Surface, Interface, and Temperature Effects on the Phase Separation and Nanoparticle Self Assembly of Bi-Metallic Ni0.5Ag0.5: A Molecular Dynamics Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
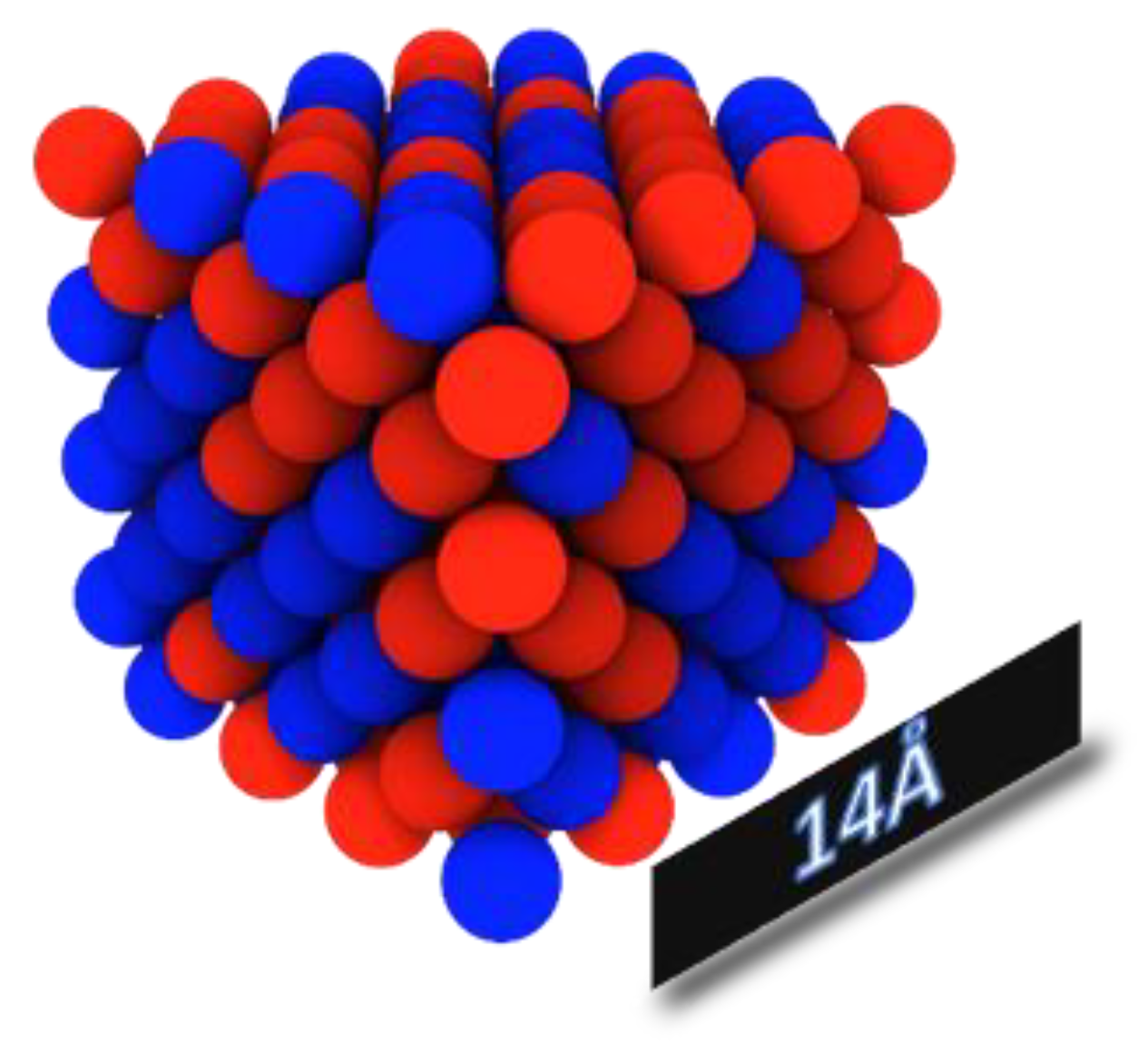
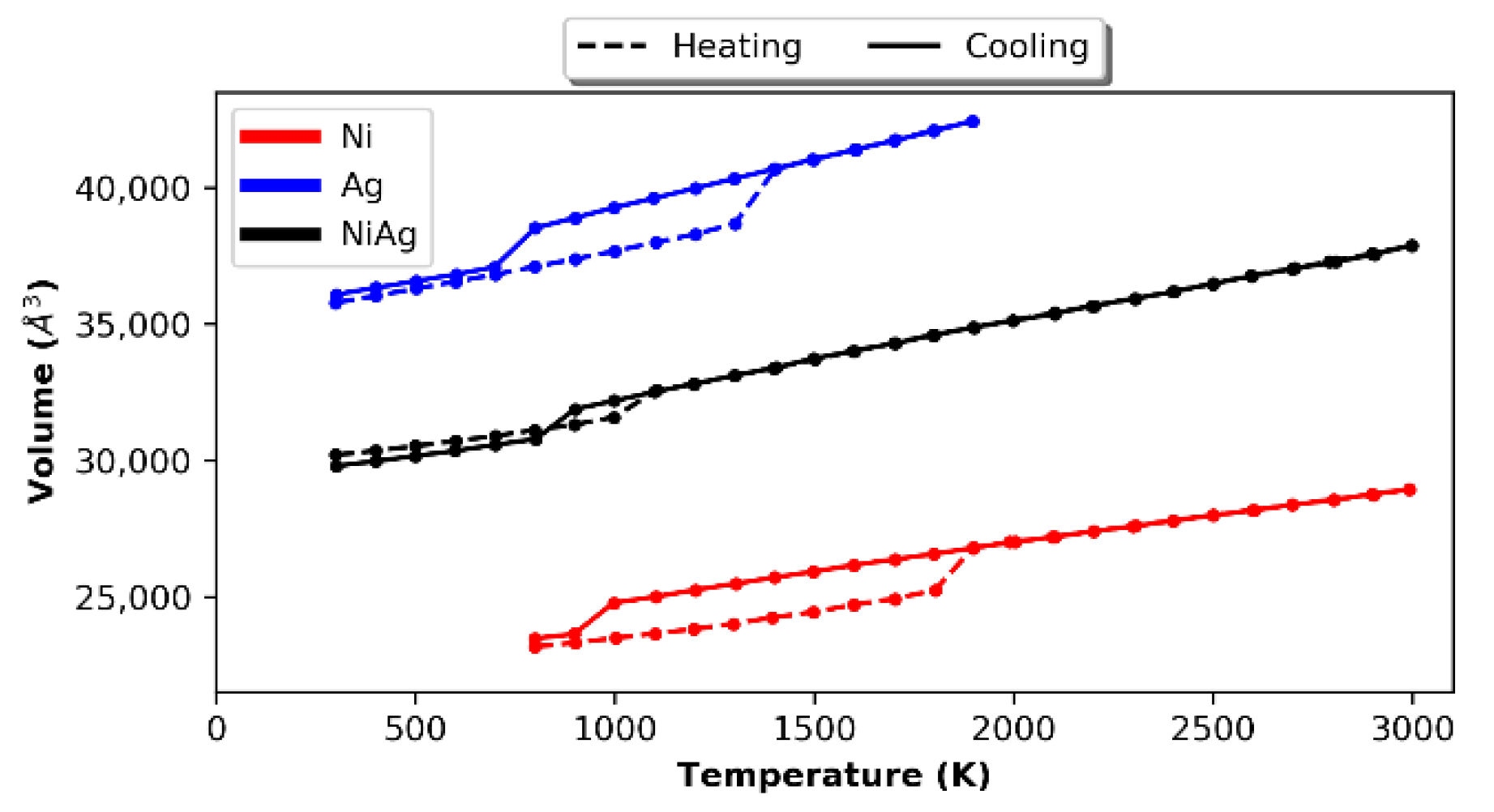
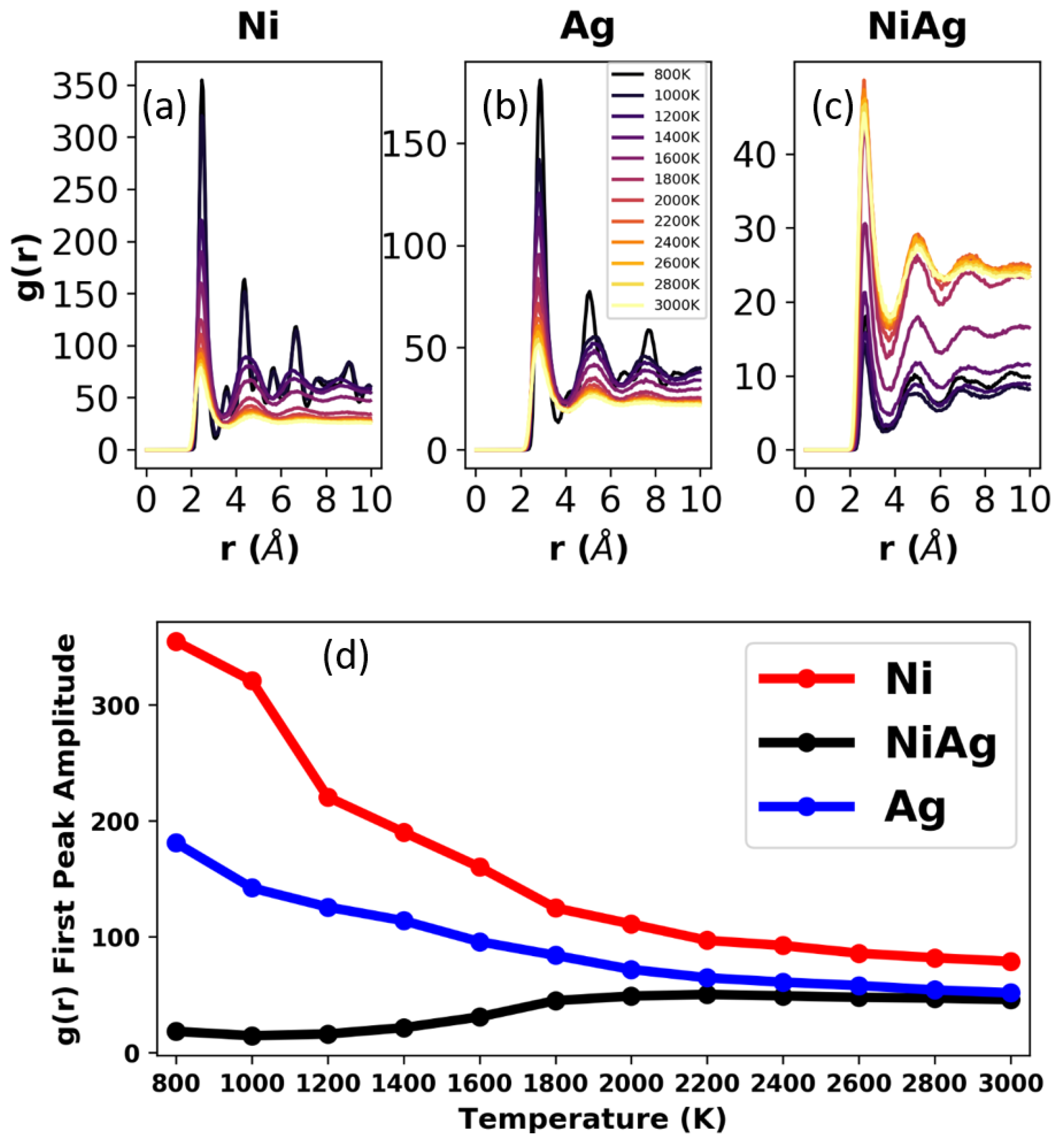
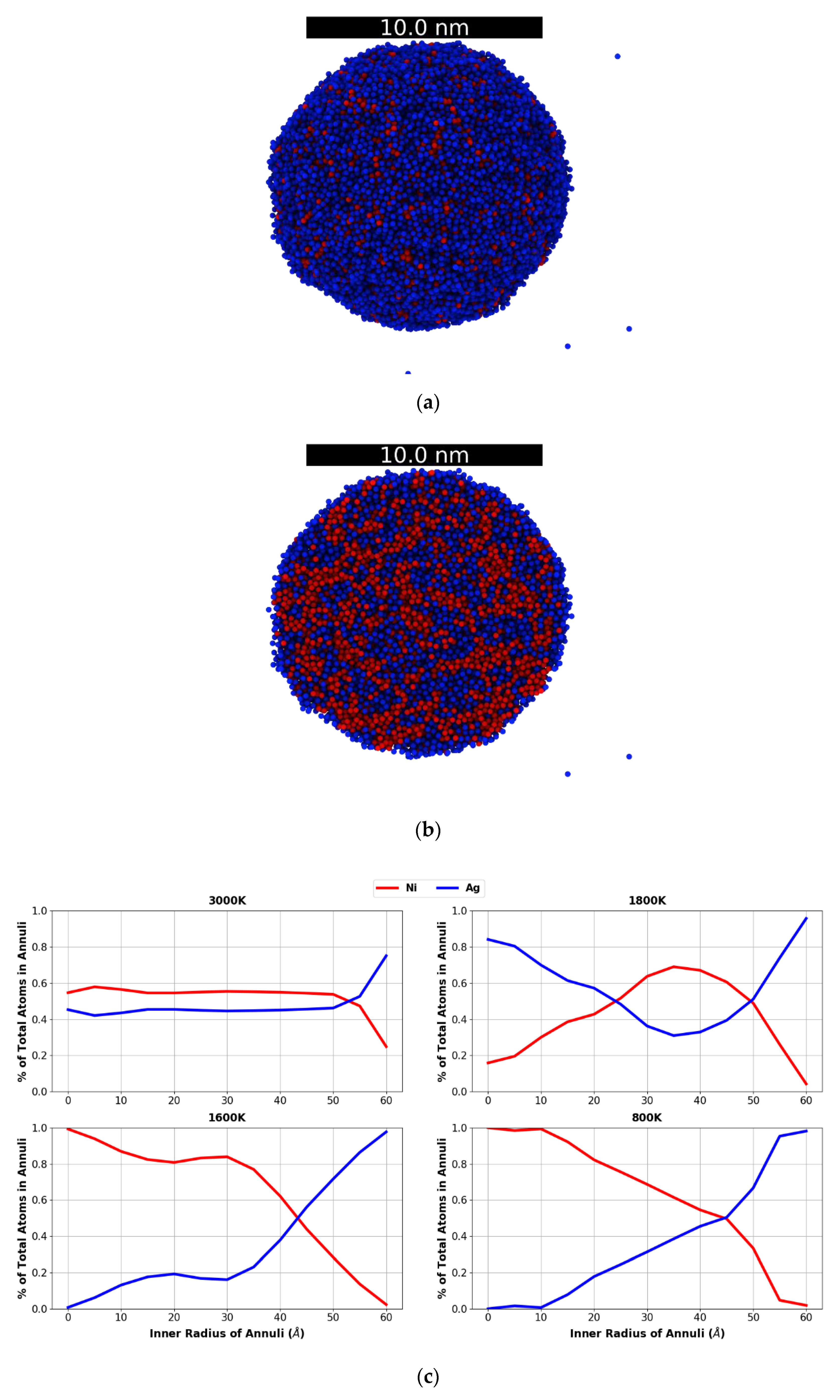
3.1. Bulk Samples
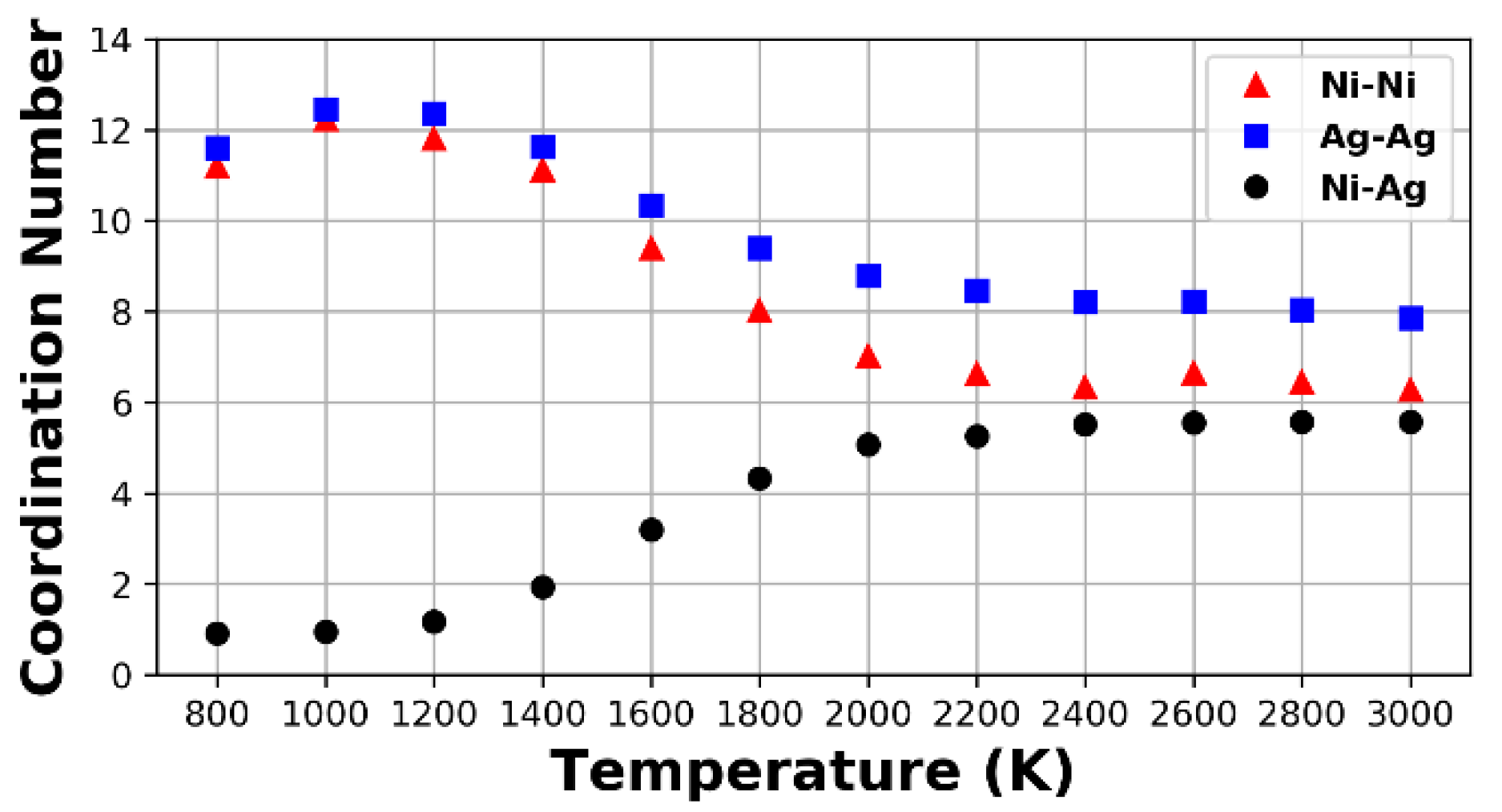
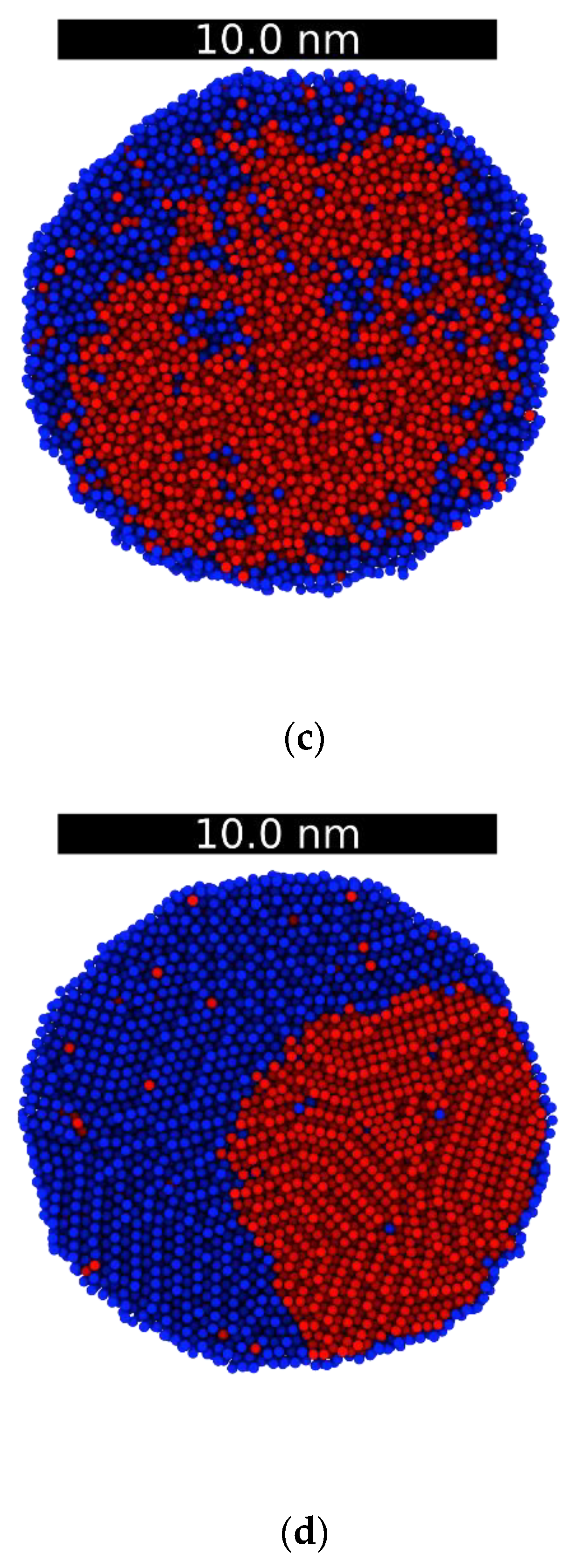
3.2. Droplets
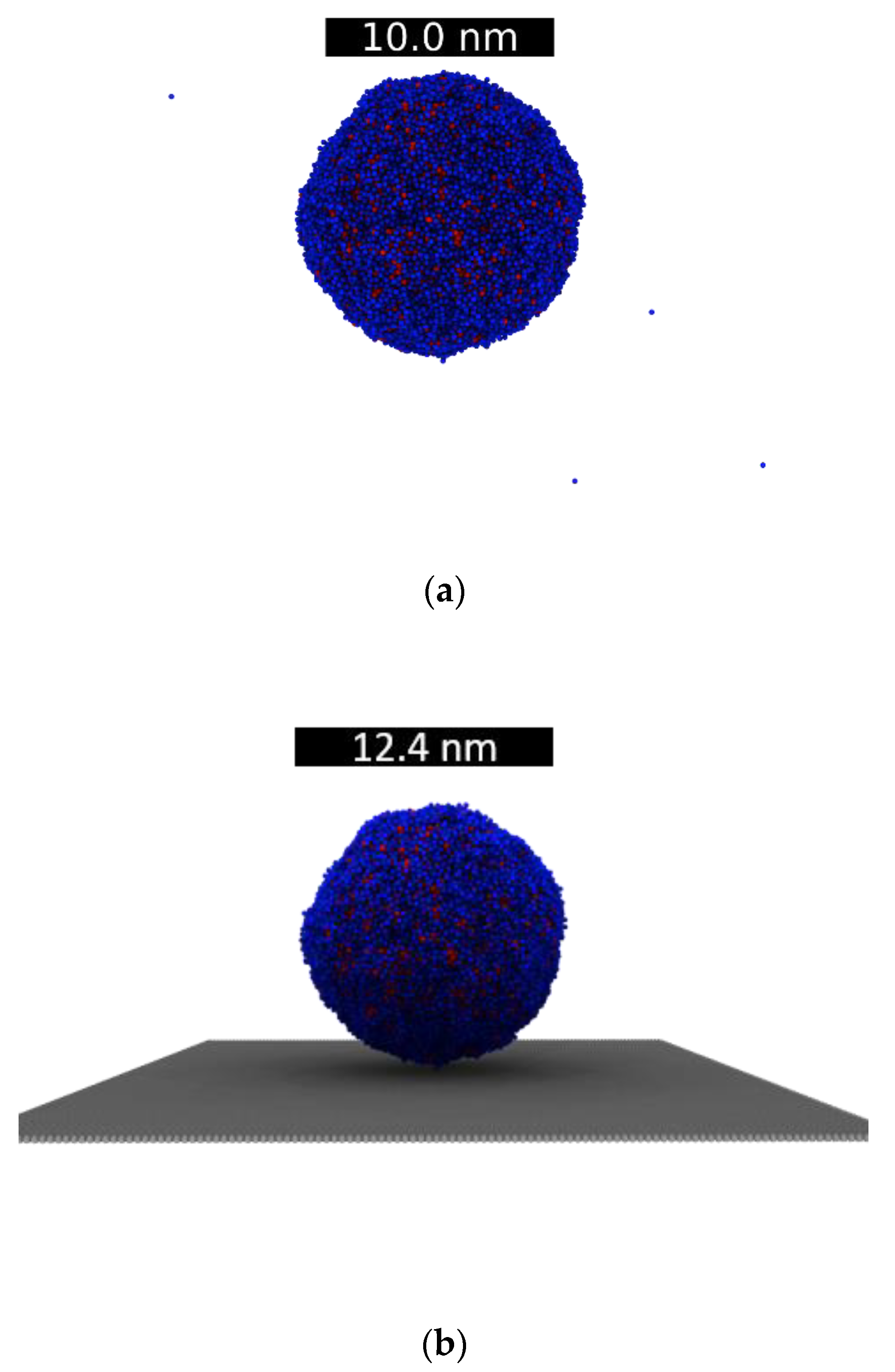
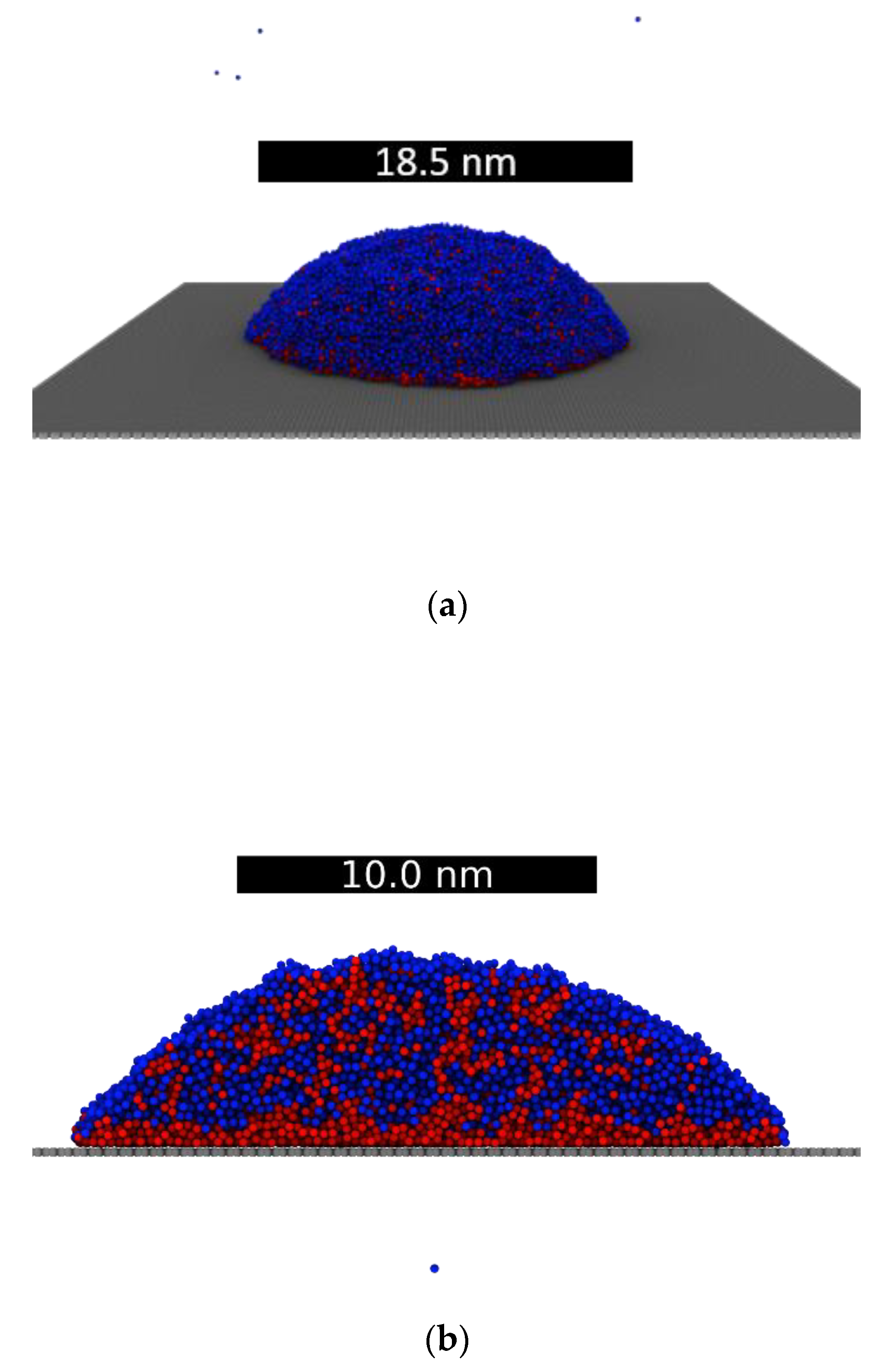
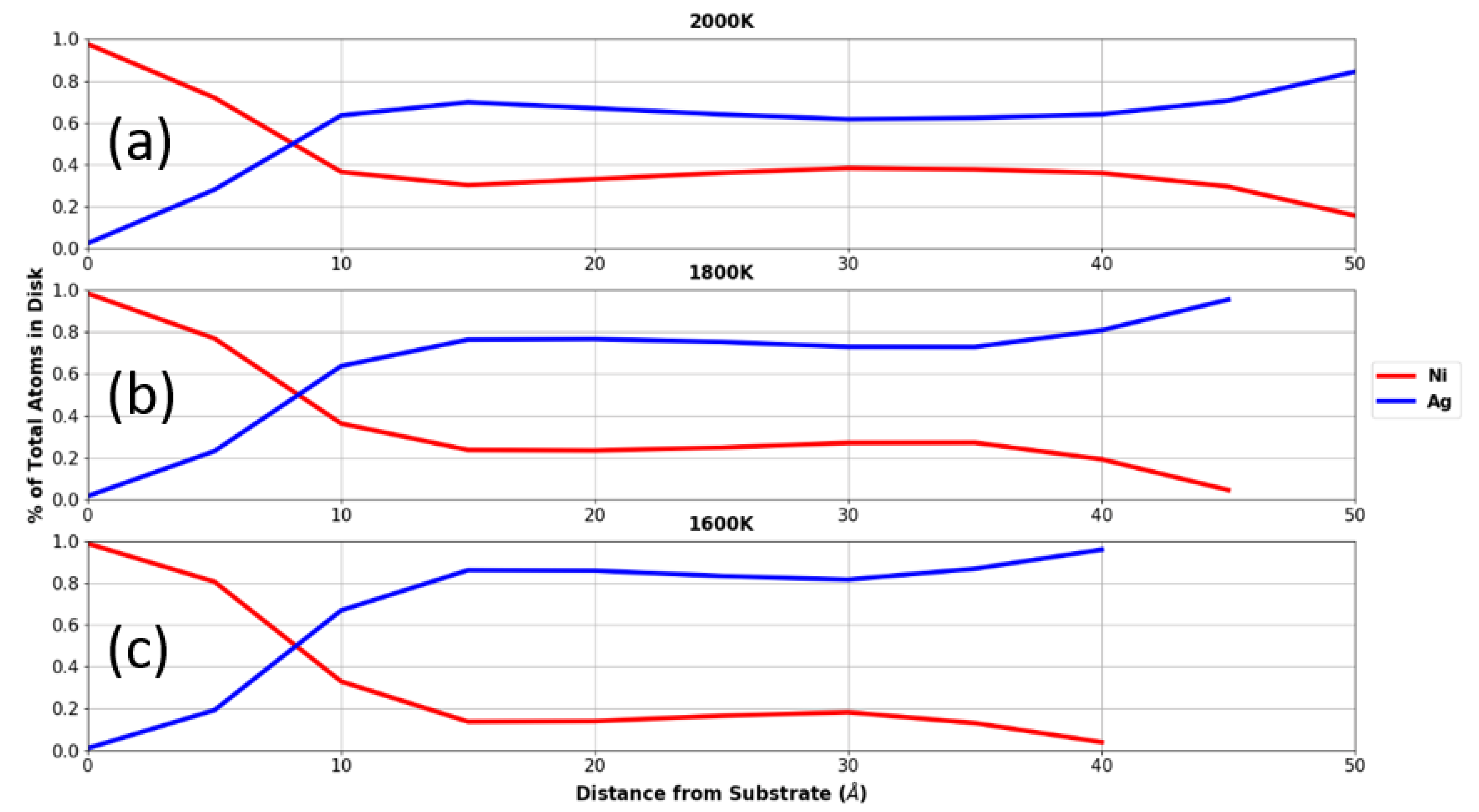
3.3. Droplets on Graphite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Favazza, C.; Kalyanaraman, R.; Sureshkumar, R. Robust nanopatterning by laser-induced dewetting of metal nanofilms. Nanotechnology 2006, 17, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Herminghaus, S.; Jacobs, K.; Mecke, K.; Bischof, J.; Fery, A.; Ibn-Elhaj, M.; Schlagowski, S. Spinodal dewetting in liquid crystal and liquid metal films. Science (New York, N.Y.) 1998, 282, 916–919. [Google Scholar] [CrossRef] [PubMed]
- McKeown, J.T.; Roberts, N.A.; Fowlkes, J.D.; Wu, Y.; LaGrange, T.; Reed, B.W.; Campbell, G.H.; Rack, P.D. Real-Time Observation of Nanosecond Liquid-Phase Assembly of Nickel Nanoparticles via Pulsed-Laser Heating. Langmuir 2012, 28, 17168–17175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruffino, F.; Pugliara, A.; Carria, E.; Bongiorno, C.; Spinella, C.; Grimaldi, M.G. Formation of nanoparticles from laser irradiated Au thin film on SiO2/Si: Elucidating the Rayleigh-instability role. Mater. Lett. 2012, 84, 27–30. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Kondic, L.; Diez, J.; Wu, Y.; Rack, P.D. Self-Assembly versus Directed Assembly of Nanoparticles via Pulsed Laser Induced Dewetting of Patterned Metal Films. Nano Lett. 2011, 11, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.D.; Roberts, N.A.; Wu, Y.; Diez, J.A.; González, A.G.; Hartnett, C.; Mahady, K.; Afkhami, S.; Kondic, L.; Rack, P.D. Hierarchical Nanoparticle Ensembles Synthesized by Liquid Phase Directed Self-Assembly. Nano Lett. 2014, 14, 774–782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, Y.F.; Pearce, R.C.; Melechko, A.V.; Hensley, D.K.; Simpson, M.L.; Rack, P.D. Pulsed laser dewetting of nickel catalyst for carbon nanofiber growth. Nanotechnology 2008, 19, 235604. [Google Scholar] [CrossRef]
- Hartnett, C.A.; Mahady, K.; Fowlkes, J.D.; Afkhami, S.; Kondic, L.; Rack, P.D. Instability of Nano- and Microscale Liquid Metal Filaments: Transition from Single Droplet Collapse to Multidroplet Breakup. Langmuir 2015, 31, 13609–13617. [Google Scholar] [CrossRef]
- Hartnett, C.A.; Seric, I.; Mahady, K.; Kondic, L.; Afkhami, S.; Fowlkes, J.D.; Rack, P.D. Exploiting the Marangoni Effect To Initiate Instabilities and Direct the Assembly of Liquid Metal Filaments. Langmuir 2017, 33, 8123–8128. [Google Scholar] [CrossRef]
- Kondic, L.; Diez, J.A.; Rack, P.D.; Guan, Y.; Fowlkes, J.D. Nanoparticle assembly via the dewetting of patterned thin metal lines: Understanding the instability mechanisms. Phys. Rev. E 2009, 79, 026302. [Google Scholar] [CrossRef]
- Rack, P.D.; Guan, Y.; Fowlkes, J.D.; Melechko, A.V.; Simpson, M.L. Pulsed laser dewetting of patterned thin metal films: A means of directed assembly. Appl. Phys. Lett. 2008, 92, 223108. [Google Scholar] [CrossRef]
- Wu, Y.; Fowlkes, J.D.; Rack, P.D.; Diez, J.A.; Kondic, L. On the Breakup of Patterned Nanoscale Copper Rings into Droplets via Pulsed-Laser-Induced Dewetting: Competing Liquid-Phase Instability and Transport Mechanisms. Langmuir 2010, 26, 11972–11979. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fowlkes, J.D.; Roberts, N.A.; Diez, J.A.; Kondic, L.; González, A.G.; Rack, P.D. Competing Liquid Phase Instabilities during Pulsed Laser Induced Self-Assembly of Copper Rings into Ordered Nanoparticle Arrays on SiO2. Langmuir 2011, 27, 13314–13323. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, G.; Cherqui, C.; Bigelow, N.W.; Thakkar, N.; Masiello, D.J.; Camden, J.P.; Rack, P.D. Electron Energy Loss Spectroscopy Study of the Full Plasmonic Spectrum of Self-Assembled Au–Ag Alloy Nanoparticles: Unraveling Size, Composition, and Substrate Effects. ACS Photonics 2016, 3, 130–138. [Google Scholar] [CrossRef]
- McKeown, J.T.; Wu, Y.; Fowlkes, J.D.; Rack, P.D.; Campbell, G.H. Simultaneous In-Situ Synthesis and Characterization of Co@Cu Core-Shell Nanoparticle Arrays. Adv. Mater. 2015, 27, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Sachan, R.; Yadavali, S.; Shirato, N.; Krishna, H.; Ramos, V.; Duscher, G.; Pennycook, S.J.; Gangopadhyay, A.K.; Garcia, H.; Kalyanaraman, R. Self-organized bimetallic Ag–Co nanoparticles with tunable localized surface plasmons showing high environmental stability and sensitivity. Nanotechnology 2012, 23, 275604. [Google Scholar] [CrossRef] [PubMed]
- Atena, A.; Khenner, M. Thermocapillary effects in driven dewetting and self assembly of pulsed-laser-irradiated metallic films. Phys. Rev. B 2009, 80, 075402. [Google Scholar] [CrossRef]
- González, A.G.; Diez, J.A.; Wu, Y.; Fowlkes, J.D.; Rack, P.D.; Kondic, L. Instability of Liquid Cu Films on a SiO2 Substrate. Langmuir 2013, 29, 9378–9387. [Google Scholar] [CrossRef]
- Trice, J.; Thomas, D.; Favazza, C.; Sureshkumar, R.; Kalyanaraman, R. Pulsed-laser-induced dewetting in nanoscopic metal films: Theory and experiments. Phys. Rev. B 2007, 75, 235439. [Google Scholar] [CrossRef]
- Fowlkes, J.; Horton, S.; Fuentes-Cabrera, M.; Rack, P.D. Signatures of the Rayleigh-Plateau Instability Revealed by Imposing Synthetic Perturbations on Nanometer-Sized Liquid Metals on Substrates. Angew. Chem. Int. Ed. 2012, 51, 8768–8772. [Google Scholar] [CrossRef]
- Fuentes-Cabrera, M.; Rhodes, B.H.; Fowlkes, J.D.; López-Benzanilla, A.; Terrones, H.; Simpson, M.L.; Rack, P.D. Molecular dynamics study of the dewetting of copper on graphite and graphene: Implications for nanoscale self-assembly. Phys. Rev. E 2011, 83, 041603. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Fuentes-Cabrera, M.; Fowlkes, J.D.; Diez, J.A.; González, A.G.; Kondic, L.; Rack, P.D. Competition between Collapse and Breakup in Nanometer-Sized Thin Rings Using Molecular Dynamics and Continuum Modeling. Langmuir 2012, 28, 13960–13967. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Fuentes-Cabrera, M.; Fowlkes, J.D.; Rack, P.D. Coexistence of spinodal instability and thermal nucleation in thin-film rupture: Insights from molecular levels. Phys. Rev. E 2014, 89, 032403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Johnson, R.A.; Wadley, H.N.G. Misfit-energy-increasing dislocations in vapor-deposited CoFe/NiFe multilayers. Phys. Rev. B 2004, 69, 144113. [Google Scholar] [CrossRef]
- Pan, Z.; Borovikov, V.; Mendelev, M.I.; Sansoz, F. Development of a semi-empirical potential for simulation of Ni solute segregation into grain boundaries in Ag. Model. Simul. Mater. Sci. Eng. 2018, 26, 075004. [Google Scholar] [CrossRef]
- Luo, S.-N.; Ahrens, T.J.; Çağın, T.; Strachan, A.; Goddard, W.A.; Swift, D.C. Maximum superheating and undercooling: Systematics, molecular dynamics simulations, and dynamic experiments. Phys. Rev. B 2003, 68, 134206. [Google Scholar] [CrossRef]
- Kumar, S. Spreading and orientation of silver nano-drop over a flat graphene substrate: An atomistic investigation. Carbon 2018, 138, 26–41. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Yaghoubi, H. Molecular dynamics simulations of silver nanocluster supported on carbon nanotube. J. Colloid Interface Sci. 2014, 418, 178–184. [Google Scholar] [CrossRef]
- Graves, D.B.; Brault, P. Molecular dynamics for low temperature plasma-surface interaction studies. J. Phys. D Appl. Phys. 2009, 42, 194011. [Google Scholar] [CrossRef]
- Schebarchov, D.; Hendy, S.C. Effects of epitaxial strain on the melting of supported nickel nanoparticles. Phys. Rev. B 2011, 84, 085407. [Google Scholar] [CrossRef]
- Tavazza, F.; Senftle, T.P.; Zou, C.; Becker, C.A.; van Duin, A.T. Molecular Dynamics Investigation of the Effects of Tip-Substrate Interactions during Nanoindentation. J. Phys. Chem. 2015, 119, 13580–13589. [Google Scholar] [CrossRef]
- Bozack, M.J.; Bell, A.E.; Swanson, L.W. Influence of surface segregation on wetting of liquid metal alloys. J. Phys. Chem. 1988, 92, 3925–3934. [Google Scholar] [CrossRef]
- Hlinka, J.; Weltsch, Z. Relation between the Wetting Property and Electrical Conduction of Silver-Gold (Ag-Au) Alloys. Period. Polytech. Transp. Eng. 2013, 41, 95–98. [Google Scholar] [CrossRef]
- Lee, J.; Seo, K.; Hirai, N.; Takahira, N.; Tanaka, T. Intrinsic contact angle and contact interaction between liquid silver and solid graphite. Met. Mater. Int. 2007, 13, 83–86. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Perevertailo, V.M.; Nevodnik, G.M. Wetting of graphite by nickel as affected by the liquid-phase dissolution process of carbon. Sov. Powder Metall. Met. Ceram. 1971, 10, 45–47. [Google Scholar] [CrossRef]
- Ricci, E.; Novakovic, R. Wetting and surface tension measurements on gold alloys. Gold Bull. 2001, 34, 41–49. [Google Scholar] [CrossRef]
- Weltsch, Z.; Lovas, A.; Takács, J.; Cziráki, Á.; Toth, A.; Kaptay, G. Measurement and modelling of the wettability of graphite by a silver-tin (Ag-Sn) liquid alloy. Appl. Surf. Sci. 2013, 268, 52–60. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]



| Element | ||
|---|---|---|
| Ni | 2.047 | 2.072 |
| Ag | 2.855 | 3.549 |
| NiAg | 1.962 | 2.815 |
| Interaction | |||
|---|---|---|---|
| Ni-C | 0.072 | 2.8 | 11.0 |
| Ag-C | 0.01 | 3.006 | 11.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaire, R.H.; Dhakane, A.; Emery, R.; Ganesh, P.; Rack, P.D.; Kondic, L.; Cummings, L.; Fuentes-Cabrera, M. Surface, Interface, and Temperature Effects on the Phase Separation and Nanoparticle Self Assembly of Bi-Metallic Ni0.5Ag0.5: A Molecular Dynamics Study. Nanomaterials 2019, 9, 1040. https://doi.org/10.3390/nano9071040
Allaire RH, Dhakane A, Emery R, Ganesh P, Rack PD, Kondic L, Cummings L, Fuentes-Cabrera M. Surface, Interface, and Temperature Effects on the Phase Separation and Nanoparticle Self Assembly of Bi-Metallic Ni0.5Ag0.5: A Molecular Dynamics Study. Nanomaterials. 2019; 9(7):1040. https://doi.org/10.3390/nano9071040
Chicago/Turabian StyleAllaire, Ryan H., Abhijeet Dhakane, Reece Emery, P. Ganesh, Philip D. Rack, Lou Kondic, Linda Cummings, and Miguel Fuentes-Cabrera. 2019. "Surface, Interface, and Temperature Effects on the Phase Separation and Nanoparticle Self Assembly of Bi-Metallic Ni0.5Ag0.5: A Molecular Dynamics Study" Nanomaterials 9, no. 7: 1040. https://doi.org/10.3390/nano9071040
APA StyleAllaire, R. H., Dhakane, A., Emery, R., Ganesh, P., Rack, P. D., Kondic, L., Cummings, L., & Fuentes-Cabrera, M. (2019). Surface, Interface, and Temperature Effects on the Phase Separation and Nanoparticle Self Assembly of Bi-Metallic Ni0.5Ag0.5: A Molecular Dynamics Study. Nanomaterials, 9(7), 1040. https://doi.org/10.3390/nano9071040







