Solvent Engineering for Intermediates Phase, All-Ambient-Air-Processed in Organic–Inorganic Hybrid Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Film and Device Characterization
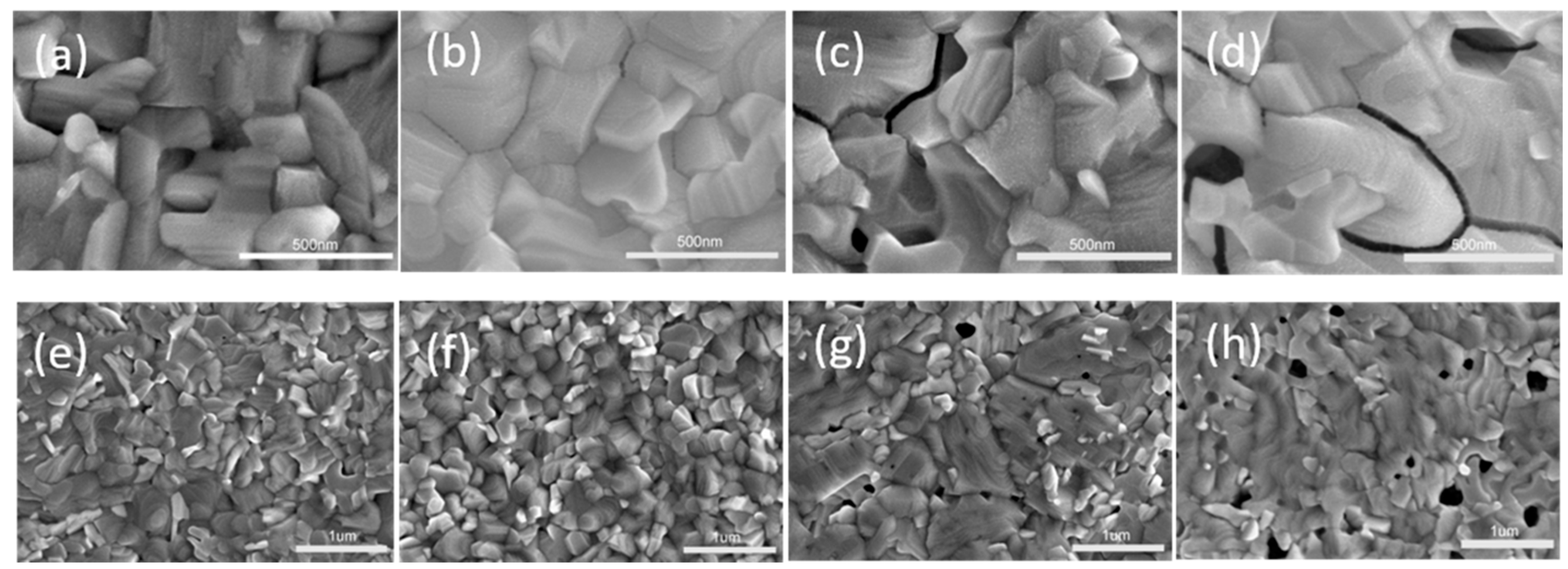
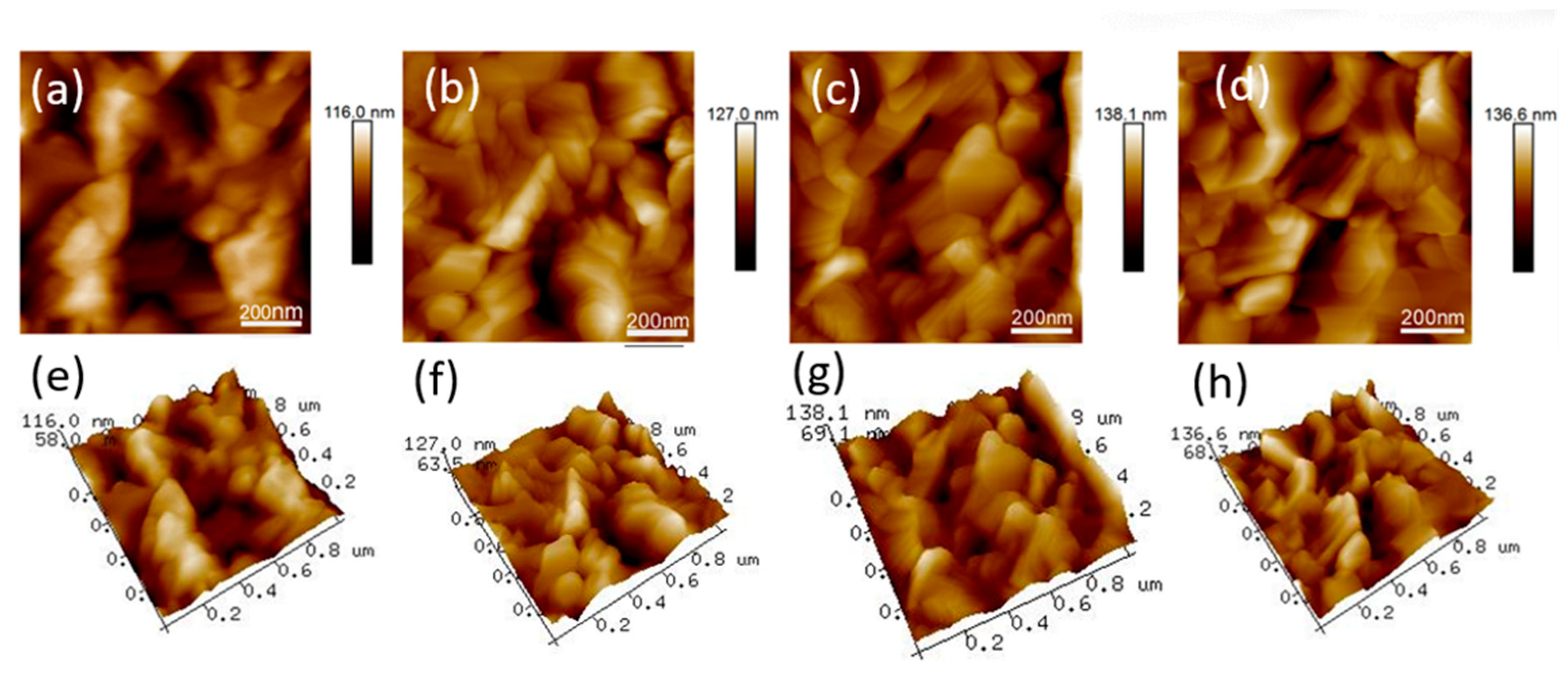
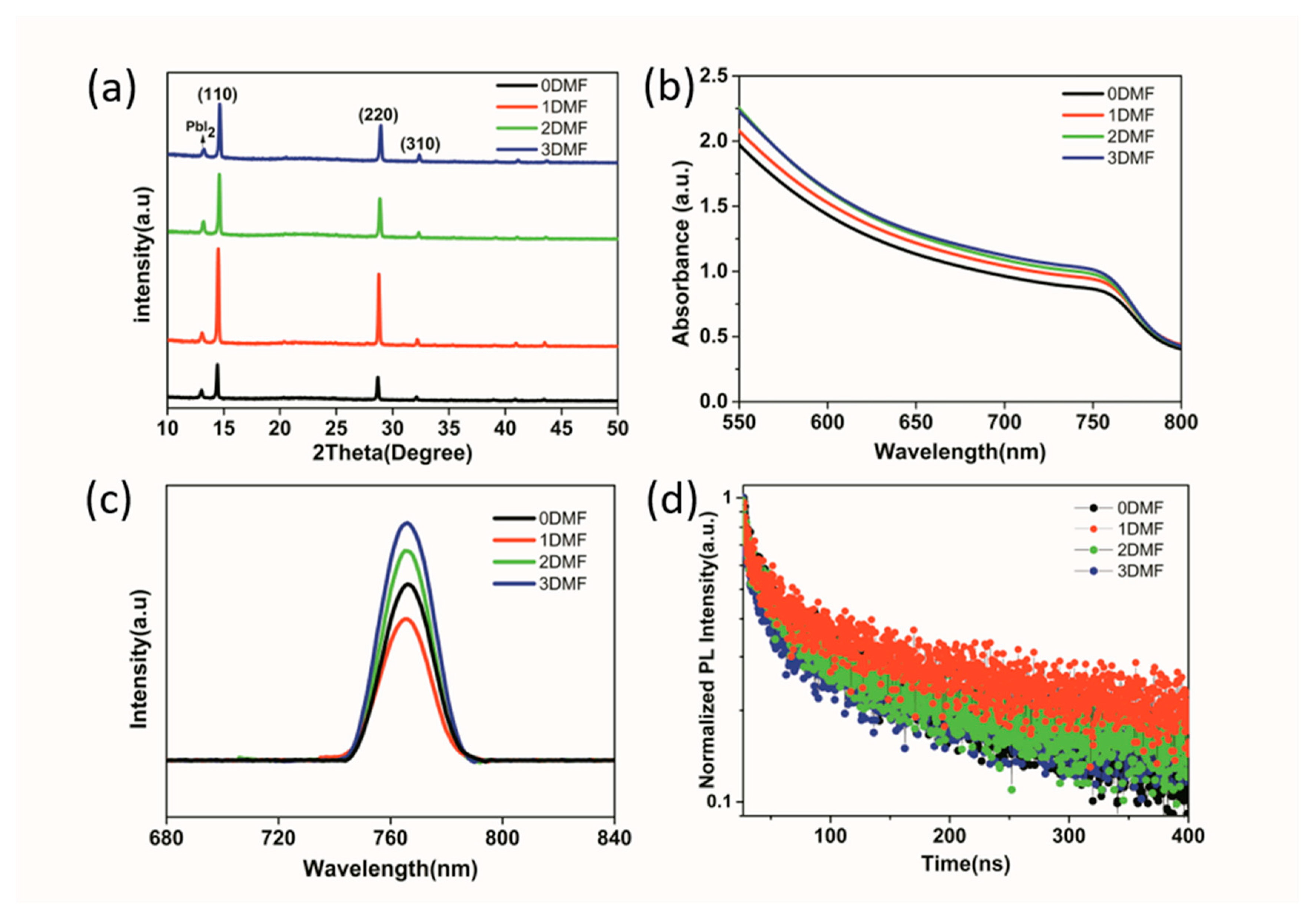
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- NREL. Best Research-Cell Efficencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-190416.pdf (accessed on 24 June 2019).
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2013, 8, 128. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths inorganic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef]
- Manda, X.; Fuzhi, H.; Wenchao, H.; Yasmina, D.; Ye, Z.; Joanne, E.; Angus, G.W.; Udo, B.; Yi-Bing, C.; Leone, S. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. 2015, 53, 9898–9903. [Google Scholar]
- Guo, Y.; Shoyama, K.; Sato, W.; Matsuo, Y.; Inoue, K.; Harano, K.; Liu, C.; Tanaka, H.; Nakamura, E. Chemical Pathways Connecting Lead(Ii) Iodide and Perovskite Via Polymeric Plumbate(Ii) Fiber. J. Am. Chem. Soc. 2015, 137, 15907–15914. [Google Scholar] [CrossRef]
- Paek, S.; Schouwink, P.; Athanasopoulou, E.N.; Cho, K.T.; Grancini, G.; Lee, Y.; Zhang, Y.; Stellacci, F.; Nazeeruddin, M.K.; Gao, P. From nano- to micrometer scale: The role of antisolvent treatment on high performance perovskite solar cells. Chem. Mater. 2017, 29, 3490–3498. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, H.-S.; Park, N.-G. Lewis acid–base adduct approach for high efficiency perovskite solar cells. Acc. Chem. Res. 2016, 49, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Dong, X.; Fang, X.; Lin, B.; Zhang, S.; Ding, J.; Yuan, N. A promising alternative solvent of perovskite to induce rapid crystallization for high-efficiency photovoltaic devices. RSC Adv. 2015, 5, 20521–20529. [Google Scholar] [CrossRef]
- Gardner, K.L.; Tait, J.G.; Merckx, T.; Qiu, W.; Paetzold, U.W.; Kootstra, L.; Jaysankar, M.; Gehlhaar, R.; Cheyns, D.; Heremans, P.; et al. Nonhazardous solvent systems for processing perovskite photovoltaics. Adv. Energy Mater. 2016, 6, 1600386. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis base adduct of lead(ii) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Tang, Z.; Zhao, Y.; Zhong, X.; Venkatesan, S.; Graham, H.; Patton, M.; Jing, Y.; Guloy, A.M.; Yao, Y. Solvent engineering towards controlled grain growth in perovskite planar heterojunction solar cells. Nanoscale 2015, 7, 10595–10599. [Google Scholar] [CrossRef]
- Rong, Y.; Venkatesan, S.; Guo, R.; Wang, Y.; Bao, J.; Li, W.; Fan, Z.; Yao, Y. Critical kinetic control of non-stoichiometric intermediate phase transformation for efficient perovskite solar cells. Nanoscale 2016, 8, 12892–12899. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Hu, C.; Zhang, T.; Meng, X.; Li, Q.; Yang, Y.; Wong, K.S.; Chen, H.; Yang, S. A pure and stable intermediate phase is key to growing aligned and vertically monolithic perovskite crystals for efficient pin planar perovskite solar cells with high processibility and stability. Nano Energy 2017, 34, 58–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Grancini, G.; Feng, Y.; Asiri, A.M.; Nazeeruddin, M.K. Optimization of stable quasi-cubic faxma1–XPbI3 perovskite structure for solar cells with efficiency beyond 20%. ACS Energy Lett. 2017, 2, 802–806. [Google Scholar] [CrossRef]
- Yin, M.; Xie, F.; Chen, H.; Yang, X.; Ye, F.; Bi, E.; Wu, Y.; Cai, M.; Han, L. Annealing-free perovskite films by instant crystallization for efficient solar cells. J. Mater. Chem. A 2016, 4, 8548–8553. [Google Scholar] [CrossRef]
- Troughton, J.; Hooper, K.; Watson, T.M. Humidity resistant fabrication of ch3nh3pbi3 perovskite solar cells and modules. Nano Energy 2017, 39, 60–68. [Google Scholar] [CrossRef]
- Yang, F.; Kapil, G.; Zhang, P.; Hu, Z.; Kamarudin, M.A.; Ma, T.; Hayase, S. Dependence of acetate-based antisolvents for high humidity fabrication of CH3NH3PbI3 perovskite devices in ambient atmosphere. Acs Appl. Mater. Interfaces 2018, 10, 16482–16489. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; van Franeker, J.J.; deQuilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The importance of moisture in hybrid lead halide perovskite thin film fabrication. ACS Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Yuan, Y.; Dong, X.; Wang, P. Anti-solvent dependent device performance in ch3nh3pbi3 solar cells: The role of intermediate phase content in the as-prepared thin films. Sustain. Energy Fuels 2017, 1, 1041–1048. [Google Scholar] [CrossRef]
- Shen, D.; Yu, X.; Cai, X.; Peng, M.; Ma, Y.; Su, X.; Xiao, L.; Zou, D. Understanding the solvent-assisted crystallization mechanism inherent in efficient organic–inorganic halide perovskite solar cells. J. Mater. Chem. A 2014, 2, 20454–20461. [Google Scholar] [CrossRef]
- Bae, S.; Han, S.J.; Shin, T.J.; Jo, W.H. Two different mechanisms of CH3NH3PbI3 film formation in one-step deposition and its effect on photovoltaic properties of opv-type perovskite solar cells. J. Mater. Chem. A 2015, 3, 23964–23972. [Google Scholar] [CrossRef]
- Cao, X.B.; Li, Y.H.; Fang, F.; Cui, X.; Yao, Y.W.; Wei, J.Q. High quality perovskite films fabricated from lewis acid–base adduct through molecular exchange. RSC Adv. 2016, 6, 70925–70931. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, H.; Qi, J.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Antisolvent-derived intermediate phases for low-temperature flexible perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 6477–6486. [Google Scholar] [CrossRef]
- Lin, N.; Qiao, J.; Dong, H.; Ma, F.; Wang, L. Morphology-controlled CH3NH3PbI3 films by hexane-assisted one-step solution deposition for hybrid perovskite mesoscopic solar cells with high reproductivity. J. Mater. Chem. A 2015, 3, 22839–22845. [Google Scholar] [CrossRef]
- Wu, Y.; Islam, A.; Yang, X.; Qin, C.; Liu, J.; Zhang, K.; Peng, W.; Han, L. Retarding the crystallization of PbI2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci. 2014, 7, 2934–2938. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, H.; Xiao, S.; Xue, Q.; Zhang, T.; Zhu, Z.; Li, Q.; Hu, C.; Yang, Y.; Hu, Z.; et al. Effects of a molecular monolayer modification of nio nanocrystal layer surfaces on perovskite crystallization and interface contact toward faster hole extraction and higher photovoltaic performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Jeong-Hyeok, I.; Chang-Ryul, L.; Jin-Wook, L.; Sang-Won, P.; Nam-Gyu, P. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.; Lv, S.; Yang, Y.; Xin, X.; Xu, Y.; Xiao, J.; Wu, H.; Luo, Y.; Li, D. Temperature-assisted controlling morphology and charge transport property for highly efficient perovskite solar cells. Nano Energy 2015, 15, 540–548. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Zhao, Y.; Shi, J.; Xu, Y.; Luo, Y.; Li, D.; Wu, H.; Meng, Q. Dmf as an additive in a two-step spin-coating method for 20% conversion efficiency in perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 26937–26947. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Masuda, T.; Miyano, K. Enhancement in efficiency and optoelectronic quality of perovskite thin films annealed in macl vapor. Sustain. Energy Fuels 2017, 1, 755–766. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Sun, P.; Ma, W.; Chen, Z.-K. Additive to regulate the perovskite crystal film growth in planar heterojunction solar cells. Appl. Phys. Lett. 2015, 106, 033901. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-performance hole-extraction layer of sol–gel-processed nio nanocrystals for inverted planar perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Leijtens, T.; Lauber, B.; Eperon, G.E.; Stranks, S.D.; Snaith, H.J. The importance of perovskite pore filling in organometal mixed halide sensitized TiO2-based solar cells. J. Phys. Chem. Lett. 2014, 5, 1096–1102. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Leung, W.W. Lead iodide thin film crystallization control for high-performance and stable solution-processed perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 14614–14619. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Degradation of encapsulated perovskite solar cells driven by deep trap states and interfacial deterioration. J. Mater. Chem. C 2018, 6, 162–170. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
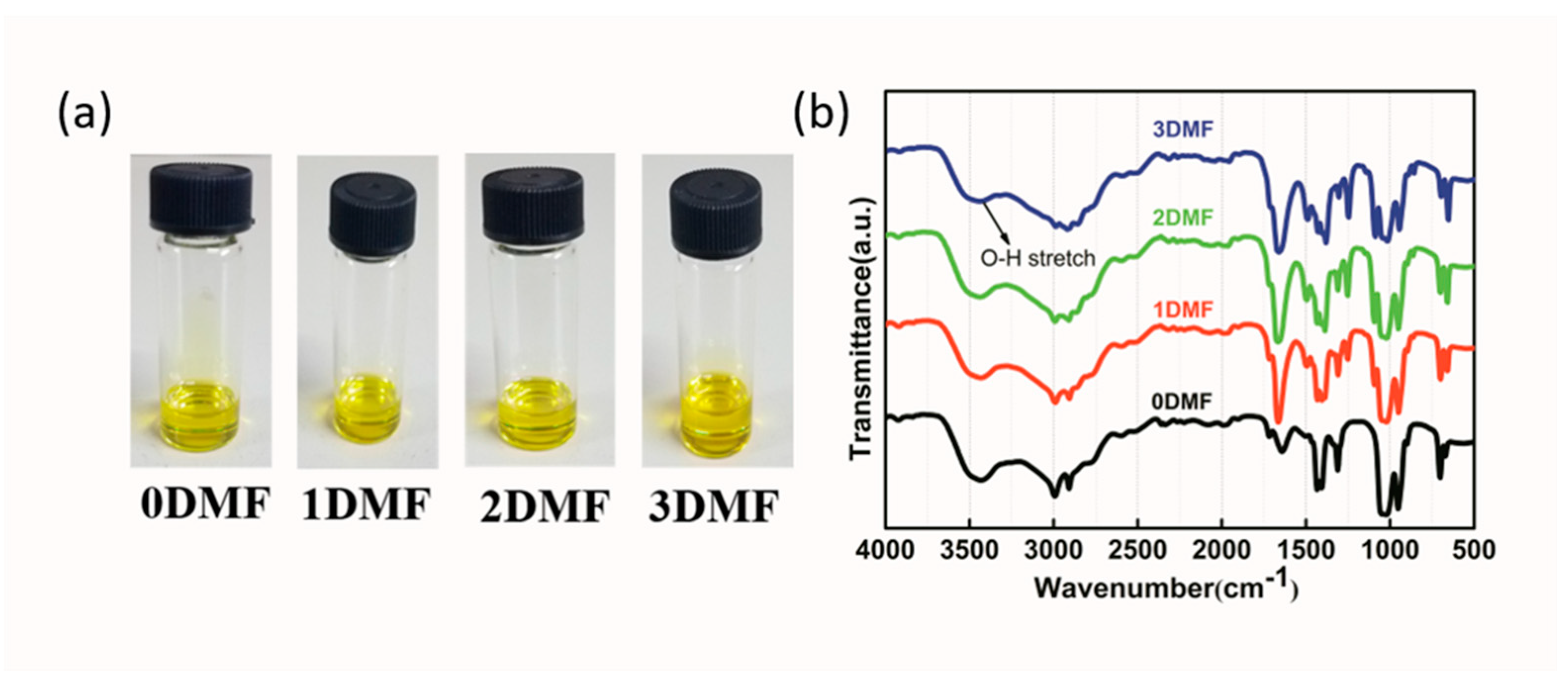
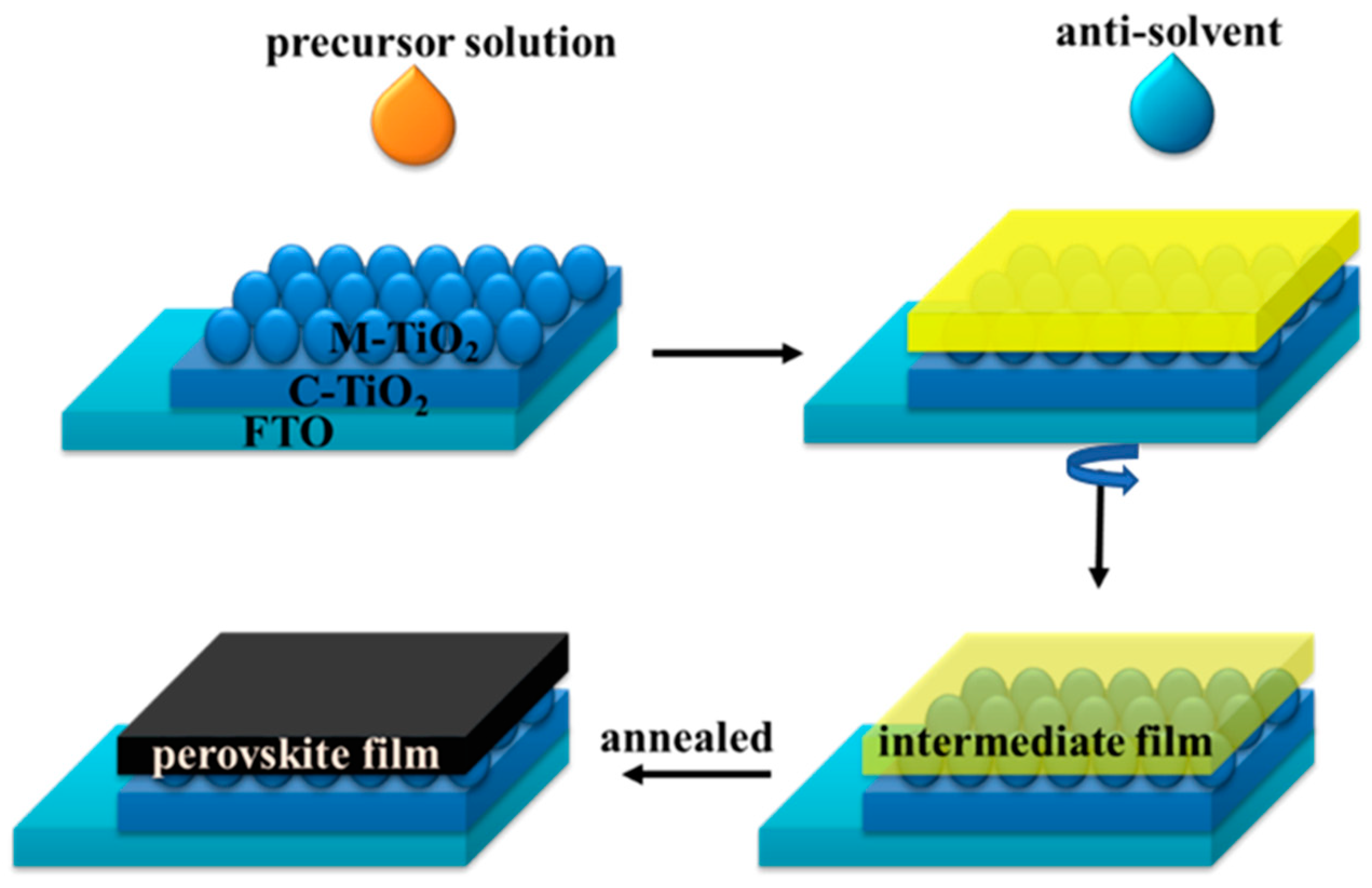
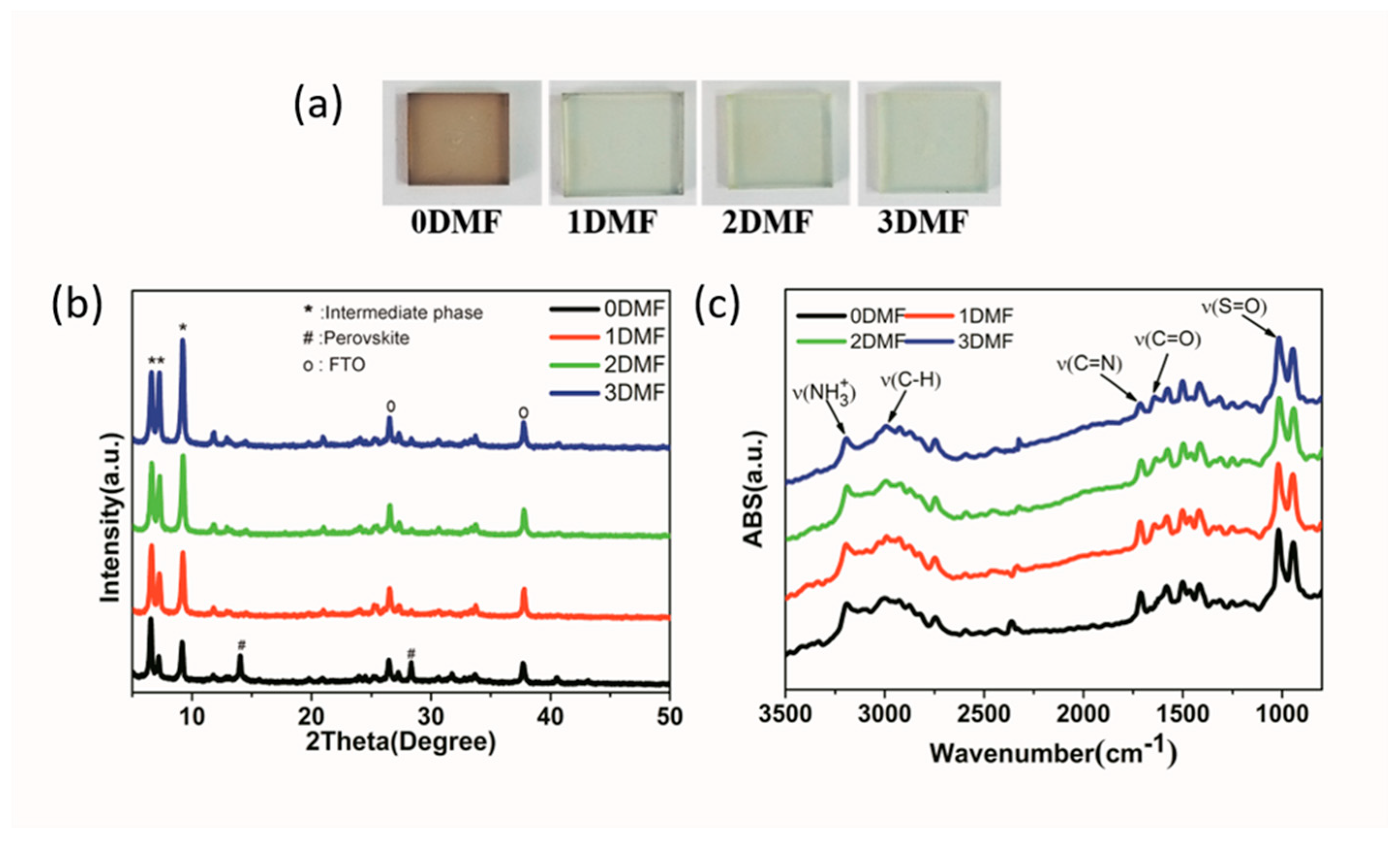
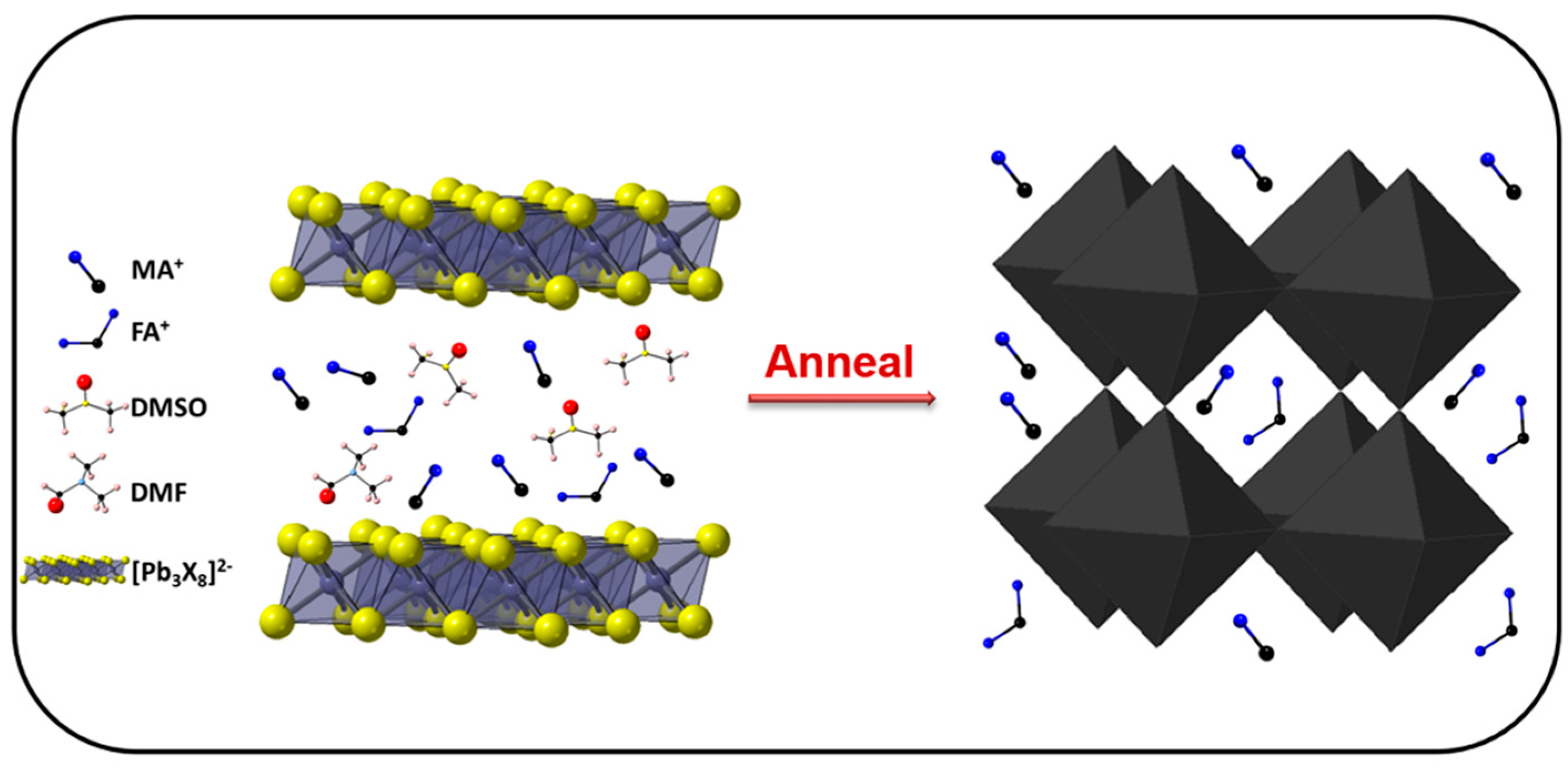
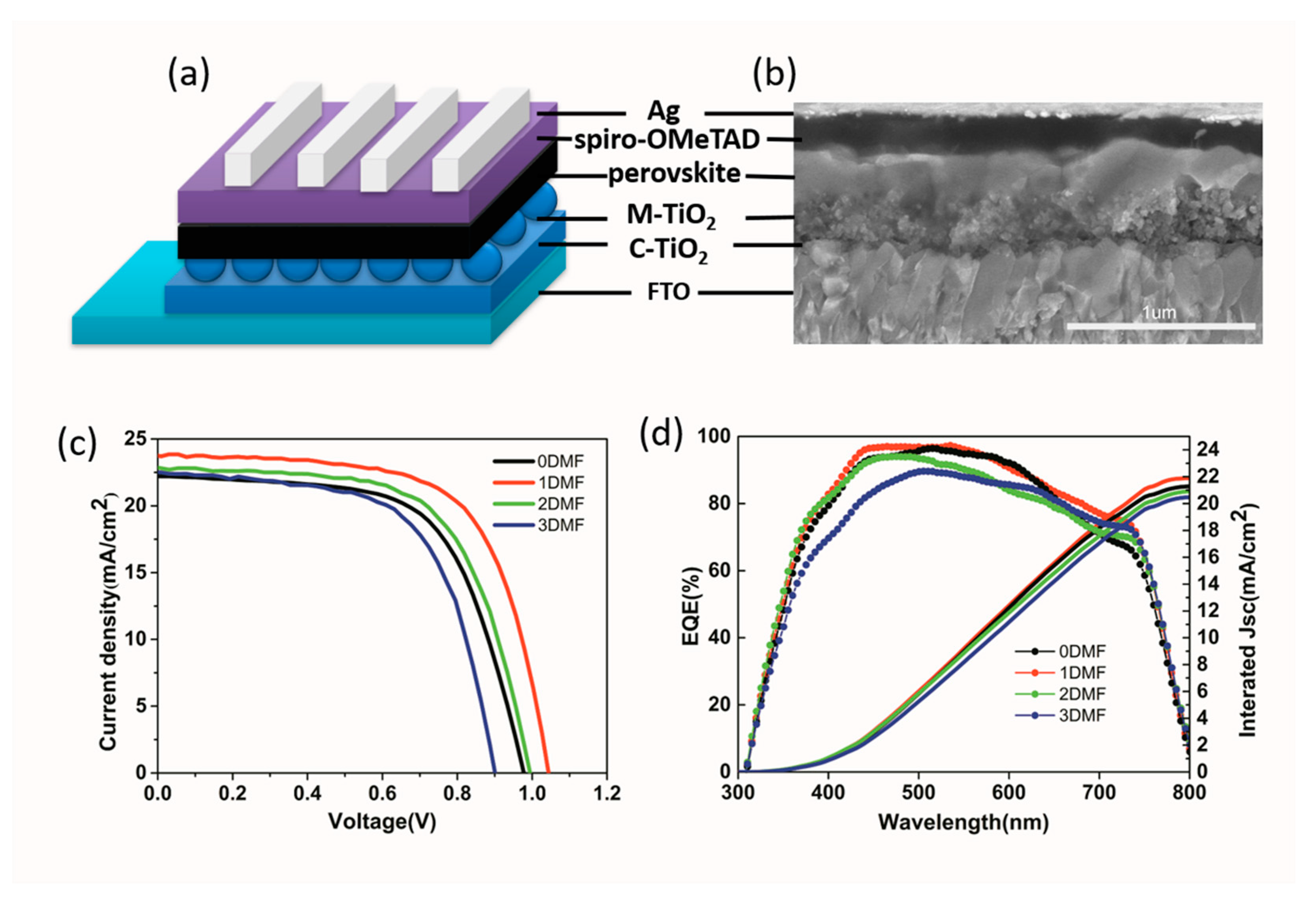
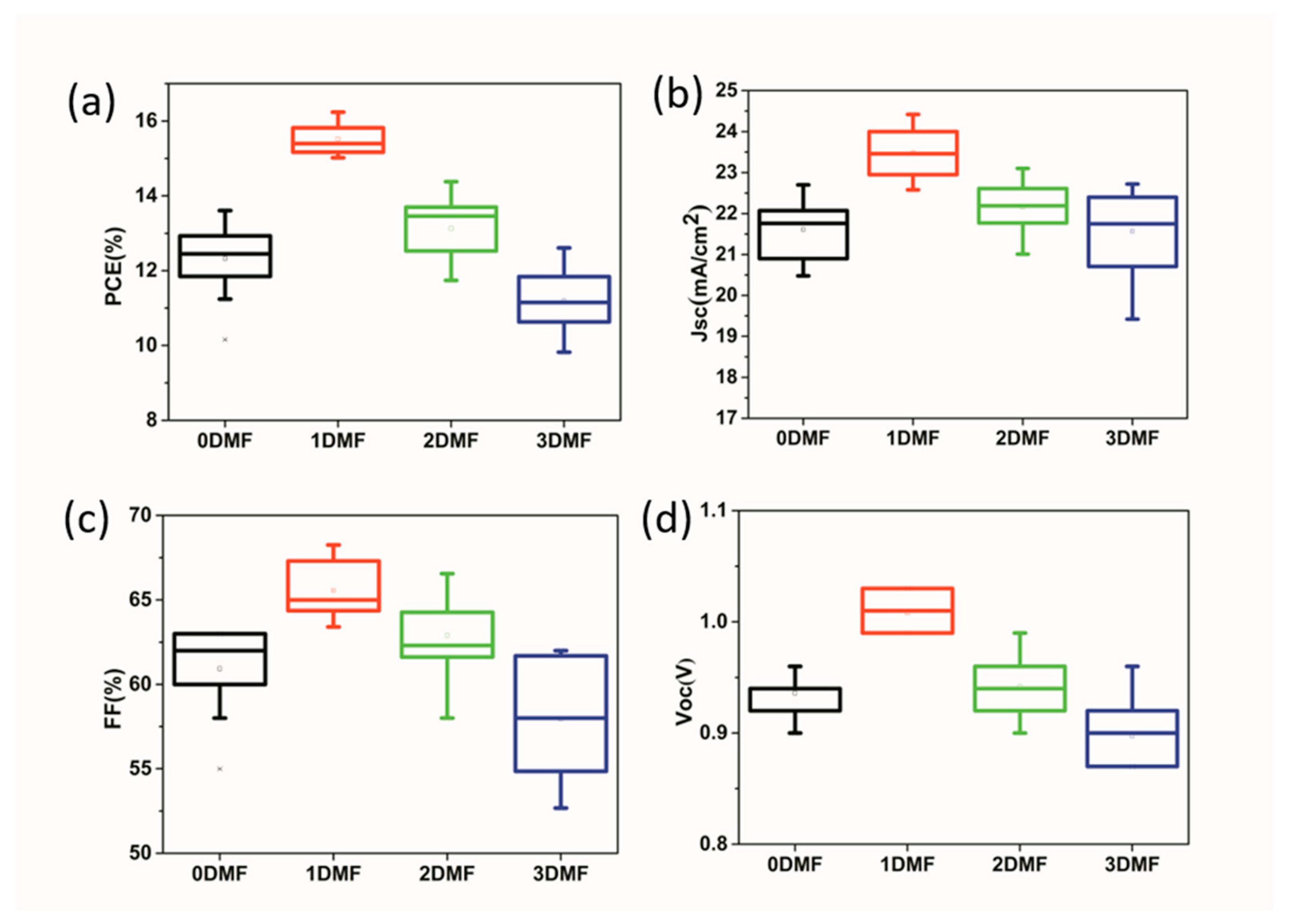
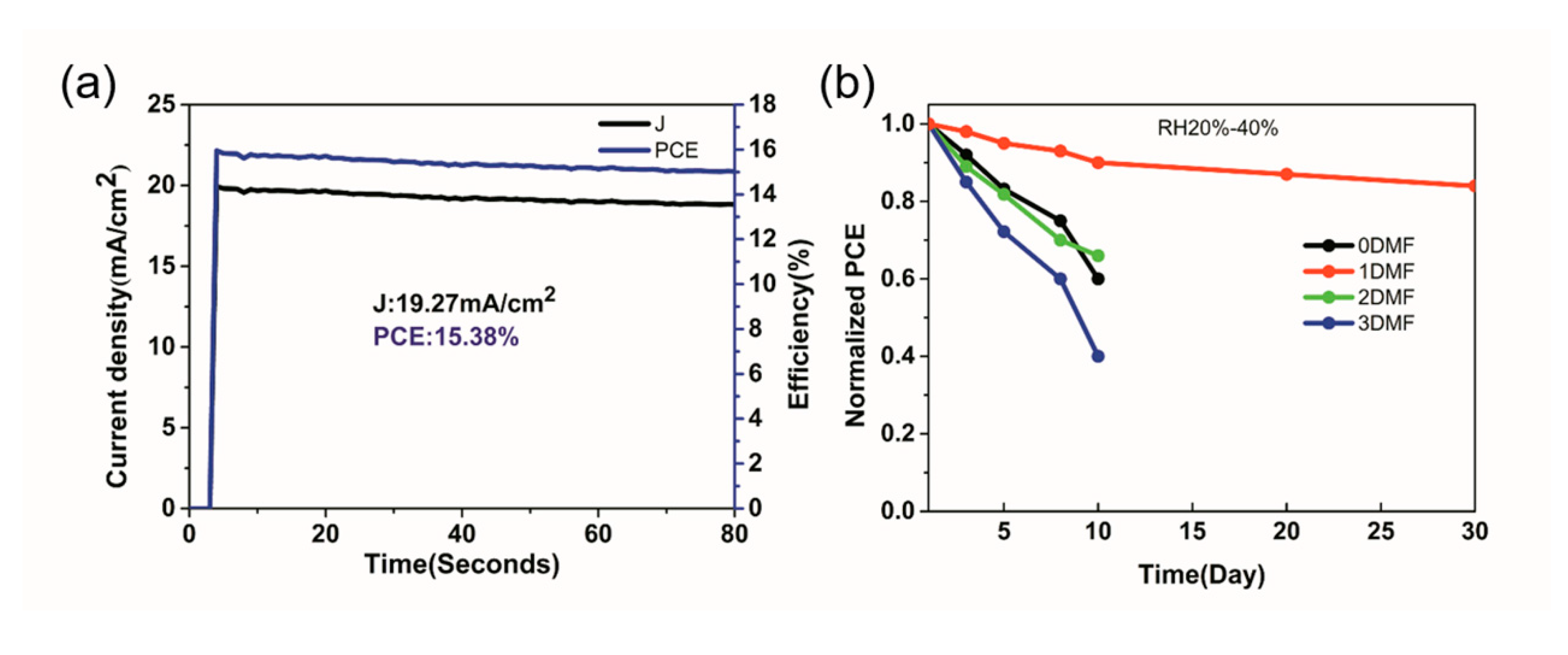
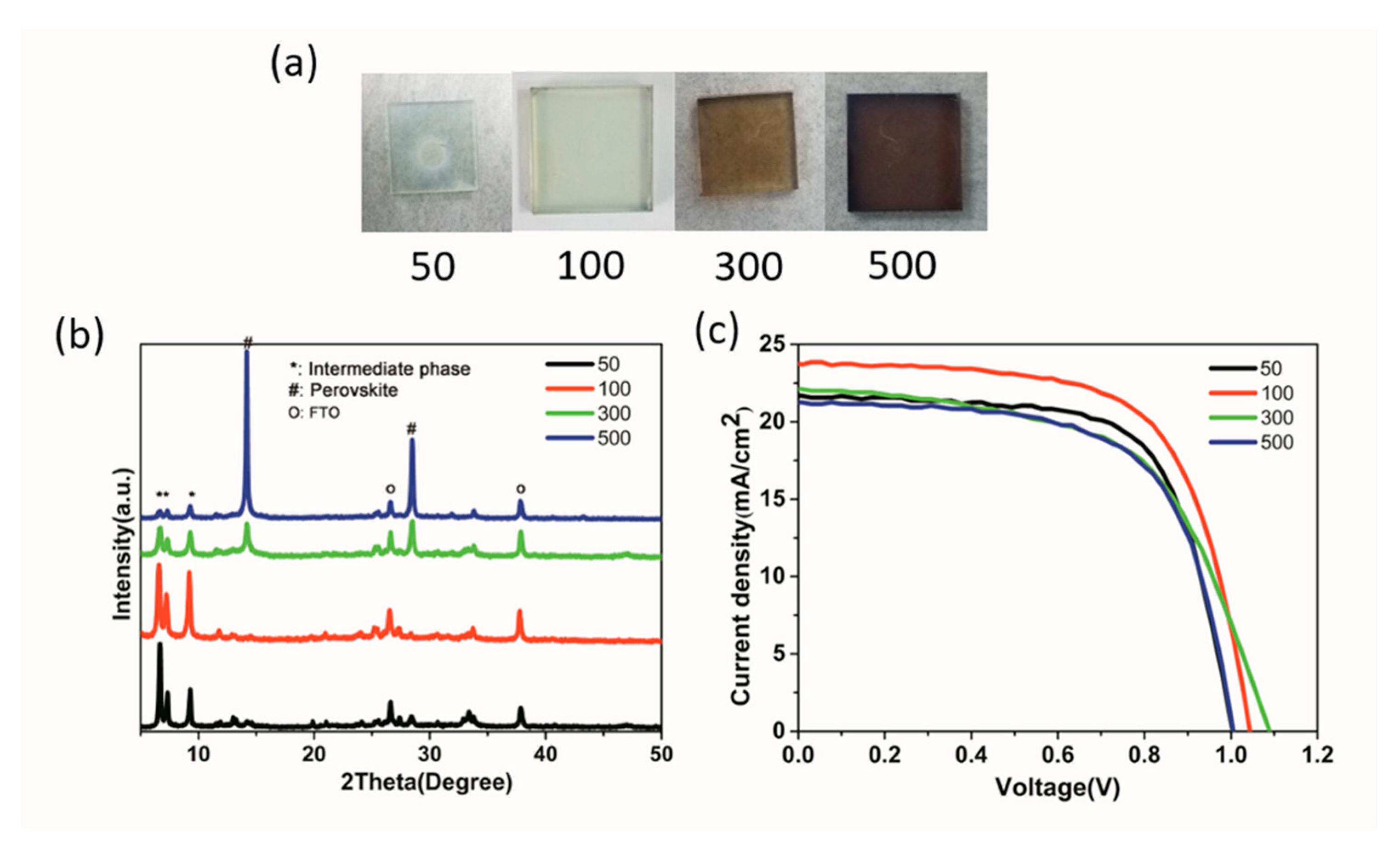
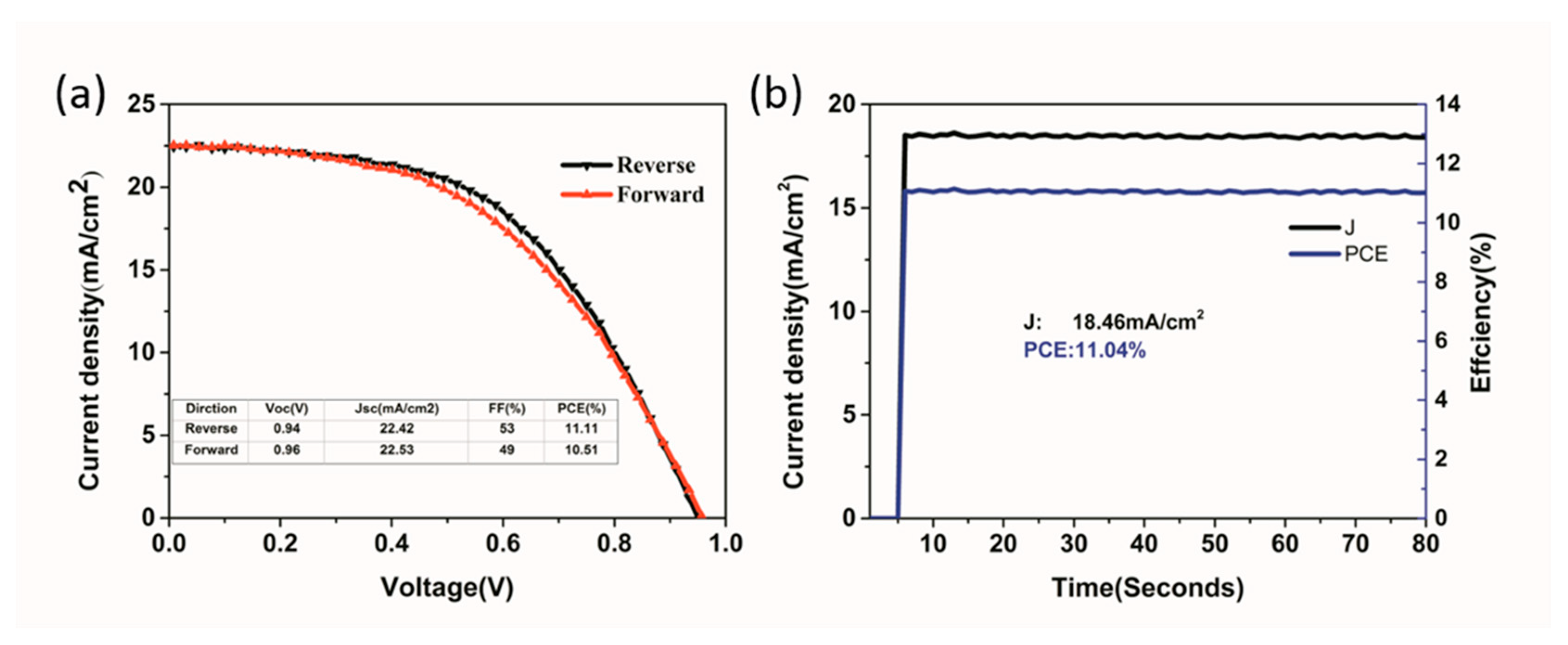
| Precursor | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0DMF | 0.96 | 22.7 | 62.5 | 13.61 |
| 1DMF | 1.03 | 23.7 | 66.5 | 16.24 |
| 2DMF | 0.99 | 22.8 | 63.7 | 14.38 |
| 3DMF | 0.90 | 22.5 | 62.3 | 12.61 |
| Anti-Solvent Volume (μL) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 50 | 0.98 | 21.68 | 71.0 | 14.97 |
| 100 | 1.03 | 23.70 | 66.5 | 16.24 |
| 300 | 1.08 | 22.11 | 58.3 | 13.93 |
| 500 | 1.01 | 21.26 | 64.3 | 13.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Hao, H.; Dong, J.; Zhong, T.; Zhang, C.; Hao, J.; Xing, J.; Liu, H. Solvent Engineering for Intermediates Phase, All-Ambient-Air-Processed in Organic–Inorganic Hybrid Perovskite Solar Cells. Nanomaterials 2019, 9, 915. https://doi.org/10.3390/nano9070915
Shi L, Hao H, Dong J, Zhong T, Zhang C, Hao J, Xing J, Liu H. Solvent Engineering for Intermediates Phase, All-Ambient-Air-Processed in Organic–Inorganic Hybrid Perovskite Solar Cells. Nanomaterials. 2019; 9(7):915. https://doi.org/10.3390/nano9070915
Chicago/Turabian StyleShi, Lei, Huiying Hao, Jingjing Dong, Tingting Zhong, Chen Zhang, Jiabin Hao, Jie Xing, and Hao Liu. 2019. "Solvent Engineering for Intermediates Phase, All-Ambient-Air-Processed in Organic–Inorganic Hybrid Perovskite Solar Cells" Nanomaterials 9, no. 7: 915. https://doi.org/10.3390/nano9070915
APA StyleShi, L., Hao, H., Dong, J., Zhong, T., Zhang, C., Hao, J., Xing, J., & Liu, H. (2019). Solvent Engineering for Intermediates Phase, All-Ambient-Air-Processed in Organic–Inorganic Hybrid Perovskite Solar Cells. Nanomaterials, 9(7), 915. https://doi.org/10.3390/nano9070915







