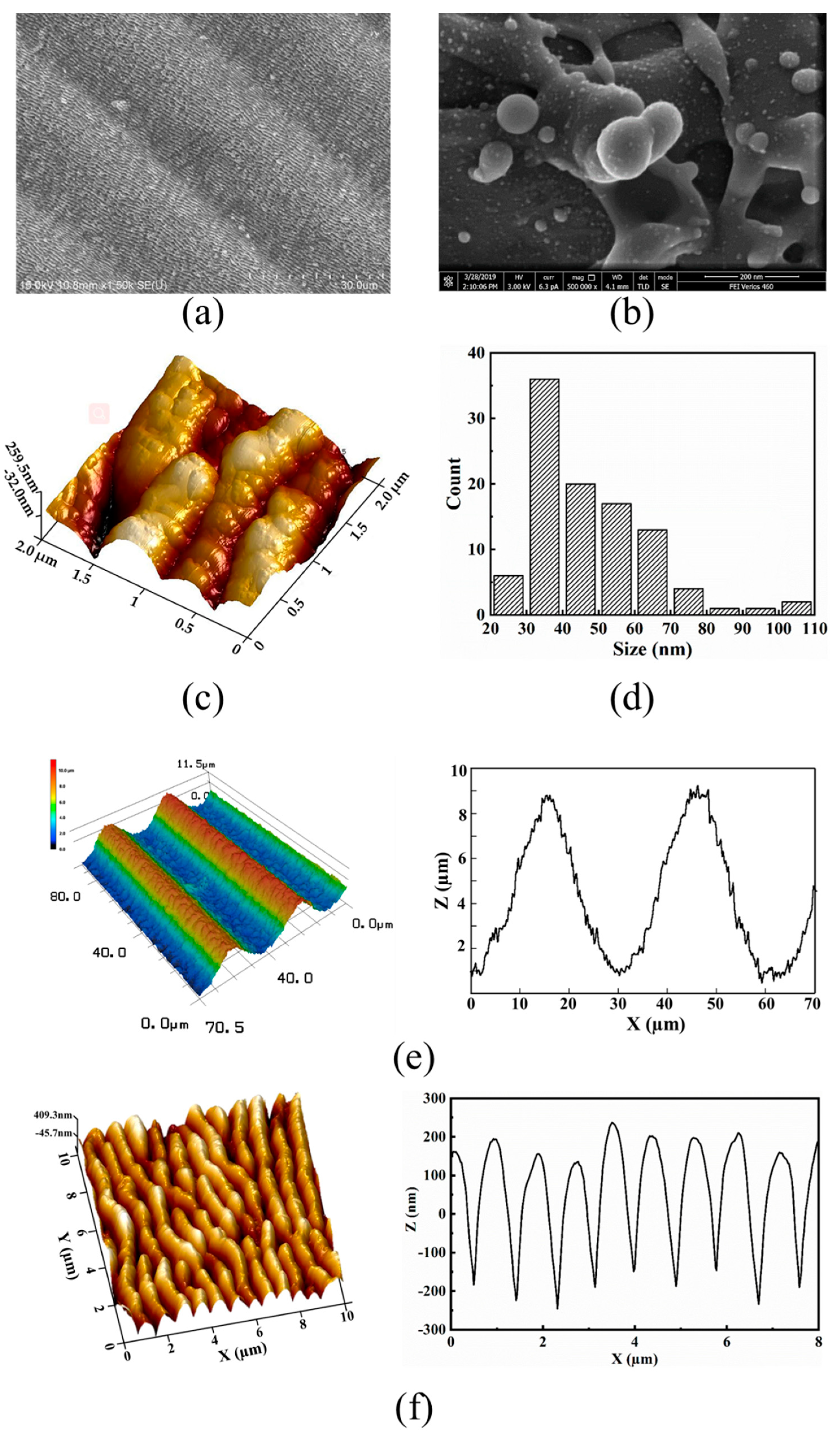
3.1. Topography of the Hierarchical LIPSS
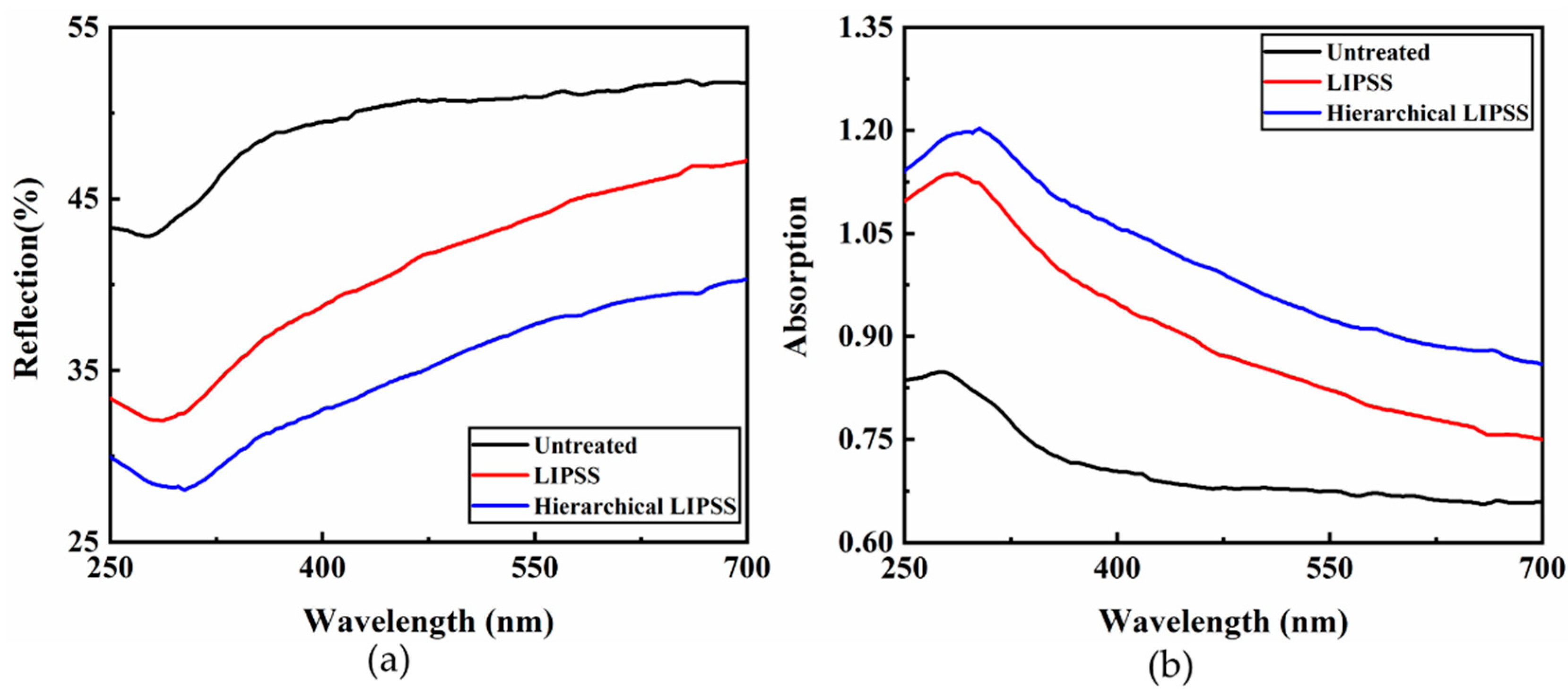
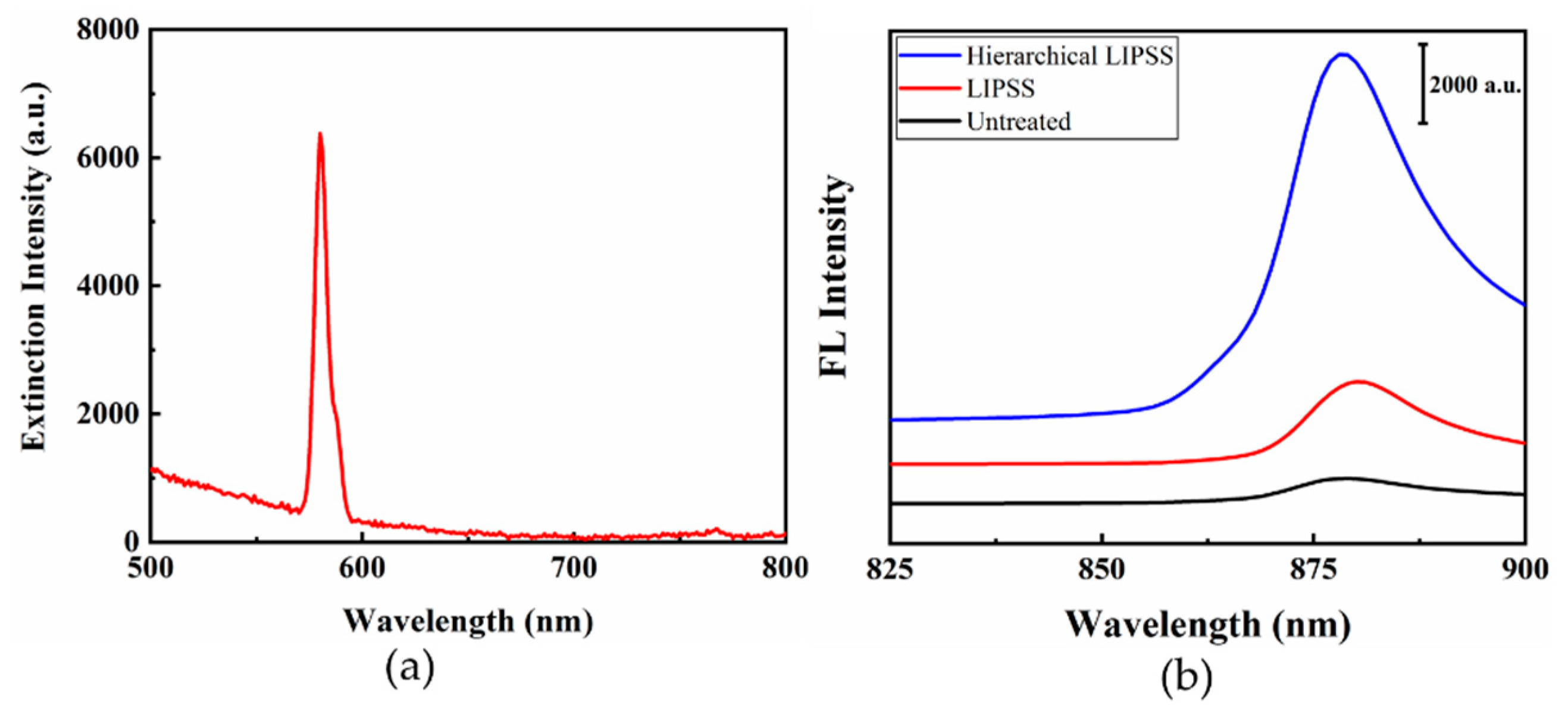
3.2. Optical Properties
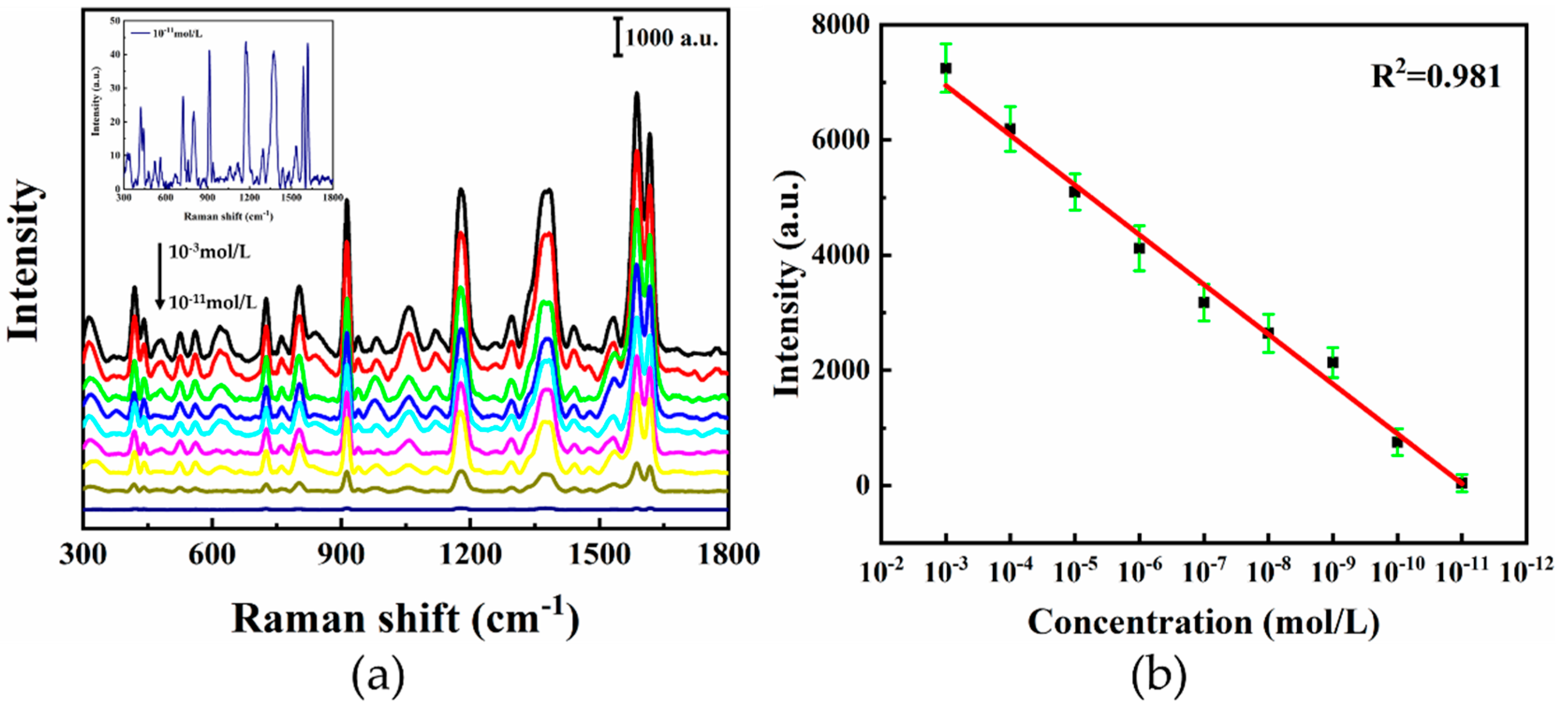
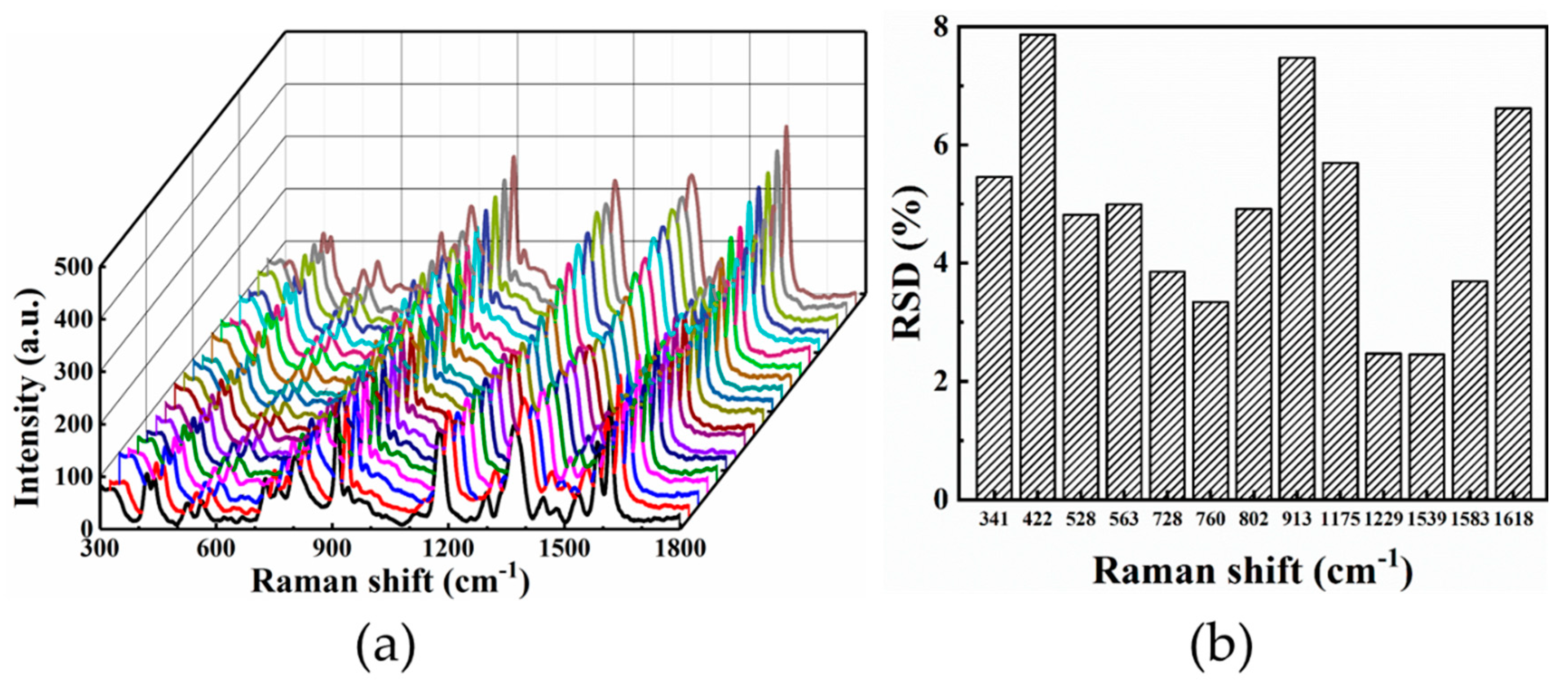
3.3. SERS Characteristics
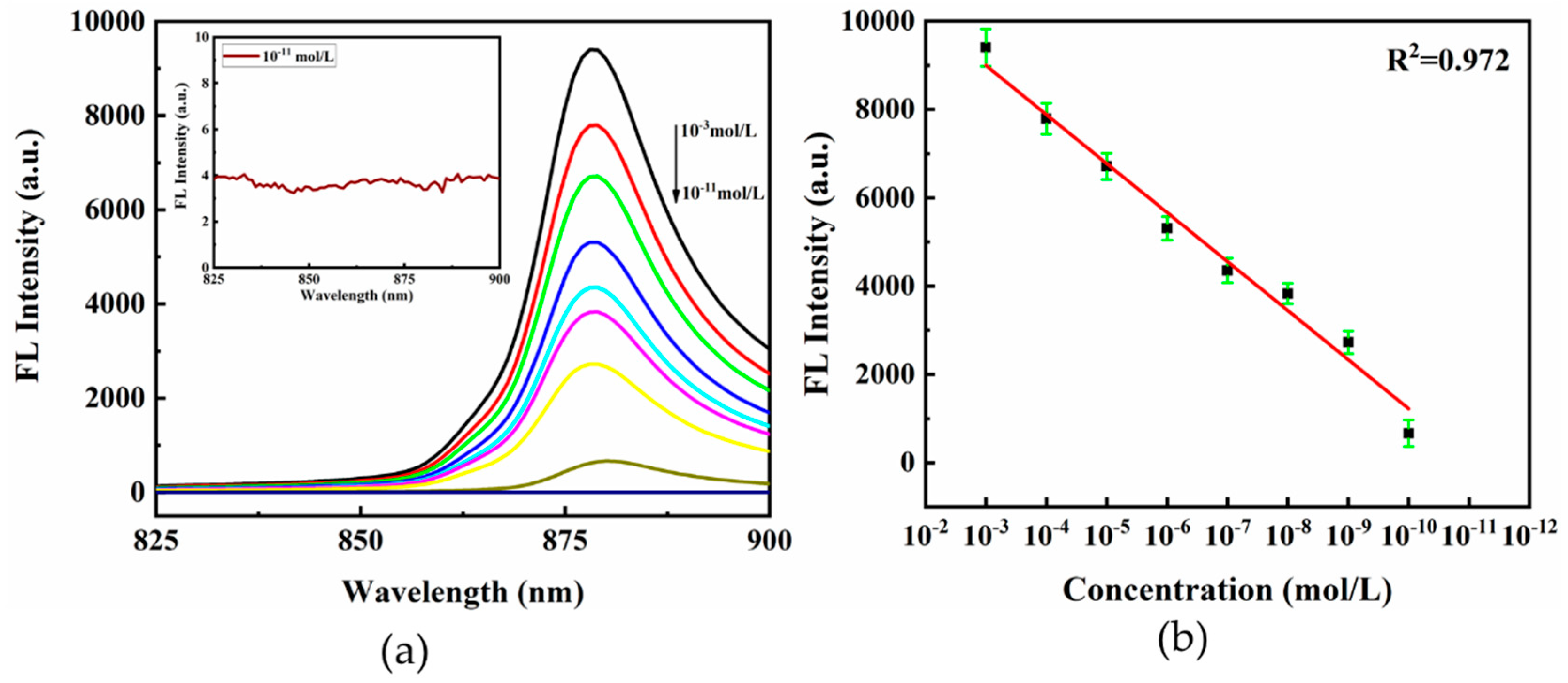
3.4. SEF Characteristics
4. Conclusions
- The hierarchical LIPSS structure consisting of micro-grooves, nano-ripples, and nano-particles with strong SPR was produced by one-step femtosecond laser processing, which may open up new possibilities in both SERS and SEF.
- Due to the combination effect of SPR and “hot spots”, the hierarchical LIPSS substrate exhibits an ultra-sensitive detectability, which shows an EFs of 7.85 × 105 for CV and LOD of 10−11 mol/L.
- The intensity of the CV fluorescence on the hierarchical LIPSS substrate was enhanced by about 14 times with the LOD of 1 × 10−10 mol/L, which is attributed to the LSPR and “hot spots” from the hierarchical LIPSS.
Author Contributions
Funding
Conflicts of Interest
References
- Huang, W.-S.; Sun, I.-W.; Huang, C.-C. Promotion of SERS and catalytic activities with bimetallic and ternary concave nanolayers. J. Mater. Chem. A 2018, 6, 13041–13049. [Google Scholar] [CrossRef]
- Lee, C.-W.; Ko, H.; Chang, S.-H.G.; Huang, C.-C. Invisible-ink-assisted pattern and written surface-enhanced Raman scattering substrates for versatile chem/biosensing platforms. Green Chem. 2018, 20, 5318–5326. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Tan, C.F.; Kang, N.; Chen, L.; Ren, L.; Thian, E.S.; Ho, G.W.; Ji, R.; Hong, M. Uniaxially stretched flexible surface Plasmon resonance film for versatile surface enhanced Raman scattering diagnostics. ACS Appl. Mater. Interfaces 2017, 9, 26341–26349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Si, T.; Liu, J.; Zhou, G. In-Situ Grown Silver Nanoparticles on Nonwoven Fabrics Based on Mussel-Inspired Polydopamine for Highly Sensitive SERS Carbaryl Pesticides Detection. Nanomaterials 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, L.; Xia, T.; Wu, Z.; Liu, Z. Construction of an Upconversion Nanoprobe with Few-Atom Silver Nanoclusters as the Energy Acceptor. Angew. Chem. Int. Ed. 2015, 54, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.; Etchegoin, P.; Le Ru, E. Ultrafast nonradiative decay rates on metallic surfaces by comparing surface-enhanced Raman and fluorescence signals of single molecules. Phys. Rev. Lett. 2009, 103, 063003. [Google Scholar] [CrossRef]
- Le Ru, E.; Etchegoin, P.; Grand, J.; Felidj, N.; Aubard, J.; Levi, G. Mechanisms of spectral profile modification in surface-enhanced fluorescence. J. Phys. Chem. C 2007, 111, 16076–16079. [Google Scholar] [CrossRef]
- Jankiewicz, B.; Jamiola, D.; Choma, J.; Jaroniec, M. Silica–metal core–shell nanostructures. Adv. Colloid Interface Sci. 2012, 170, 28–47. [Google Scholar] [CrossRef]
- Burns, A.; Ow, H.; Wiesner, U. Fluorescent core–shell silica nanoparticles: Towards “Lab on a Particle” architectures for nanobiotechnology. Chem. Soc. Rev. 2006, 35, 1028–1042. [Google Scholar] [CrossRef]
- Kamalieva, A.N.; Toropov, N.A.; Bogdanov, K.V.; Vartanyan, T.A. Enhancement of Fluorescence and Raman Scattering in Cyanine-Dye Molecules on the Surface of Silicon-Coated Silver Nanoparticles. Opt. Spectrosc. 2018, 124, 319–322. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, X.; Cui, Q.; Yang, Y.; Li, L. Synthesis and application of bifunctional gold/gelatin nanocomposites with enhanced fluorescence and Raman scattering. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 117–125. [Google Scholar] [CrossRef]
- Cyrankiewicz, M.; Wybranowski, T.; Kruszewski, S. Silver Nanoparticles as Enhancing Substrates for Raman and Fluorescence Spectroscopy. Acta Phys. Pol. A 2014, 125. [Google Scholar] [CrossRef]
- Chang, S.; Eichmann, S.L.; Huang, T.-Y.S.; Yun, W.; Wang, W. Controlled Design and Fabrication of SERS–SEF Multifunctional Nanoparticles for Nanoprobe Applications: Morphology-Dependent SERS Phenomena. J. Phys. Chem. C 2017, 121, 8070–8076. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, F.; Cheng, Y.; Xu, N.; Xu, Z. Origin of Laser-Induced Near-Subwavelength Ripples: Interference between Surface Plasmons and Incident Laser. ACS Nano 2009, 3, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Preston, J.; Van Driel, H.; Sipe, J. Laser-induced periodic surface structure. II. Experiments on Ge, Si, Al, and brass. Phys. Rev. B 1983, 27, 1155. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, S.; Wang, S.; Hu, A. Ultra-Short Pulsed Laser Manufacturing and Surface Processing of Microdevices. Engineering 2018, 4, 779–786. [Google Scholar] [CrossRef]
- Chang, C.-L.; Cheng, C.-W.; Chen, J.-K. Femtosecond laser-induced periodic surface structures of copper: Experimental and modeling comparison. Appl. Surf. Sci. 2019, 469, 904–910. [Google Scholar] [CrossRef]
- Sztainbuch, I.W. The effects of Au aggregate morphology on surface-enhanced Raman scattering enhancement. J. Chem. Phys. 2006, 125, 124707. [Google Scholar] [CrossRef]
- Perez, D.; Lewis, L.J. Molecular-dynamics study of ablation of solids under femtosecond laser pulses. Phys. Rev. B 2003, 67, 184102. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, U.; Naik, P.; Mukherjee, C.; Kumbhare, S.; Gupta, P. Formation of metal nanoparticles of various sizes in plasma plumes produced by Ti: Sapphire laser pulses. J. Appl. Phys. 2010, 108, 053107. [Google Scholar] [CrossRef]
- Serkov, A.A.; Rakov, I.I.; Simakin, A.V.; Kuzmin, P.G.; Shafeev, G.A.; Mikhailova, G.N.; Antonova, L.K.; Troitskii, A.V.; Kuzmin, G.P. Influence of external magnetic field on laser-induced gold nanoparticles fragmentation. Appl. Phys. Lett. 2016, 109, 053107. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013, 2013, 49. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Guo, C. Spectral and polarization responses of femtosecond laser-induced periodic surface structures on metals. J. Appl. Phys. 2008, 103, 043513. [Google Scholar] [CrossRef]
- Vorobyev, A.; Makin, V.; Guo, C. Periodic ordering of random surface nanostructures induced by femtosecond laser pulses on metals. J. Appl. Phys. 2007, 101, 034903. [Google Scholar] [CrossRef]
- Toma, M.; Loget, G.; Corn, R.M. Fabrication of Broadband Antireflective Plasmonic Gold Nanocone Arrays on Flexible Polymer Films. Nano Lett. 2013, 13, 6164–6169. [Google Scholar] [CrossRef]
- Bosman, M.; Anstis, G.R.; Keast, V.J.; Clarke, J.D.; Cortie, M.B. Light Splitting in Nanoporous Gold and Silver. ACS Nano 2012, 6, 319–326. [Google Scholar] [CrossRef]
- Song, J.; Huang, Y.; Fan, Y.; Zhao, Z.; Yu, W.; Rasco, B.; Lai, K. Detection of prohibited fish drugs using silver nanowires as substrate for surface-enhanced Raman scattering. Nanomaterials 2016, 6, 175. [Google Scholar] [CrossRef]
- Fu, L.-H.; Liu, B.; Meng, L.-Y.; Ma, M.-G. Comparative study of cellulose/Ag nanocomposites using four cellulose types. Mater. Lett. 2016, 171, 277–280. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.; Padilla, A.; Hernandez-Rivera, S. Surface enhanced Raman scattering (SERS) studies of gold and silver nanoparticles prepared by laser ablation. Nanomaterials 2013, 3, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Cardoso-Avila, P.; Pichardo-Molina, J. Concave gold nanocubes on Al-6063 alloy as a simple and efficient SERS substrate. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 66–73. [Google Scholar] [CrossRef]
- Kim, W.; Kim, Y.-H.; Park, H.-K.; Choi, S. Facile Fabrication of a Silver Nanoparticle Immersed, Surface-Enhanced Raman Scattering Imposed Paper Platform through Successive Ionic Layer Absorption and Reaction for On-Site Bioassays. ACS Appl. Mater. Interfaces 2015, 7, 27910–27917. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q. Rapid fabrication of silver nanoparticle-coated filter paper as SERS substrate for low-abundance molecules detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Patze, S.; Hidi, I.J.; Knipper, R.; Radu, A.I.; Mühlig, A.; Yüksel, S.; Peksa, V.; Weber, K.; Mayerhöfer, T. Plasmonic nanostructures for surface enhanced spectroscopic methods. Analyst 2016, 141, 756–793. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Cialla-May, D.; Zheng, X.-S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef]
- Jiaru, Z.; Guoqing, H.; Libin, L.; Yingchun, G.; Hong, M. Enhancing protein fluorescence detection through hierarchical biometallic surface structuring. Opt. Lett. 2019, 44, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pu, H.; Sun, D.-W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2800–2813. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Fan, F.; Xu, H.; Wang, Z.L. Light propagation in curved silver nanowire plasmonic waveguides. Nano Lett. 2011, 11, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Al-Shalalfeh, M.M.; Al-Saadi, A.A. Silver loaded graphene as a substrate for sensing 2-thiouracil using surface-enhanced Raman scattering. Sens. Actuators B Chem. 2018, 254, 1110–1117. [Google Scholar] [CrossRef]
- Carpi, F.; Rossi, D.D. Improvement of electromechanical actuating performances of a silicone dielectric elastomer by dispersion of titanium dioxide powder. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 835–843. [Google Scholar] [CrossRef]
- Zeyada, H.; Makhlouf, M. Electrical conduction mechanisms and dielectric constants of nanostructured methyl violet 2B thin films. Appl. Phys. A 2015, 119, 1109–1118. [Google Scholar] [CrossRef]
- Torosyan, G.; Rau, C.; Pradarutti, B.; Beigang, R. Excitation and Propagation of Surface Plasmon Polaritons on Metallic Periodic Structures; Springer: Berlin/Heidelberg, Germany, 2005; pp. 661–663. [Google Scholar]
- Luo, Z.; Chen, L.; Liang, C.; Wei, Q.; Chen, Y.; Wang, J. Porous carbon films decorated with silver nanoparticles as a sensitive SERS substrate, and their application to virus identification. Microchim. Acta 2017, 184, 3505–3511. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, S.; Wang, P.; Zhang, L.; Ren, X.; Cui, L.; Zhai, T.; Chen, J.; Wang, Y.; Wang, X.; et al. Demonstration of a 3D Radar-Like SERS Sensor Micro- and Nanofabricated on an Optical Fiber. Adv. Opt. Mater. 2015, 3, 1232–1239. [Google Scholar] [CrossRef]
- Gabudean, A.M.; Focsan, M.; Astilean, S. Gold nanorods performing as dual-modal nanoprobes via metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS). J. Phys. Chem. C 2012, 116, 12240–12249. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Xiang, X.; Zhang, P.; Peng, Z.; Cao, Z.; Mu, Q.; Xuan, L. Highly effective surface-enhanced fluorescence substrates with roughened 3D flowerlike silver nanostructures fabricated in liquid crystalline phase. Appl. Surf. Sci. 2017, 401, 297–305. [Google Scholar] [CrossRef]
- Fort, E.; Grésillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2007, 41, 013001. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed]
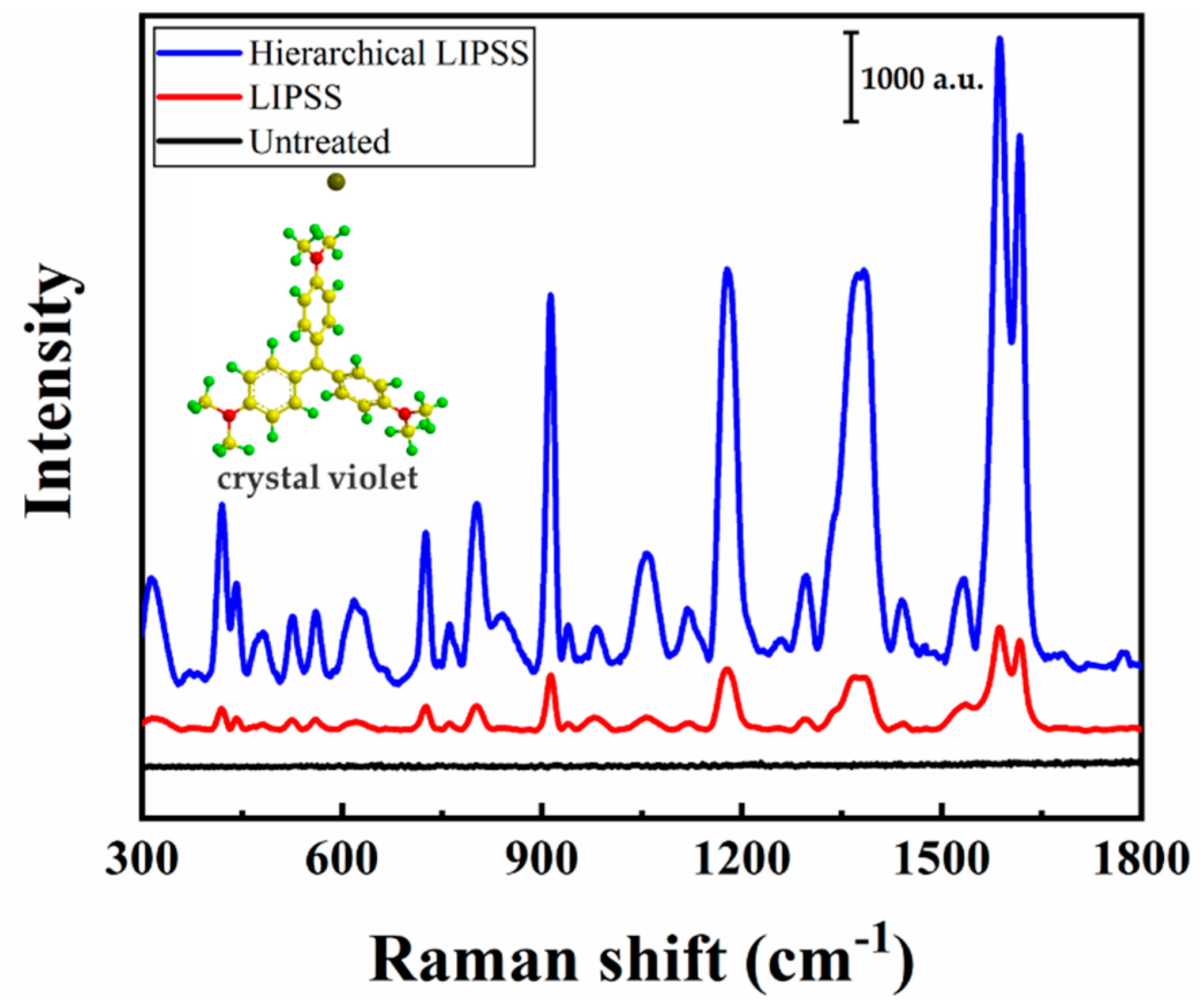

| Peak (cm−1) | 913 | 1175 | 1229 | 1539 | 1587 | 1618 |
|---|---|---|---|---|---|---|
| LIPSS | 8.83 × 103 | 1.99 × 104 | 2.15 × 103 | 8.29 × 103 | 3.59 × 104 | 2.16 × 104 |
| Hierarchical LIPSS | 2.82 × 104 | 6.27 × 104 | 7.85 × 105 | 7.28 × 104 | 2.32 × 105 | 1.36 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Zhang, J.; Jiao, L.; Guan, Y. Large-Scale Fabrication of Nanostructure on Bio-Metallic Substrate for Surface Enhanced Raman and Fluorescence Scattering. Nanomaterials 2019, 9, 916. https://doi.org/10.3390/nano9070916
Lu L, Zhang J, Jiao L, Guan Y. Large-Scale Fabrication of Nanostructure on Bio-Metallic Substrate for Surface Enhanced Raman and Fluorescence Scattering. Nanomaterials. 2019; 9(7):916. https://doi.org/10.3390/nano9070916
Chicago/Turabian StyleLu, Libin, Jiaru Zhang, Lishi Jiao, and Yingchun Guan. 2019. "Large-Scale Fabrication of Nanostructure on Bio-Metallic Substrate for Surface Enhanced Raman and Fluorescence Scattering" Nanomaterials 9, no. 7: 916. https://doi.org/10.3390/nano9070916
APA StyleLu, L., Zhang, J., Jiao, L., & Guan, Y. (2019). Large-Scale Fabrication of Nanostructure on Bio-Metallic Substrate for Surface Enhanced Raman and Fluorescence Scattering. Nanomaterials, 9(7), 916. https://doi.org/10.3390/nano9070916






