Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Methods
2.3.1. Production of Nanoparticles
2.3.2. Particle Size Measurement
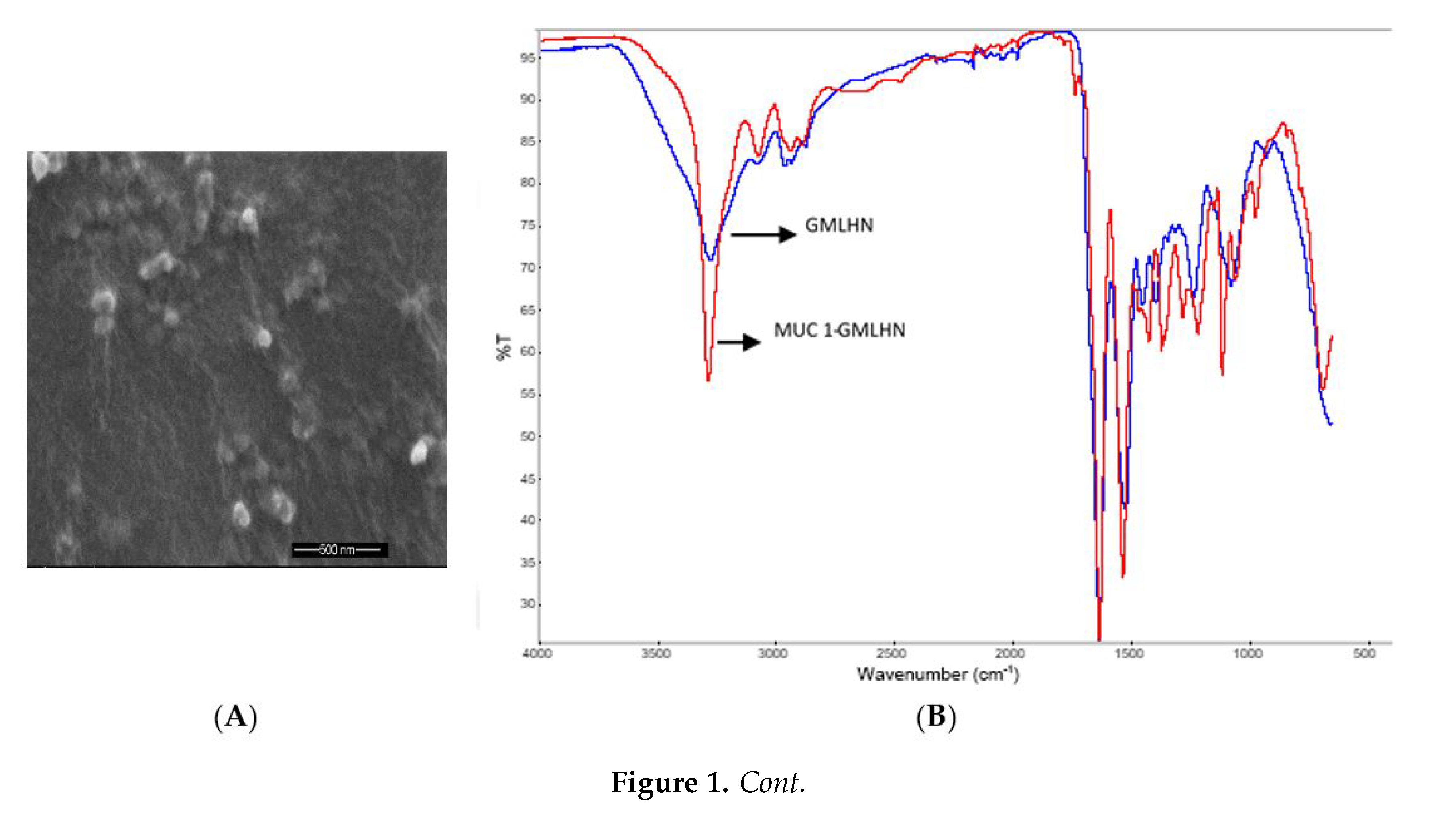
2.3.3. Fourier Transform-infrared Spectroscopy (FT-IR)
2.3.4. Scanning Electron Microscopy (SEM)
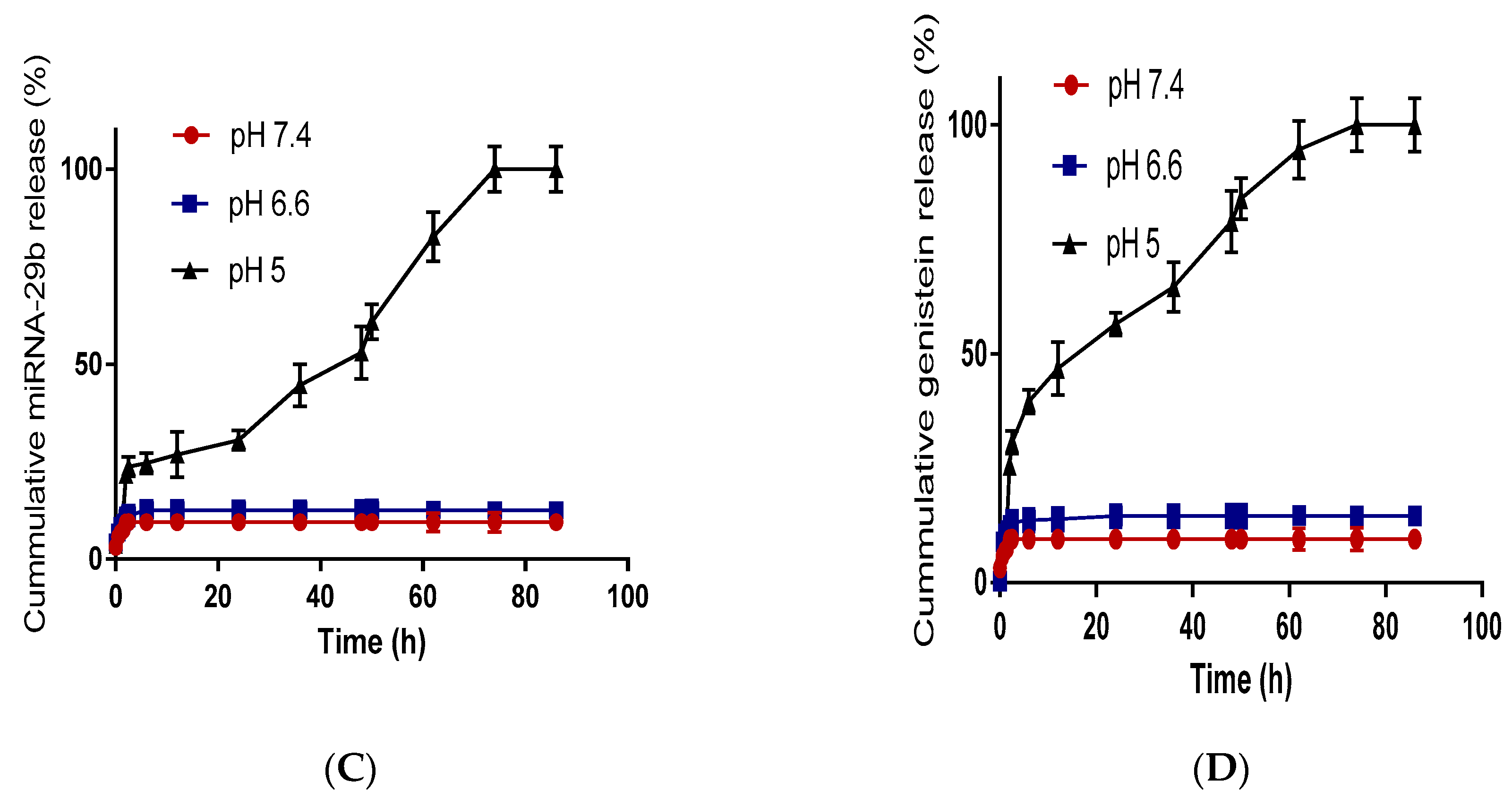
2.3.5. Drug Release Study
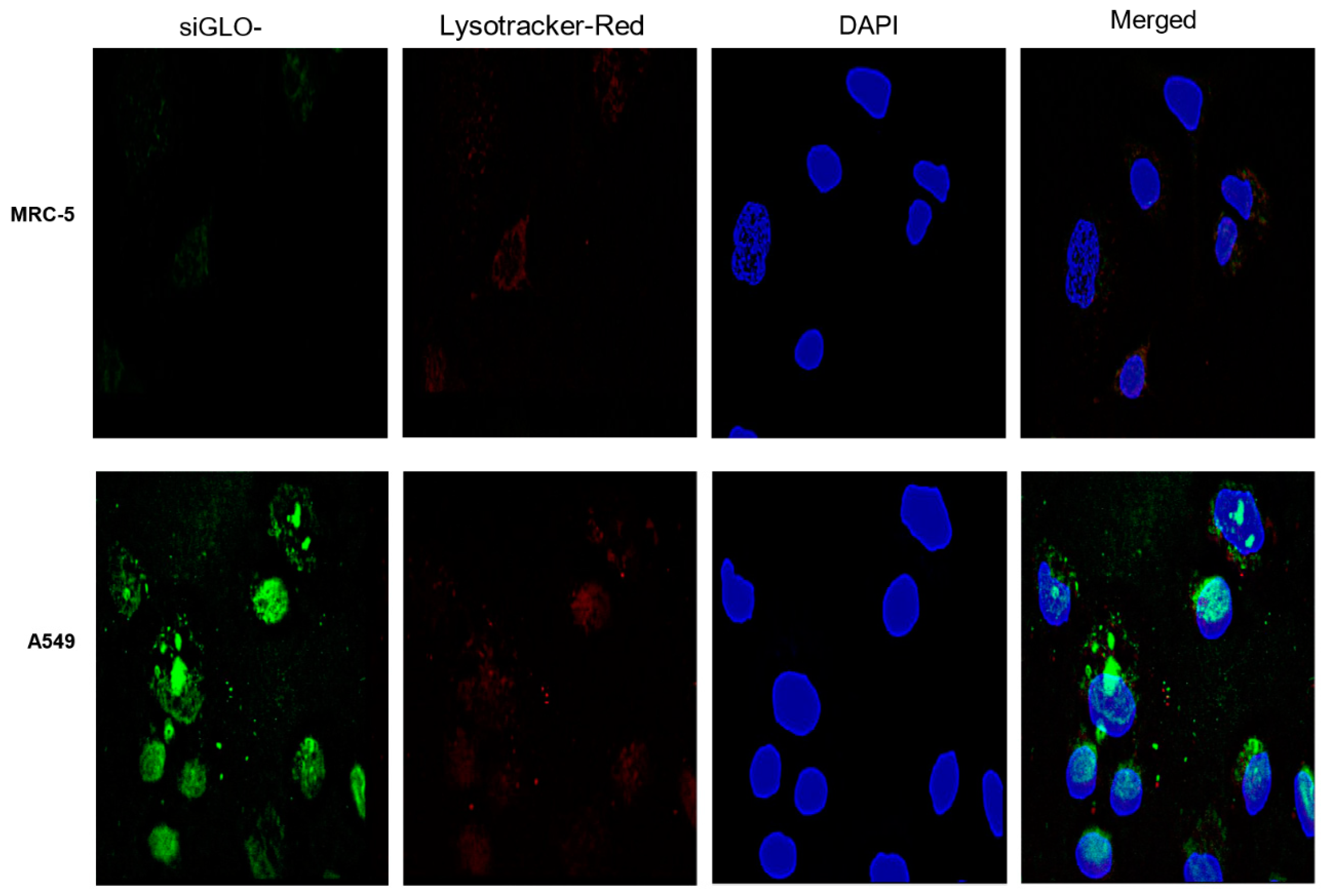
2.3.6. Fluorescence Microscopy
2.3.7. Cell Viability Study
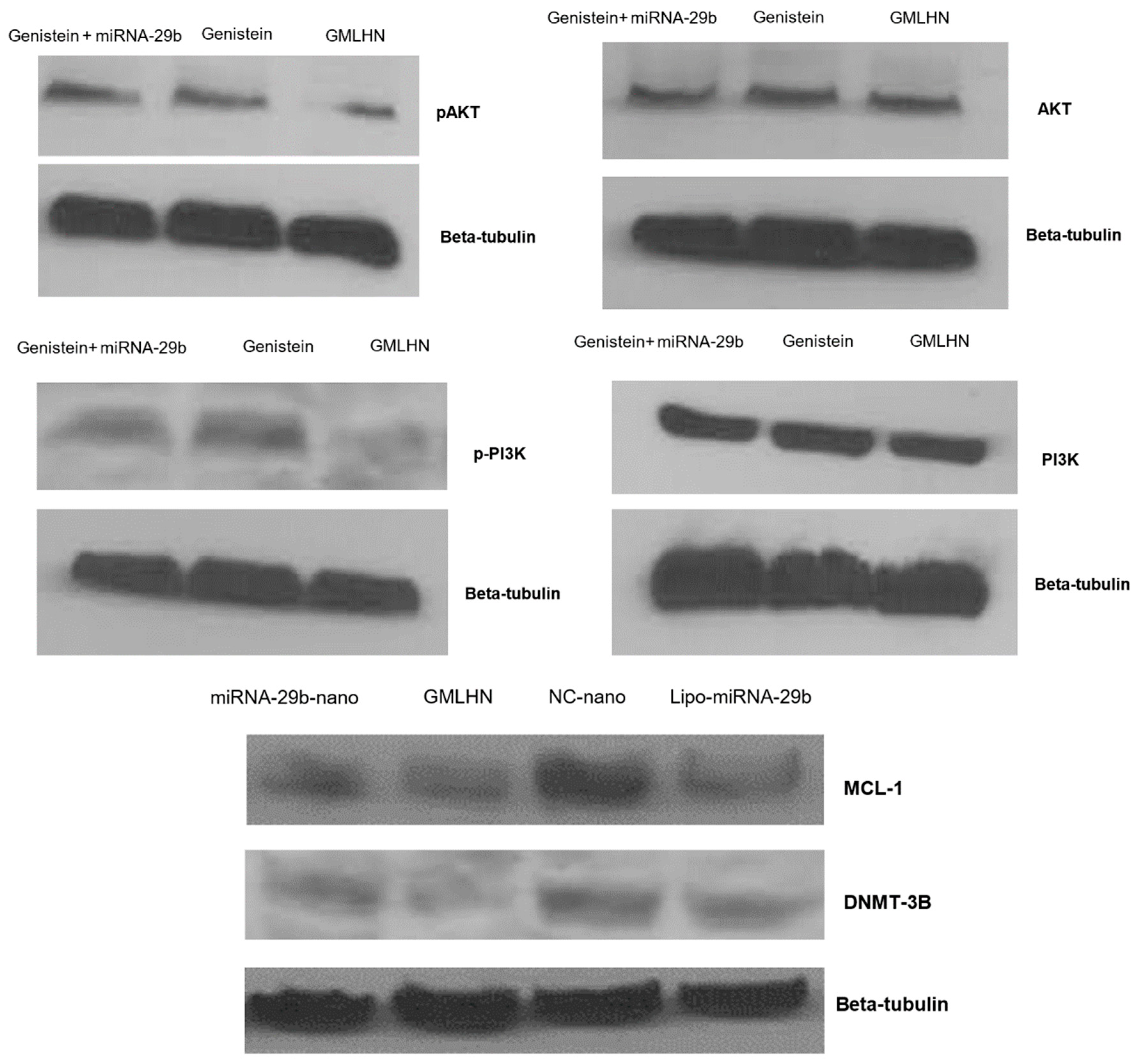
2.3.8. Western Blot Analysis
2.3.9. Cell-Death Detection ELISA
2.3.10. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
3.2. Cellular Uptake of MUC 1-Aptamer Functionalized GMLHN
3.3. Western Blot Analysis
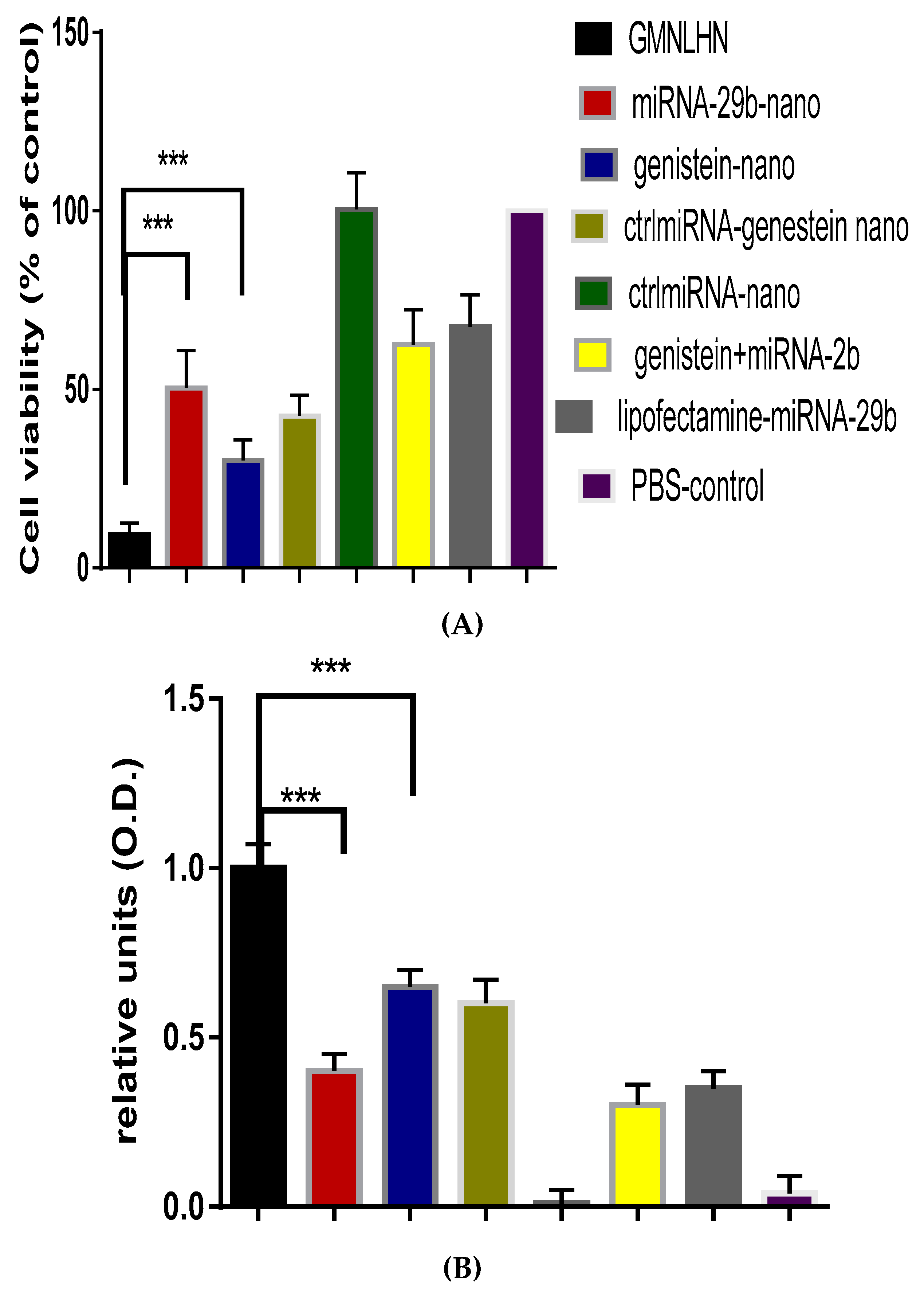
3.4. Antiproliferation Effect of GMLHN in A549 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Srinivasan, A.R.; Lakshmikuttyamma, A.; Shoyele, S.A. Investigation of the stability and cellular uptake of self-associated monoclonal antibody (MAb) nanoparticles by non-small lung cancer cells. Mol. Pharm. 2013, 10, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikuttyamma, A.; Sun, Y.; Lu, B.; Undieh, A.S.; Shoyele, S.A. Stable and Efficient Transfection of siRNA for Mutated KRAS Silencing using Novel Hybrid Nanoparticles. Mol. Pharm. 2014, 11, 4415–4424. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.R.; Shoyele, S.A. Self-associated submicron IgG1 particles for pulmonary delivery: Effects of non-ionic surfactants on size, shape, stability, and aerosol performance. AAPS PharmSciTech 2013, 14, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Athawale, R.; Bejay, A.; Shrikhande, S.; Goel, P.N.; Gude, R.P. Studies on stabilization mechanism and stealth effect of poloxamer 188 onto PLGA nanoparticles. Colloids Surf. B Biointerfaces 2013, 109, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liv, J.P.; Chen, Z.Q. Stealth tenshinone IIA-loaded solid lipid nanoparticles in rats. Acta Pharm. Sin. 2009, 44, 1422–1428. [Google Scholar]
- Wang, Y.; Zhang, X.; Li, H.; Yu, J.; Ren, X. The role of miRNA-29 family in cancer. Eur. J. Cell Biol. 2013, 92, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yan, L.; Wang, L.; Tai, W.; Wang, W.; Yang, C. Genistein enhances the effect of cisplatin on the inhibition of non-small cell lung cancer A549 cell growth in vitro and in vivo. Oncol. Lett. 2004, 8, 2806–2810. [Google Scholar] [CrossRef]
- Yang, Y.; Zang, A.; Jin, Y.; Shang, Y.; Zhang, Z.; Ge, K.; Zhang, J.; Fan, W.; Wang, B. Genistein inhibits A549 cells human lung cancer cells via miR-27a and MET signaling. Oncol. Lett. 2016, 12, 2189–2193. [Google Scholar] [CrossRef]
- Kharbanda, A.; Rajabi, H.; Jin, C.; Alam, M.; Wong, K.K.; Kufe, D. Muc 1 confers EMT and KRAS independence in mutant KRAS lung cancer cells. Oncotarget 2014, 5, 8893–8904. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Y.; Duan, J.; Yuan, W.; Wang, C.; Xu, H.; Yang, X.D. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer to MUC1-positive cancer cells in vitro. PLoS ONE 2011, 6, e24077. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lin, B.L.; Chen, C.H. An aptamer targeting shared tumor-specific peptide antigen of MAGE-A3 in multiple cancers. Int. J. Cancer 2016, 138, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.R.; Shoyele, S.A. Influence of Surface Modification and the pH on the Release Mechanisms and Kinetics of Erlotinib from Antibody-Functionalized Chitosan Nanoparticles. Ind. Eng. Chem. Res. 2014, 53, 2987–2993. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Margue, C.; Behrmann, I.; Kreis, S. miRNA-29: A microRNA family with tumor-suppressing and immune-modulating properties. Curr. Mod. Med. 2012, 13, 572–585. [Google Scholar] [CrossRef]
- Yan, B.; Guo, Q.; Fu, F.J.; Wang, Z.; Yin, Z.; Wei, Y.B.; Yang, J.R. The role of miR-29b in cancer: Regulation, function, and signaling. Onco. Target. Ther. 2015, 8, 539–548. [Google Scholar]
- Steele, R.; Mott, J.L.; Ray, R.B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens and matrix metalloproteinase-2 in prostate cancer cells. Genes. Cancer 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Mao, Y.; Robert, J.L.; Davis, I.C.; Elton, T.S.; Lee, L.J.; Nana-Sinkam, S.P. Therapeutic delivery of microRNA-29b by cationic lipoplexes for lung cancer. Mol. Ther. Nucleic Acids 2013, 2, e84. [Google Scholar] [CrossRef]
- Garzon, R.; Heaphy, C.E.; Havelange, V.; Fabbri, M.; Volinia, S.; Tsao, T.; Zanesi, N.; Kornblau, S.M.; Marcucci, G.; Calin, G.A.; et al. MicroRNA 29b function in acute myeloid leukemia. Blood 2009, 114, 5331–5341. [Google Scholar] [CrossRef]
- Gagnon, V.; Themsche, C.; Turner, S.; Leblanc, V.; Asselin, E. AKT and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin doxorubicin and taxol. Apopotosis 2008, 13, 259–271. [Google Scholar] [CrossRef]
- McDonald, G.T.; Sullivan, R.; Pave, G.C.; Graham, C.H. Inhibition of phosphatidylinositol 3-kinase promotes tumor cell resistance to chemotherapeutic agents via a mechanism involving delay in cell cycle progression. Exp. Cell Res. 2010, 316, 3197–3206. [Google Scholar] [CrossRef]
- Cicenas, J. The potential role of AKT phosphorylation in human cancers. Int. J. Biol. Markers 2008, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perepelyuk, M.; Maher, C.; Lakshmikuttyamma, A.; Shoyele, S.A. Aptamer-hybrid Nanoparticle Bioconjugate Efficiently Delivers miRNA-29b to Non-Small cell Lung Cancer Cells and Inhibits Growth by Downregulating Essential Oncoproteins. Int. J. Nanomed. 2016, 11, 3533–3544. [Google Scholar]
- Perepelyuk, M.; Sacko, K.; Thangavel, K.; Shoyele, S.A. Evaluation of MUC1-Aptamer Functionalized Hybrid Nanoparticles for Targeted Delivery of miRNA-29b to Nonsmall Cell Lung Cancer. Mol. Pharm. 2018, 15, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ming, X.; Nakagawa, O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug. Chem. 2013, 23, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2008, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Sultana, S.; Gupta, K.P. Involvement of epigenetics and microRNA-29b in the urethane induced inception and establishment of mouse lung tumors. Exp. Mol. Pthol. 2014, 96, 61–70. [Google Scholar] [CrossRef] [PubMed]
| Samples | Particle Size ± SD (nm) | PDI * ± SD | Zeta Potential ± SD (ζ, mV) |
|---|---|---|---|
| GMLHN | 240.8 ± 41.2 | 0.242 ± 0.021 | −2.2 ± 1.8 |
| MUC 1-aptamer functionalized GMLHN | 598 ± 34.1 | 0.414 ± 0.241 | 4.2 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacko, K.; Thangavel, K.; Shoyele, S.A. Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates. Nanomaterials 2019, 9, 1052. https://doi.org/10.3390/nano9071052
Sacko K, Thangavel K, Shoyele SA. Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates. Nanomaterials. 2019; 9(7):1052. https://doi.org/10.3390/nano9071052
Chicago/Turabian StyleSacko, Koita, Karthik Thangavel, and Sunday A. Shoyele. 2019. "Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates" Nanomaterials 9, no. 7: 1052. https://doi.org/10.3390/nano9071052
APA StyleSacko, K., Thangavel, K., & Shoyele, S. A. (2019). Codelivery of Genistein and miRNA-29b to A549 Cells Using Aptamer-Hybrid Nanoparticle Bioconjugates. Nanomaterials, 9(7), 1052. https://doi.org/10.3390/nano9071052




