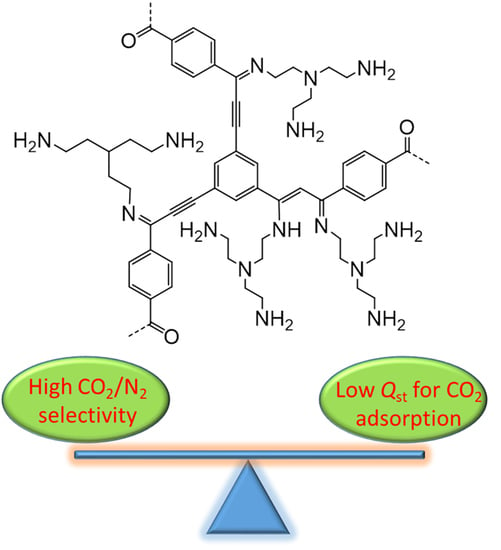
Synthesis of Porous Organic Polymers with Tunable Amine Loadings for CO2 Capture: Balanced Physisorption and Chemisorption
Abstract
1. Introduction
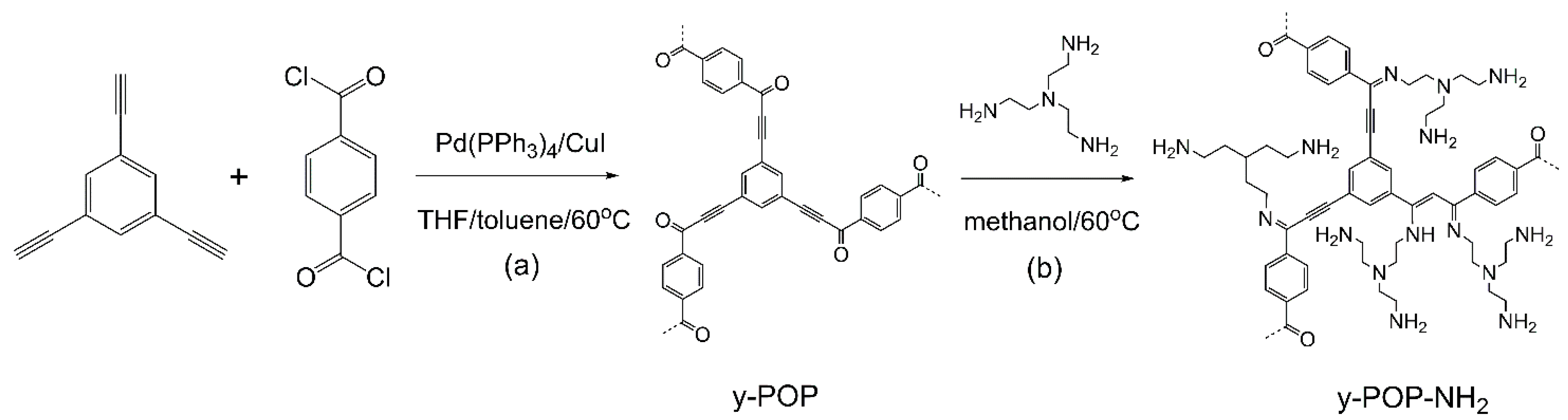
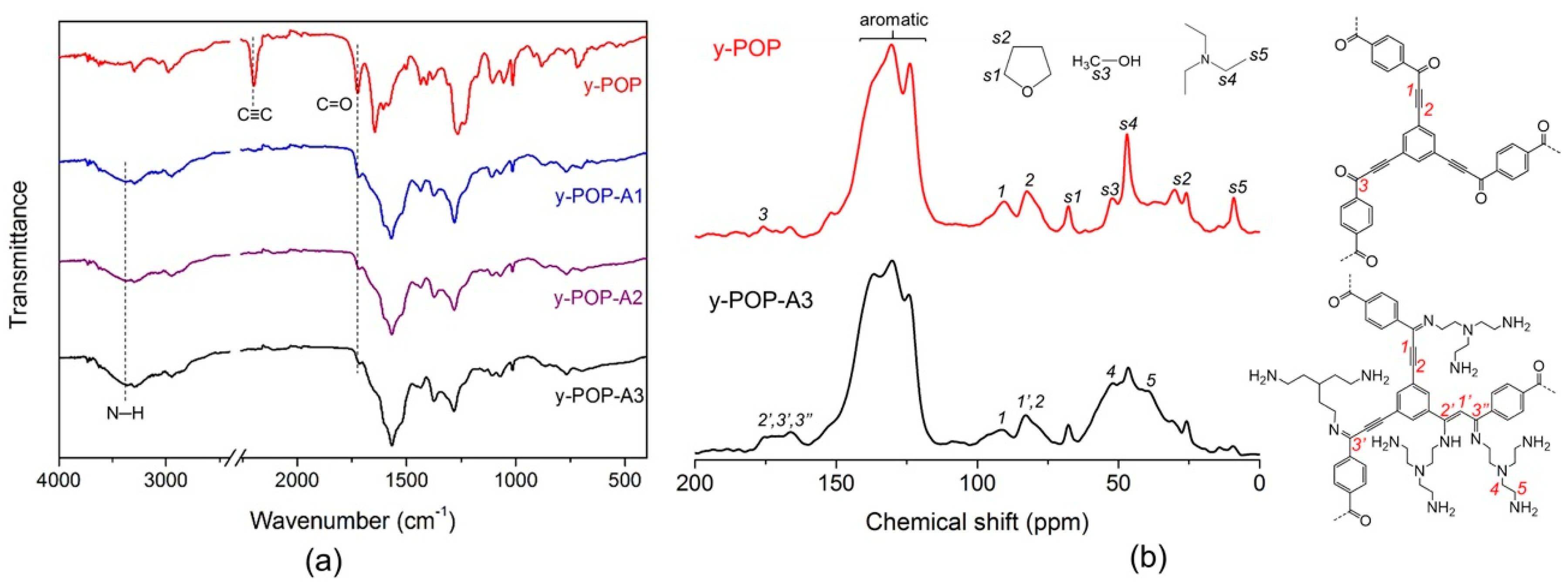
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014.
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Agarwal, A.; Biegler, L.T.; Zitney, S.E. A superstructure-based optimal synthesis of PSA cycles for post-combustion CO2 capture. AlChE J. 2010, 56, 1813–1828. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- CCS in Norway Entering a New Phase. Available online: https://www.gassnova.no/en/ccs-in-norway-entering-a-new-phase (accessed on 29 June 2018).
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Chue, K.T.; Kim, J.N.; Yoo, Y.J.; Cho, S.H.; Yang, R.T. Comparison of Activated Carbon and Zeolite 13X for CO2 Recovery from Flue Gas by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 1995, 34, 591–598. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, K.; Singh, R.; Xiao, G.; Gu, Q.; Zhao, Q.; Li, G.; Xiao, P.; Webley, P.A. Li+/ZSM-25 Zeolite as a CO2 Capture Adsorbent with High Selectivity and Improved Adsorption Kinetics, Showing CO2-Induced Framework Expansion. J. Phys. Chem. C 2018, 122, 18933–18941. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, J.; Xiao, P.; He, Y.; Zhao, Q.; Chu, Z.; Liu, Y.; Li, Z.; Webley, P.A. Simultaneous biogas purification and CO2 capture by vacuum swing adsorption using zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture. Carbon 2015, 93, 68–80. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R.; Al-Jumialy, A.S.M.; Fuertes, A.B. Optimization of the Pore Structure of Biomass-Based Carbons in Relation to Their Use for CO2 Capture under Low- and High-Pressure Regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, C.-Q.; Li, Y.; Lindh, J.; Strømme, M. High-Performance Activated Carbons Synthesized from Nanocellulose for CO2 Capture and Extremely Selective Removal of Volatile Organic Compounds. Adv. Sustain. Syst. 2018, 2, 1700147. [Google Scholar] [CrossRef]
- Xu, C.; Strømme, M. Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture. Nanomaterials 2019, 9, 103. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, M.; Hammershøj, P.; Zhou, D.; Han, Y.; Laursen, B.W.; Yan, C.-G.; Han, B.-H. Microporous Polycarbazole with High Specific Surface Area for Gas Storage and Separation. J. Am. Chem. Soc. 2012, 134, 6084–6087. [Google Scholar] [CrossRef]
- Dawson, R.; Stöckel, E.; Holst, J.R.; Adams, D.J.; Cooper, A.I. Microporous organic polymers for carbon dioxide capture. Energy Environ. Sci. 2011, 4, 4239–4245. [Google Scholar] [CrossRef]
- Shan, M.; Liu, X.; Wang, X.; Yarulina, I.; Seoane, B.; Kapteijn, F.; Gascon, J. Facile manufacture of porous organic framework membranes for precombustion CO2 capture. Sci. Adv. 2018, 4, eaau1698. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Wang, Z. Highly Selective Separation of CO2, CH4, and C2–C4 Hydrocarbons in Ultramicroporous Semicycloaliphatic Polyimides. ACS Appl. Mater. Interfaces 2018, 10, 26618–26627. [Google Scholar] [CrossRef]
- Gao, H.; Ding, L.; Bai, H.; Li, L. Microporous Organic Polymers Based on Hyper-Crosslinked Coal Tar: Preparation and Application for Gas Adsorption. ChemSusChem 2017, 10, 618–623. [Google Scholar] [CrossRef]
- Tan, M.X.; Zhang, Y.; Ying, J. Mesoporous Poly(Melamine-Formaldehyde) Solid Sorbent for Carbon Dioxide Capture. ChemSusChem 2013, 6, 1186–1190. [Google Scholar] [CrossRef]
- Xu, C.; Hedin, N. Microporous adsorbents for CO2 capture – a case for microporous polymers? Mater. Today 2014, 17, 397–403. [Google Scholar] [CrossRef]
- Chaoui, N.; Trunk, M.; Dawson, R.; Schmidt, J.; Thomas, A. Trends and challenges for microporous polymers. Chem. Soc. Rev. 2017, 46, 3302–3321. [Google Scholar] [CrossRef]
- Rabbani, M.G.; El-Kaderi, H.M. Template-Free Synthesis of a Highly Porous Benzimidazole-Linked Polymer for CO2Capture and H2Storage. Chem. Mater. 2011, 23, 1650–1653. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.-C. Sulfonate-Grafted Porous Polymer Networks for Preferential CO2 Adsorption at Low Pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef]
- Xie, L.-H.; Suh, M.P.; Xie, L. High CO2-Capture Ability of a Porous Organic Polymer Bifunctionalized with Carboxy and Triazole Groups. Chem. A Eur. J. 2013, 19, 11590–11597. [Google Scholar] [CrossRef]
- Mohanty, P.; Kull, L.D.; Landskron, K. Porous covalent electron-rich organonitridic frameworks as highly selective sorbents for methane and carbon dioxide. Nat. Commun. 2011, 2, 401. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- Haikal, R.R.; Hassan, Y.S.; Emwas, A.-H.; Belmabkhout, Y.; Alkordi, M.H. Poly-functional porous-organic polymers to access functionality—CO2 sorption energetic relationships. J. Mater. Chem. A 2015, 3, 22584–22590. [Google Scholar]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.-C. Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef]
- Guillerm, V.; Weselinski, L.J.; Alkordi, M.; Mohideen, M.I.H.; Belmabkhout, Y.; Cairns, A.J.; Eddaoudi, M. Porous organic polymers with anchored aldehydes: A new platform for post-synthetic amine functionalization en route for enhanced CO2 adsorption properties. Chem. Commun. 2014, 50, 1937. [Google Scholar] [CrossRef]
- Ratvijitvech, T.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Post-synthetic modification of conjugated microporous polymers. Polymer 2014, 55, 321–325. [Google Scholar] [CrossRef]
- Sun, L.-B.; Kang, Y.-H.; Shi, Y.-Q.; Jiang, Y.; Liu, X.-Q. Highly Selective Capture of the Greenhouse Gas CO2 in Polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Lee, Y.-R.; Ahn, W.-S. Microporous amine-functionalized aromatic polymers and their carbonized products for CO2 adsorption. Chem. Eng. J. 2017, 319, 65–74. [Google Scholar] [CrossRef]
- Xu, C.; Bacsik, Z.; Hedin, N. Adsorption of CO2 on a micro-/mesoporous polyimine modified with tris(2-aminoethyl)amine. J. Mater. Chem. A 2015, 3, 16229–16234. [Google Scholar] [CrossRef]
- Sung, S.; Suh, M.P. Highly efficient carbon dioxide capture with a porous organic polymer impregnated with polyethylenimine. J. Mater. Chem. A 2014, 2, 13245–13249. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Zhou, H.-C. Carbon Dioxide Capture from Air Using Amine-Grafted Porous Polymer Networks. J. Phys. Chem. C 2013, 117, 4057–4061. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Sprick, R.S.; Jiang, J.-X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable Organic Photocatalysts for Visible-Light-Driven Hydrogen Evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef]
- Chen, L.; Honsho, Y.; Seki, S.; Jiang, D. Light-Harvesting Conjugated Microporous Polymers: Rapid and Highly Efficient Flow of Light Energy with a Porous Polyphenylene Framework as Antenna. J. Am. Chem. Soc. 2010, 132, 6742–6748. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Su, F.; Niu, H.; Wood, C.D.; Campbell, N.L.; Khimyak, Y.Z.; Cooper, A.I. Conjugated microporous poly(phenylene butadiynylene)s. Chem. Commun. 2008, 486–488. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene) Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Choi, J.; Ko, J.H.; Kang, C.W.; Lee, S.M.; Kim, H.J.; Ko, Y.-J.; Yang, M.; Son, S.U. Enhanced redox activity of a hollow conjugated microporous polymer through the generation of carbonyl groups by carbonylative Sonogashira coupling. J. Mater. Chem. A 2018, 6, 6233–6237. [Google Scholar] [CrossRef]
- Karabiyikoglu, S.; Kelgokmen, Y.; Zora, M. Facile synthesis of iodopyridines from N-propargylic β-enaminones via iodine-mediated electrophilic cyclization. Tetrahedron 2015, 71, 4324–4333. [Google Scholar] [CrossRef]
- Kelgokmen, Y.; Zora, M. Facile synthesis of heavily-substituted alkynylpyridines via a Sonogashira approach. RSC Adv. 2016, 6, 4608–4621. [Google Scholar] [CrossRef]
- Hoven, B.G.V.D.; Alper, H. Innovative Synthesis of 4-Carbaldehydepyrrolin-2-ones by Zwitterionic Rhodium Catalyzed Chemo- and Regioselective Tandem Cyclohydrocarbonylation/CO Insertion of α-Imino Alkynes. J. Am. Chem. Soc. 2001, 123, 10214–10220. [Google Scholar] [CrossRef]
- Waldo, J.P.; LaRock, R.C. The Synthesis of Highly Substituted Isoxazoles by Electrophilic Cyclization. An Efficient Synthesis of Valdecoxib. J. Org. Chem. 2007, 72, 9643–9647. [Google Scholar] [CrossRef]
- Liu, X.; Hong, D.; She, Z.; Hersh, W.H.; Yoo, B.; Chen, Y. Complementary regioselective synthesis of 3,5-disubstituted isoxazoles from ynones. Tetrahedron 2018, 74, 6593–6606. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Xu, C.; Hedin, N. Synthesis of microporous organic polymers with high CO2-over-N2 selectivity and CO2 adsorption. J. Mater. Chem. A 2013, 1, 3406. [Google Scholar] [CrossRef]
- Bacsik, Z.; Atluri, R.; Garcia-Bennett, A.E.; Hedin, N. Temperature-Induced Uptake of CO2 and Formation of Carbamates in Mesocaged Silica Modified with n-Propylamines. Langmuir 2010, 26, 10013–10024. [Google Scholar] [CrossRef]
- Knoöfel, C.; Martin, C.; Hornebecq, V.; Llewellyn, P.L. Study of Carbon Dioxide Adsorption on Mesoporous Aminopropylsilane-Functionalized Silica and Titania Combining Microcalorimetry and in Situ Infrared Spectroscopy. J. Phys. Chem. C 2009, 113, 21726–21734. [Google Scholar]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. J. Phys. Chem. C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Aziz, B.; Hedin, N.; Bacsik, Z. Quantification of chemisorption and physisorption of carbon dioxide on porous silica modified by propylamines: Effect of amine density. Micropor. Mesopor. Mater. 2012, 159, 42–49. [Google Scholar] [CrossRef]
- Fu, F.-N.; DeOliveira, D.B.; Trumble, W.R.; Sarkar, H.K.; Singh, B.R. Secondary Structure Estimation of Proteins Using the Amide III Region of Fourier Transform Infrared Spectroscopy: Application to Analyze Calcium-Binding-Induced Structural Changes in Calsequestrin. Appl. Spectrosc. 1994, 48, 1432–1441. [Google Scholar] [CrossRef]
- Wang, X.; Schwartz, V.; Clark, J.C.; Ma, X.; Overbury, S.H.; Xu, X.; Song, C. Infrared Study of CO2 Sorption over “Molecular Basket” Sorbent Consisting of Polyethylenimine-Modified Mesoporous Molecular Sieve. J. Phys. Chem. C 2009, 113, 7260–7268. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Micropor. Mesopor. Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Bacsik, Z.; Ahlsten, N.; Ziadi, A.; Zhao, G.; Garcia-Bennett, A.E.; Martín-Matute, B.; Hedin, N. Mechanisms and Kinetics for Sorption of CO2 on Bicontinuous Mesoporous Silica Modified with n-Propylamine. Langmuir 2011, 27, 11118–11128. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Patel, H.H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; D’Alessandro, D.M.; Krishna, R.; Long, J.R. Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal–organic framework CuBTTri. Chem. Sci. 2011, 2, 2022–2028. [Google Scholar] [CrossRef]
- Choi, S.; Gray, M.L.; Jones, C.W. Amine-Tethered Solid Adsorbents Coupling High Adsorption Capacity and Regenerability for CO2 Capture from Ambient Air. ChemSusChem 2011, 4, 628–635. [Google Scholar] [CrossRef] [PubMed]


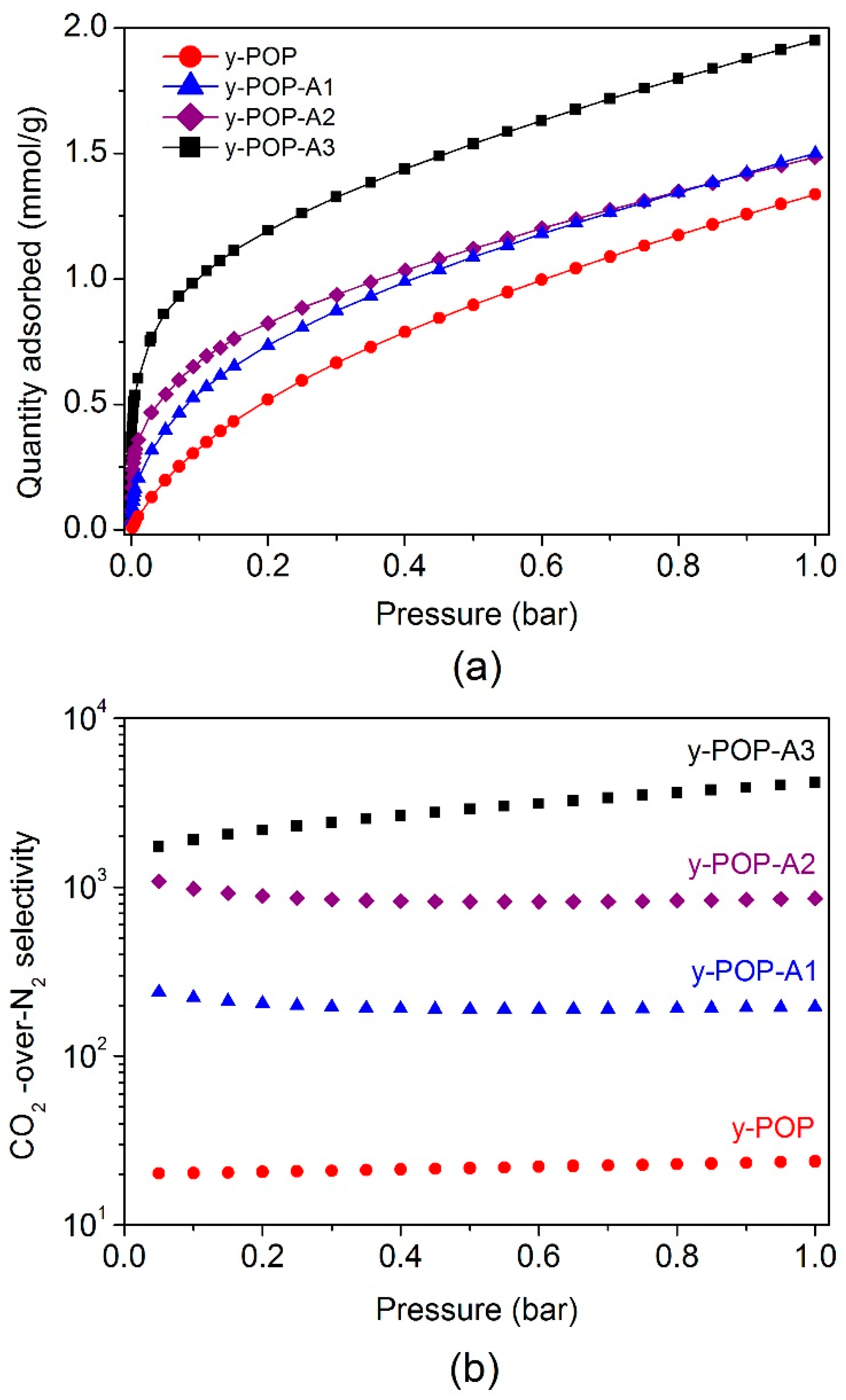
| Sample | Amine Loading | SBET (m2 g−1) | CO2 Uptake (mmol g−1) | CO2/N2 Selectivity | Qst (kJ mol−1) | ||
|---|---|---|---|---|---|---|---|
| 0.15 bar | 1 bar | IAST | Henry’s Law | ||||
| y-POP | 0 | 226 | 0.43 | 1.34 | 20 | 22 | 29.0 |
| y-POP-A1 | 12% | 145 | 0.65 | 1.50 | 239 | 216 | 46.8 |
| y-POP-A2 | 16% | 107 | 0.76 | 1.49 | 1083 | 750 | 62.2 |
| y-POP-A3 | 19% | 84 | 1.11 | 1.95 | 4154 | 3806 | 76.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.; Li, S.; Strømme, M.; Xu, C. Synthesis of Porous Organic Polymers with Tunable Amine Loadings for CO2 Capture: Balanced Physisorption and Chemisorption. Nanomaterials 2019, 9, 1020. https://doi.org/10.3390/nano9071020
Kong X, Li S, Strømme M, Xu C. Synthesis of Porous Organic Polymers with Tunable Amine Loadings for CO2 Capture: Balanced Physisorption and Chemisorption. Nanomaterials. 2019; 9(7):1020. https://doi.org/10.3390/nano9071020
Chicago/Turabian StyleKong, Xueying, Shangsiying Li, Maria Strømme, and Chao Xu. 2019. "Synthesis of Porous Organic Polymers with Tunable Amine Loadings for CO2 Capture: Balanced Physisorption and Chemisorption" Nanomaterials 9, no. 7: 1020. https://doi.org/10.3390/nano9071020
APA StyleKong, X., Li, S., Strømme, M., & Xu, C. (2019). Synthesis of Porous Organic Polymers with Tunable Amine Loadings for CO2 Capture: Balanced Physisorption and Chemisorption. Nanomaterials, 9(7), 1020. https://doi.org/10.3390/nano9071020