Facile Construction of Functionalized GO Nanocomposites with Enhanced Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Characterization
2.3. Release Behavior
2.4. Antibacterial Activity
2.4.1. Inhibition of Growth Effect
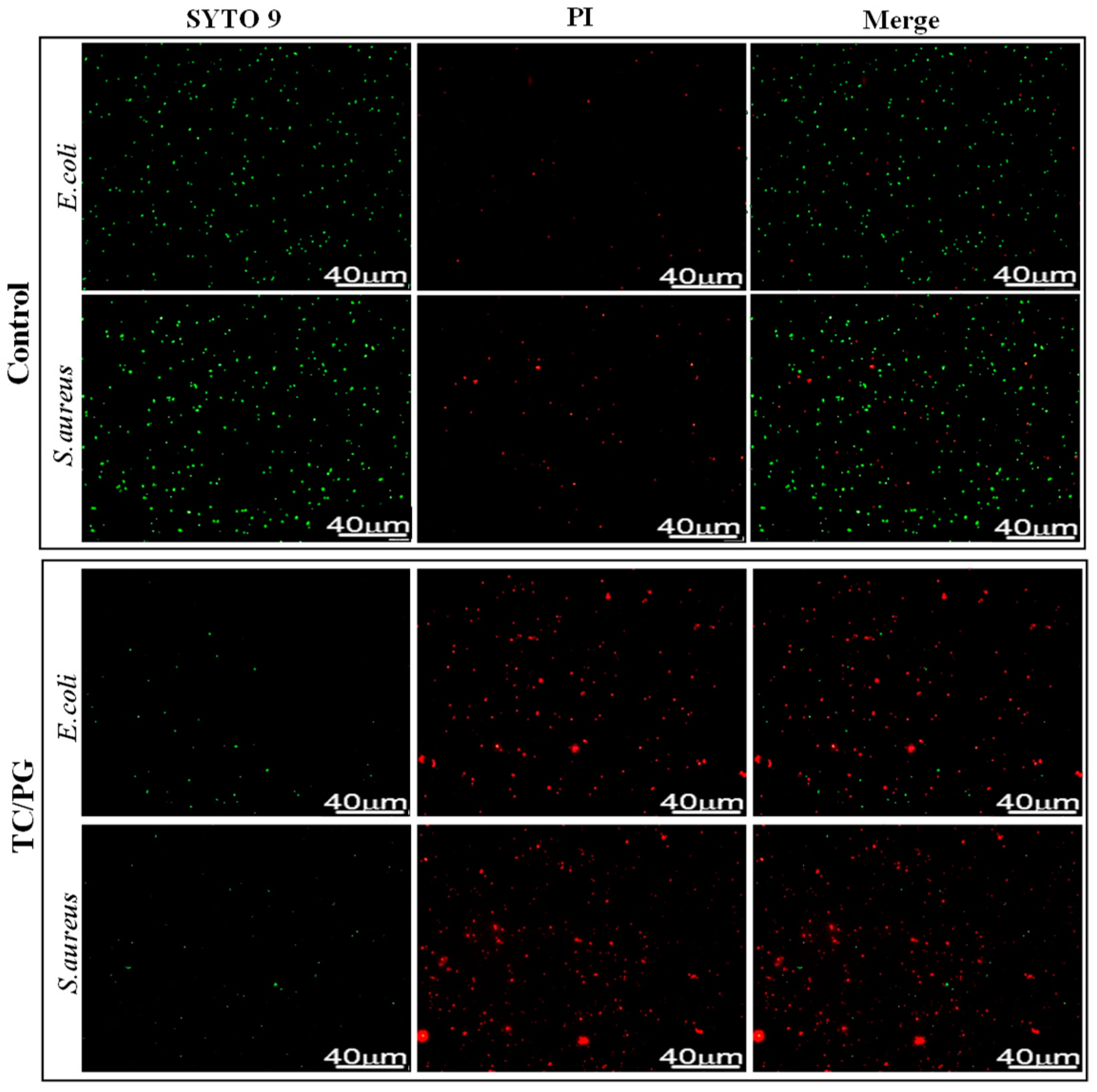
2.4.2. Fluorescent Stain
2.4.3. Inhibition Zone Behavior
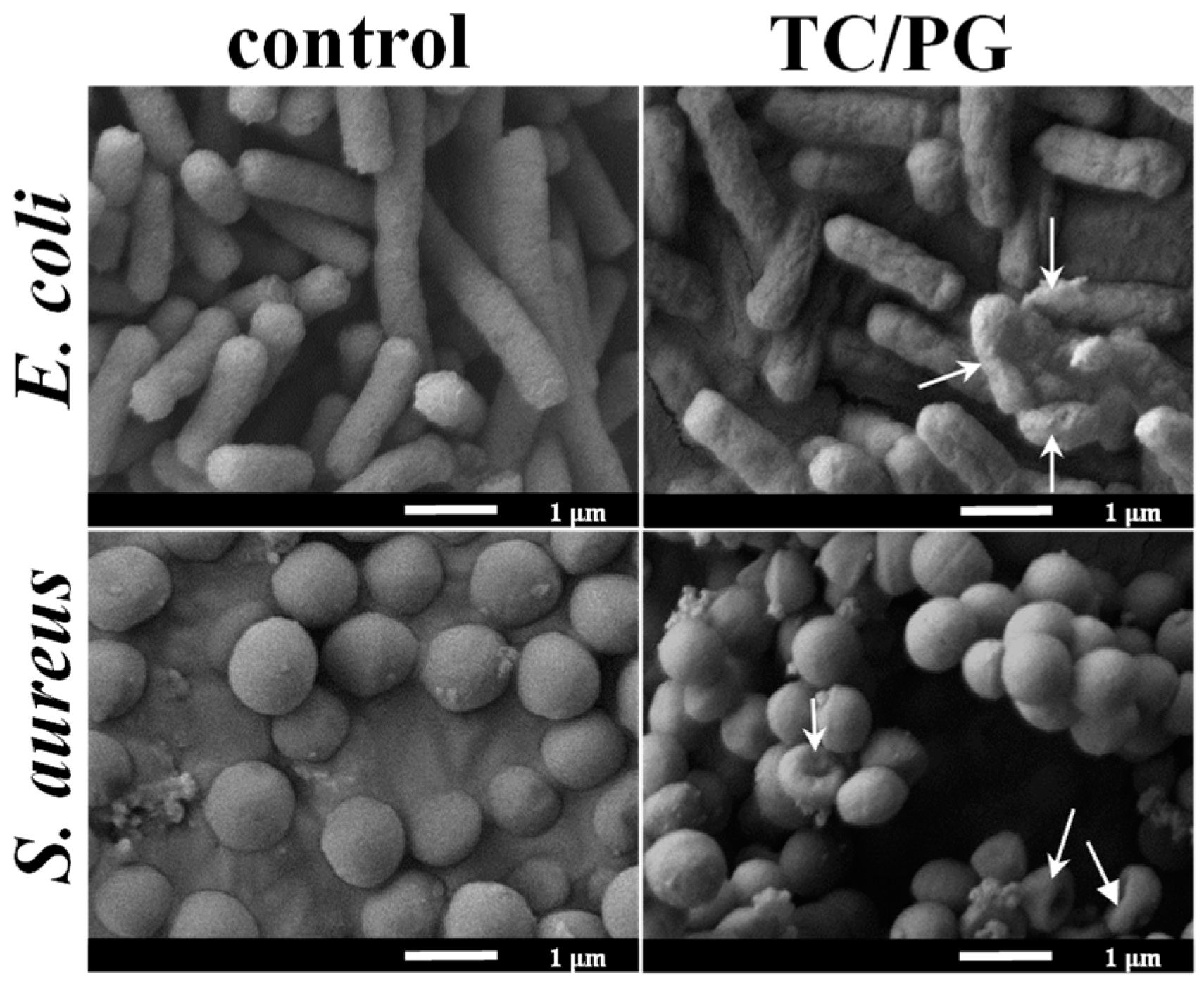
2.4.4. Bacterial Morphologies
2.4.5. Protein Leakage
2.4.6. Detection of Reactive Oxygen Species (ROS) Production
2.5. Statistical Analysis
3. Results and Discussion
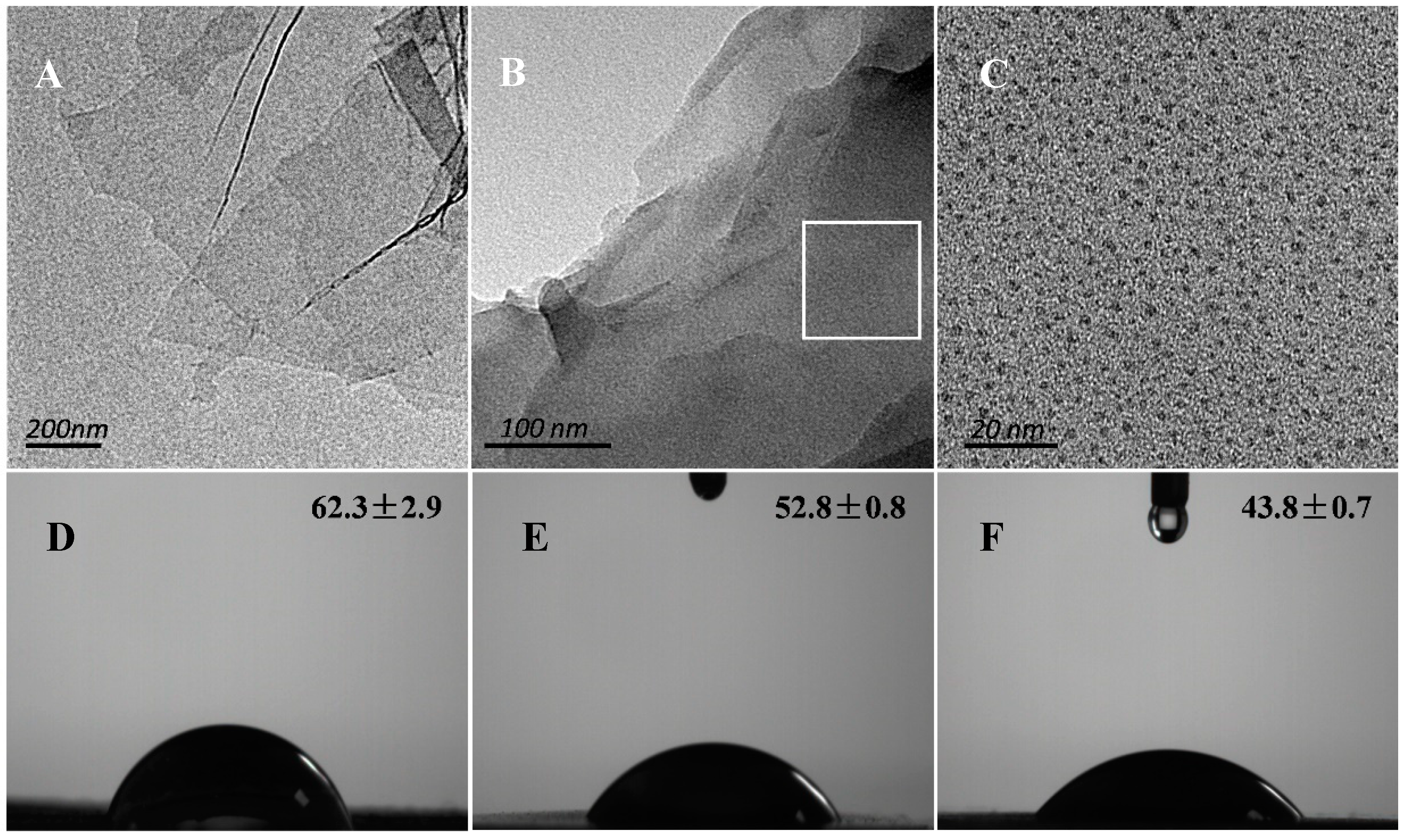
3.1. Morphology
3.2. FTIR and Raman Analysis
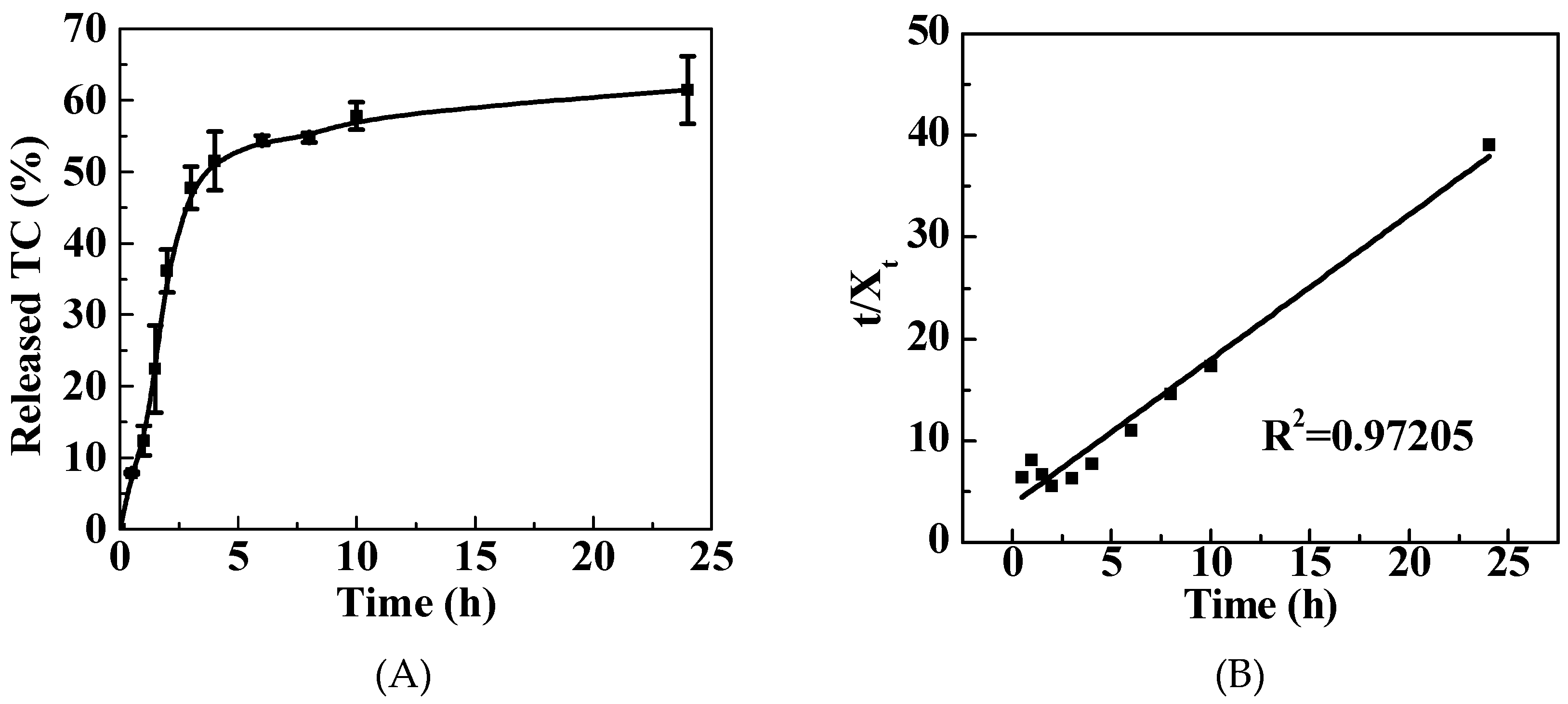
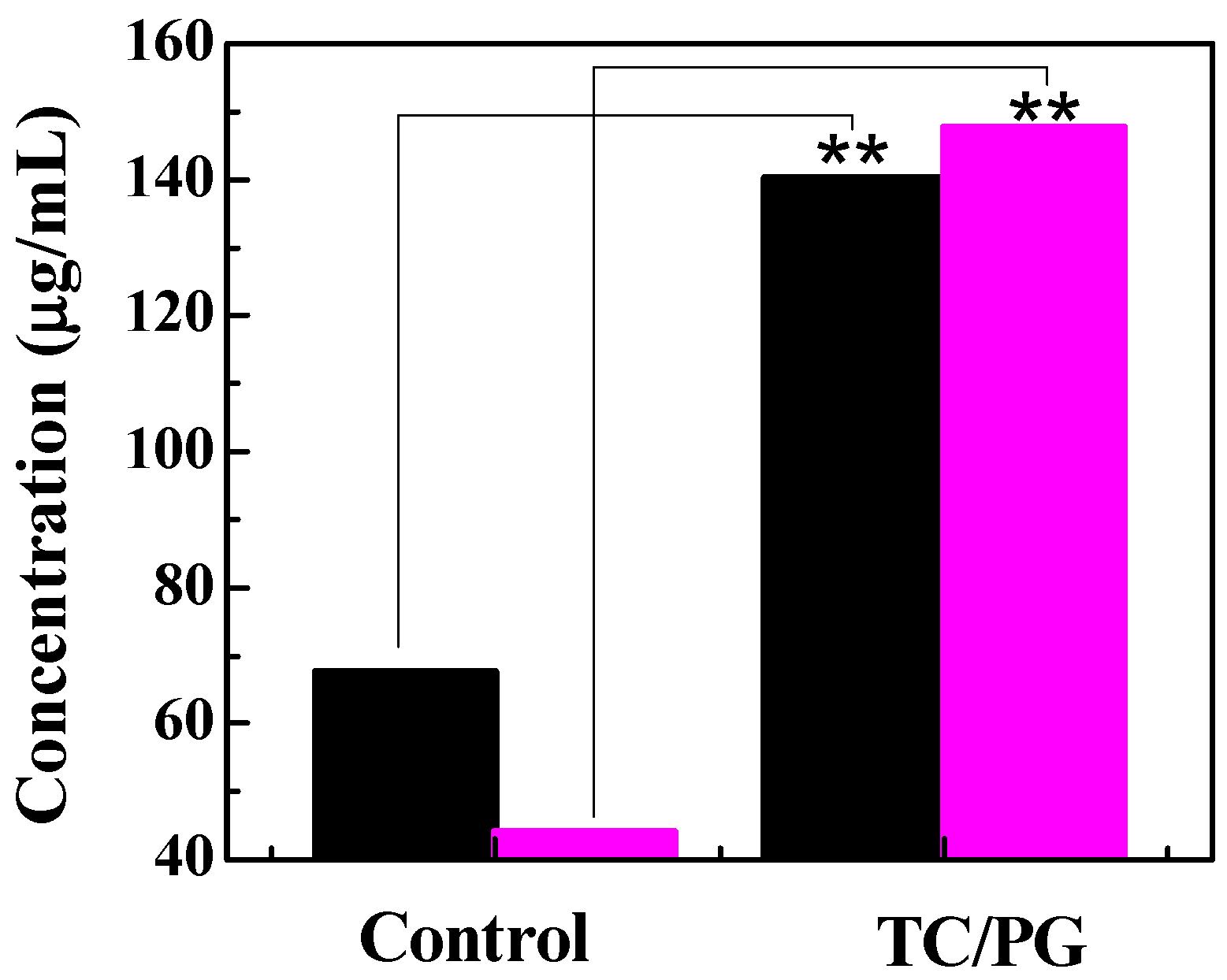
3.3. TC Release Performance
3.4. Antibacterial Performance
3.5. Protein Leakage
3.6. Oxidative Stress-Mediated Antimicrobial Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xie, Q.; Zhang, Y.; Zhao, P. Facile fabrication of honeycomb-like restacking-inhibited graphene architecture with superior electrochemical performance for energy storage. Mater. Lett. 2018, 225, 93–96. [Google Scholar] [CrossRef]
- Ye, M.; Gao, J.; Xiao, Y.; Xu, T.; Zhao, Y.; Qu, L. Metal/graphene oxide batteries. Carbon 2017, 125, 299–307. [Google Scholar] [CrossRef]
- Pandit, S.; Cao, Z.; Mokkapati, V.R.; Celauro, E.; Yurgens, A.; Lovmar, M.; Westerlund, F.; Sun, J.; Mijakovic, I. Vertically Aligned Graphene Coating is Bactericidal and Prevents the Formation of Bacterial Biofilms. Adv. Mater. Interfaces 2018, 5, 1701331. [Google Scholar] [CrossRef]
- He, D.; Song, L.; Lv, L.; Zhang, Z.; Qu, L. Superelastic air-bubbled graphene foam monoliths as structural buffer for compressible high-capacity anode materials in lithium-ion batteries. Chem. Eng. J. 2018, 331, 704–711. [Google Scholar] [CrossRef]
- Şenol, A.M.; Onganer, Y.; Meral, K. An unusual “off-on” fluorescence sensor for iron(III) detection based on fluorescein–reduced graphene oxide functionalized with polyethyleneimine. Sens. Actuators B Chem. 2017, 239, 343–351. [Google Scholar] [CrossRef]
- Nayak, G.S.; Zybala, R.; Kozinski, R.; Woluntarski, M.; Telle, R.; Schickle, K. Immobilization of reduced graphene oxide nano-flakes on inert ceramic surfaces using self-assembled monolayer technique. Mater. Lett. 2018, 225, 109–112. [Google Scholar] [CrossRef]
- Qian, W.; Wang, Z.; He, D.; Huang, X.; Su, J. Ornidazole-loaded graphene paper for combined antibacterial materials. J. Saudi Chem. Soc. 2018, 22, 581–587. [Google Scholar] [CrossRef]
- Gong, P.; Ji, S.; Wang, J.; Dai, D.; Wang, F.; Tian, M.; Zhang, L.; Guo, F.; Liu, Z. Fluorescence-switchable ultrasmall fluorinated graphene oxide with high near-infrared absorption for controlled and targeted drug delivery. Chem. Eng. J. 2018, 348, 438–446. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, N.; Prasad, P.; Shirbhate, S.; Sehrawat, S.; Lochab, B. Stable Dispersions of Covalently Tethered Polymer Improved Graphene Oxide Nanoconjugates as an Effective Vector for siRNA Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14577–14593. [Google Scholar] [CrossRef]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater. Sci. Eng. C 2017, 74, 177–185. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, L.; Zhu, H.; Liu, X. Combination types between graphene oxide and substrate affect the antibacterial activity. Bioact. Mater. 2018, 3, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wang, Y.; Li, W.; Liu, F.; Wang, L.; Sui, X.; Riaz, M.A.; Chen, Y. Antimicrobial graphene materials: The interplay of complex materials characteristics and competing mechanisms. Biomater. Sci. 2018, 6, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Yuan, H.Y.; Von Dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H.J. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Ye, X.L.; Feng, J.; Zhang, J.X.; Yang, X.J.; Liao, X.Y.; Shi, Q.S.; Tan, S.Z. Controlled release and long-term antibacterial activity of reduced graphene oxide/quaternary ammonium salt nanocomposites prepared by non-covalent modification. Colloid Surf. B Biointerfaces 2017, 149, 322–329. [Google Scholar] [CrossRef]
- Pan, N.Y.; Liu, Y.; Fan, X.Y.; Jiang, Z.M.; Ren, X.H.; Liang, J. Preparation and characterization of antibacterial graphene oxide functionalized with polymeric N-halamine. J. Mater. Sci. 2017, 52, 1996–2006. [Google Scholar] [CrossRef]
- Pang, J.; Kang, Z.; Wang, R.; Xu, B.; Nie, X.; Fan, L.; Zhang, F.; Du, X.; Feng, S.; Sun, D. Exploring the sandwich antibacterial membranes based on UiO-66/graphene oxide for forward osmosis performance. Carbon 2019, 144, 321–332. [Google Scholar] [CrossRef]
- Nayamadi Mahmoodabadi, A.; Kompany, A.; Mashreghi, M. Characterization, antibacterial and cytotoxicity studies of graphene-Fe3O4 nanocomposites and Fe3O4 nanoparticles synthesized by a facile solvothermal method. Mater. Chem. Phys. 2018, 213, 285–294. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, D.S.; Cui, J.H. A graphene oxide/silver nanoparticle composite as a novel agricultural antibacterial agent against Xanthomonas oryzae pv. oryzae for crop disease management. New J. Chem. 2017, 41, 13692–13699. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Samal, M.; Yun, K. Synthesis of ZnO nanoparticles-decorated spindle-shaped graphene oxide for application in synergistic antibacterial activity. J. Photochem. Photobiol. B Biol. 2018, 183, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Holoidovsky, L.; Thamaraiselvan, C.; Thakur, A.K.; Singh, S.P.; Meijler, M.M.; Arnusch, C.J. Silver-doped laser-induced graphene for potent surface antibacterial activity and anti-biofilm action. Chem. Commun. (Camb. Engl.) 2019. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-H.; Gong, J.-L.; Zeng, G.-M.; Zhang, P.; Song, B.; Zhang, X.-G.; Liu, H.-Y.; Huan, S.-Y. Graphene sponge decorated with copper nanoparticles as a novel bactericidal filter for inactivation of Escherichia coli. Chemosphere 2017, 184, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.H.; Qiu, J.J.; Su, J.S.; Liu, X.Y. Minocycline hydrochloride loaded on titanium by graphene oxide: An excellent antibacterial platform with the synergistic effect of contact-killing and release-killing. Biomater. Sci. 2018, 6, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jia, F.; Yang, B.; Song, S. Efficient adsorption of Au(CN) 2− from gold cyanidation with graphene oxide-polyethylenimine hydrogel as adsorbent. Results Phys. 2017, 7, 4089–4095. [Google Scholar] [CrossRef]
- Chen, B.A.; Liu, M.; Zhang, L.M.; Huang, J.; Yao, J.L.; Zhang, Z.J. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Zhou, X.; Laroche, F.; Lamers, G.E.M.; Torraca, V.; Voskamp, P.; Lu, T.; Chu, F.Q.; Spaink, H.P.; Abrahams, J.P.; Liu, Z.F. Ultra-small graphene oxide functionalized with polyethylenimine (PEI) for very efficient gene delivery in cell and zebrafish embryos. Nano Res. 2012, 5, 703–709. [Google Scholar] [CrossRef]
- Wang, N.; Hu, B.; Chen, M.L.; Wang, J.H. Polyethylenimine mediated silver nanoparticle-decorated magnetic graphene as a promising photothermal antibacterial agent. Nanotechnology 2015, 26, 195703. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Su, C.; Ye, S.; Wu, J.; Zhu, Z.; Wen, Y.; Zhang, R.; Shao, W. Synergistic antibacterial effect of tetracycline hydrochloride loaded functionalized graphene oxide nanostructures. Nanotechnology 2018, 29, 505102. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hong, J.; Liu, Y.; Wang, B.; Hua, Q.; Liu, L.; Ying, D. Urea Controlled-Release Fertilizer Based on Gelatin Microspheres. J. Polym. Environ. 2017, 26, 1930–1939. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.; Chen, L.; Huang, L.; Cao, S.; Wang, H. Preparation of microfibrillated cellulose/chitosan-benzalkonium chloride biocomposite for enhancing antibacterium and strength of sodium alginate films. J. Agric. Food Chem. 2013, 61, 6562–6567. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.-T.; Whiteley, C.G.; Lee, C.-K. Hydrophobically Modified Chitosan-Grafted Magnetic Nanoparticles for Bacteria Removal. Ind. Eng. Chem. Res. 2015, 54, 9270–9277. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Valenzuela-Medina, D.; Du, W.-X.; Gregorski, K.S.; Williams, T.G.; Wood, D.F.; Glenn, G.M.; Orts, W.J. Solution blow spun poly(lactic acid)/hydroxypropyl methylcellulose nanofibers with antimicrobial properties. Eur. Polym. J. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly Stable Graphene-Based Nanocomposite (GO-PEI-Ag) with Broad-Spectrum, Long-Term Antimicrobial Activity and Antibiofilm Effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Yang, L.; Zhan, Y.; Zou, L.; Ye, B. Nonenzymatic H2O2 Electrochemical Sensor Based on SnO2-NPs Coated Polyethylenimine Functionalized Graphene. Electroanalysis 2017, 29, 2044–2052. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef]
- Li, G.W.; Yu, S.M.; Xue, W.; Ma, D.; Zhang, W. Chitosan-graft-PAMAM loading nitric oxide for efficient antibacterial application. Chem. Eng. J. 2018, 347, 923–931. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Kwon, B.; Yoon, J.; Park, I.J.; Bang, G.S.; Park, Y.; Seo, Y.S.; Choi, S.Y. Antibacterial Activities of Graphene Oxide-Molybdenum Disulfide Nanocomposite Films. ACS Appl. Mater. Interfaces 2017, 9, 7908–7917. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhu, Z.; Wen, Y.; Ye, S.; Su, C.; Zhang, R.; Shao, W. Facile Construction of Functionalized GO Nanocomposites with Enhanced Antibacterial Activity. Nanomaterials 2019, 9, 913. https://doi.org/10.3390/nano9070913
Jiang L, Zhu Z, Wen Y, Ye S, Su C, Zhang R, Shao W. Facile Construction of Functionalized GO Nanocomposites with Enhanced Antibacterial Activity. Nanomaterials. 2019; 9(7):913. https://doi.org/10.3390/nano9070913
Chicago/Turabian StyleJiang, Lei, Zhongjie Zhu, Yanyi Wen, Shan Ye, Chen Su, Rui Zhang, and Wei Shao. 2019. "Facile Construction of Functionalized GO Nanocomposites with Enhanced Antibacterial Activity" Nanomaterials 9, no. 7: 913. https://doi.org/10.3390/nano9070913
APA StyleJiang, L., Zhu, Z., Wen, Y., Ye, S., Su, C., Zhang, R., & Shao, W. (2019). Facile Construction of Functionalized GO Nanocomposites with Enhanced Antibacterial Activity. Nanomaterials, 9(7), 913. https://doi.org/10.3390/nano9070913




