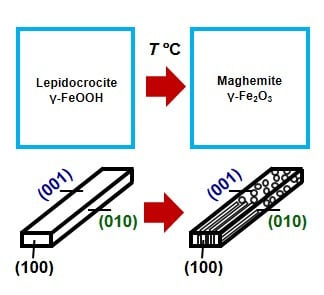
Fabrication of Maghemite Nanoparticles with High Surface Area
Abstract
:1. Introduction
2. Materials and Methods
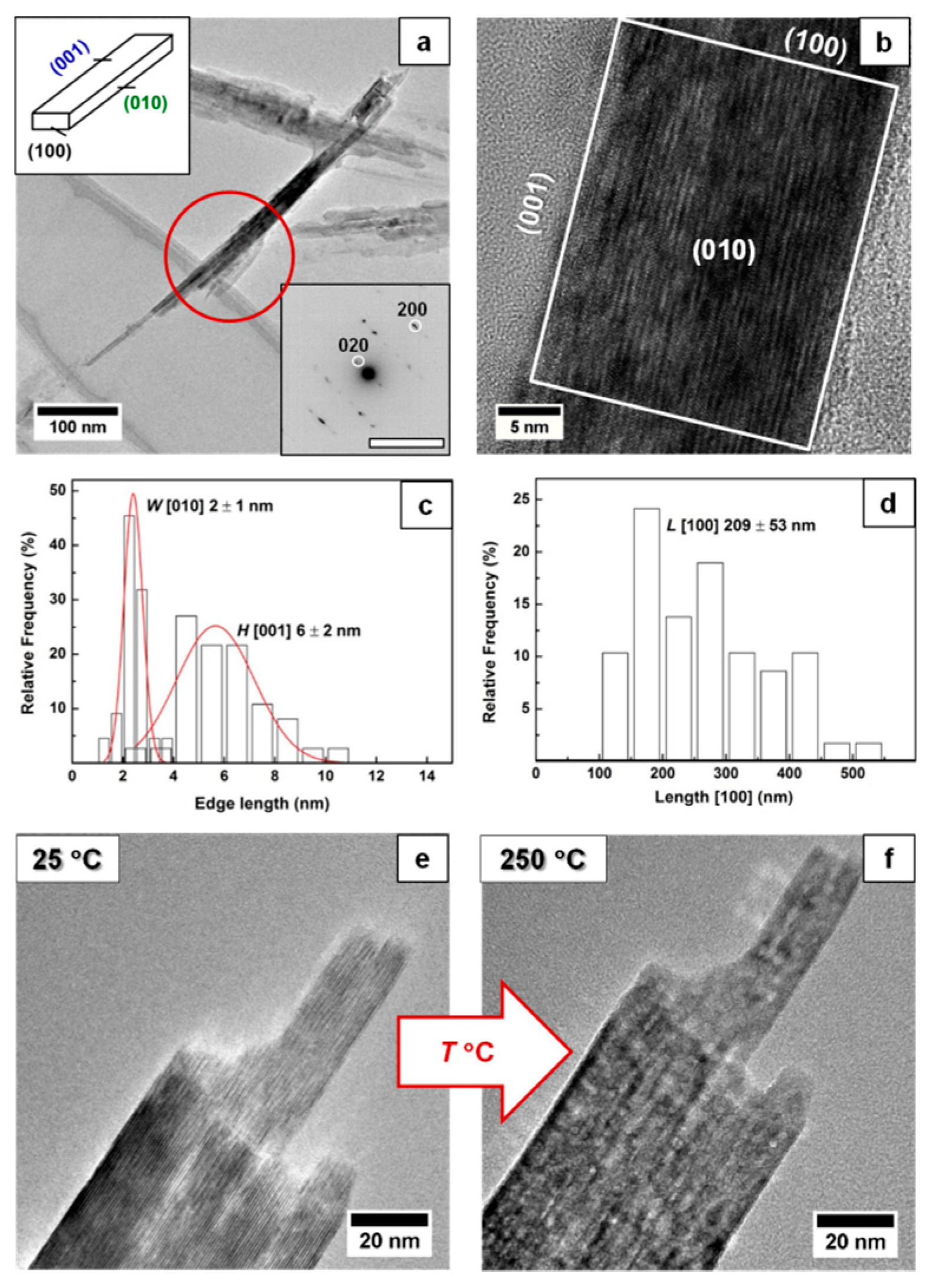
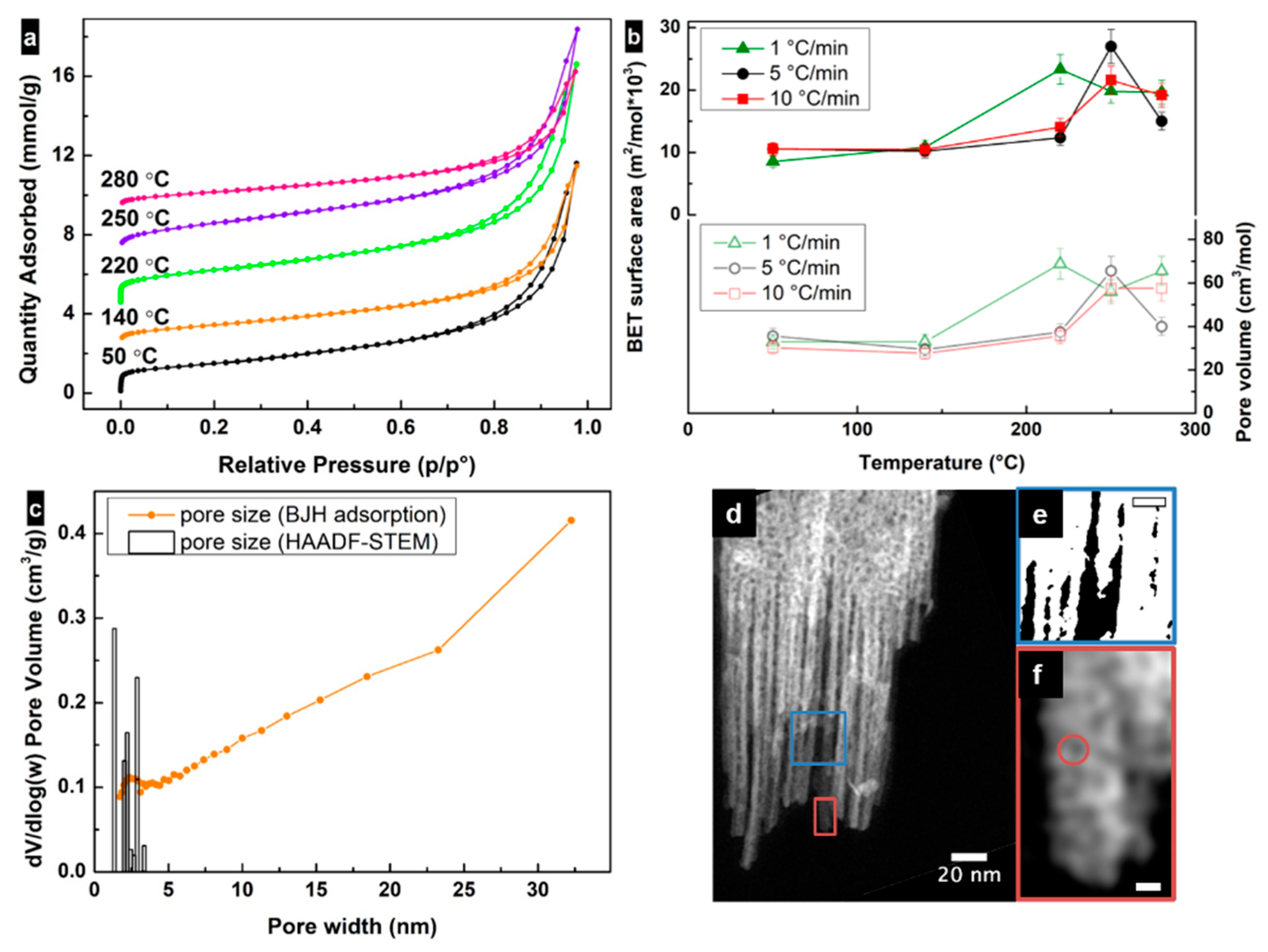
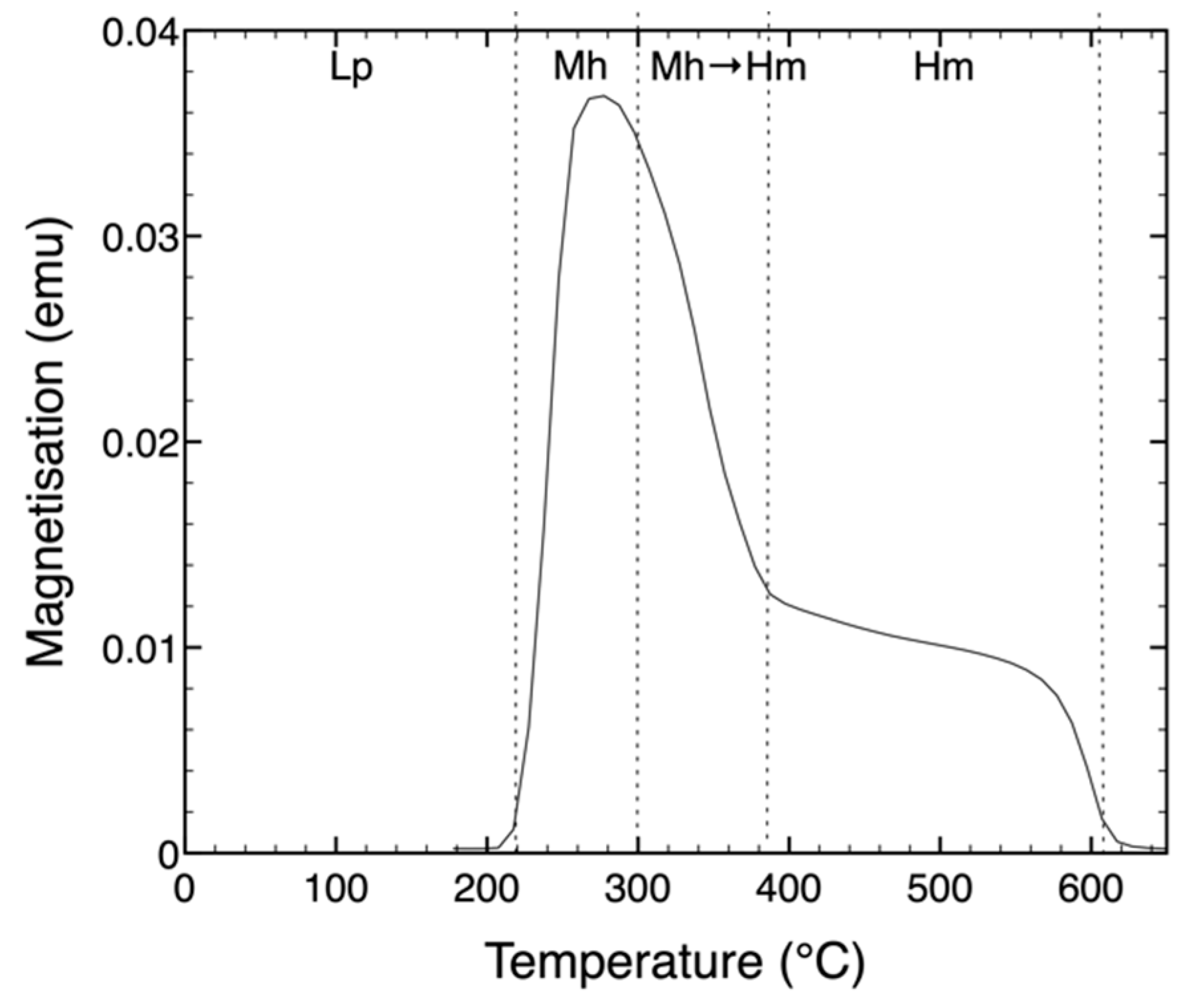
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKenna, K.P.; Hofer, F.; Gilks, D.; Lazarov, V.K.; Chen, C.; Wang, Z.; Ikuhara, Y. Atomic-scale structure and properties of highly stable antiphase boundary defects in Fe3O4. Nat. Commun. 2014, 5, 5740. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar]
- Cudennec, Y.; Lecerf, A. Topotactic transformations of goethite and lepidocrocite into hematite and maghemite. Solid State Sci. 2005, 7, 520–529. [Google Scholar] [CrossRef]
- Vernekar, D.; Jagadeesan, D. Tunable acid–base bifunctional catalytic activity of FeOOH in an orthogonal tandem reaction. Catal. Sci. Technol. 2015, 5, 4029–4038. [Google Scholar] [CrossRef]
- Agarwal, A.; Joshi, H.; Kumar, A. Synthesis, characterization and application of nano lepidocrocite and magnetite in the degradation of carbon tetrachloride. S. Afr. J. Chem. 2011, 64, 218–224. [Google Scholar]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Cotin, G.; Piant, S.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Iron Oxide Nanoparticles for Biomedical Applications: Synthesis, Functionalization, and Application. In Iron Oxide Nanoparticles for Biomedical Applications; Mahmoudi, M., Laurent, S., Eds.; Elsevier Ltd.: Cambridge, MA, USA, 2017; pp. 43–88. ISBN 9780081019252. [Google Scholar]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Morris, R.V.; Golden, D.C.; Shelfer, T.D.; Lauer, H.V. Lepidocrocite to maghemite to hematite: A pathway to magnetic and hematitic Martian soil. Meteorit. Planet. Sci. 1998, 33, 743–751. [Google Scholar] [CrossRef]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wu, M.C.; Zhao, T.S.; Zeng, Y.K.; Ren, Y.X. An aqueous alkaline battery consisting of inexpensive all-iron redox chemistries for large-scale energy storage. Appl. Energy 2018, 215, 98–105. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U.; Björefors, F.; Edström, K. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Koo, B.; Xiong, H.; Slater, M.D.; Prakapenka, V.B.; Balasubramanian, M.; Podsiadlo, P.; Johnson, C.S.; Rajh, T.; Shevchenko, E.V. Hollow Iron Oxide Nanoparticles for Application in Lithium Ion Batteries. Nano Lett. 2012, 12, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Wu, X.; Zhang, Y. Goethite nanorods as anode electrode materials for rechargeable Li-ion batteries. Electrochem. Commun. 2009, 11, 1696–1699. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination 2010, 263, 240–248. [Google Scholar] [CrossRef]
- Mähler, J.; Persson, I. Rapid adsorption of arsenic from aqueous solution by ferrihydrite-coated sand and granular ferric hydroxide. Appl. Geochem. 2013, 37, 179–189. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Tailoring size and structural distortion of Fe3O4 nanoparticles for the purification of contaminated water. Bioresour. Technol. 2009, 100, 4139–4146. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P.; Knappe, D.R.U. Intraparticle diffusion and adsorption of arsenate onto granular ferric hydroxide (GFH). Water Res. 2004, 38, 4002–4012. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.D.; Dasgupta, D.R.; Mackay, A.L. Oriented Transformations in Iron Oxides and Hydroxides. Nature 1957, 180, 645–647. [Google Scholar] [CrossRef]
- Giovanoli, R.; Brütsch, R. Kinetics and mechanism of the dehydration of γ-FeOOH. Thermochim. Acta 1975, 13, 15–36. [Google Scholar] [CrossRef]
- Song, X.; Boily, J.F. Surface and Bulk Thermal Dehydroxylation of FeOOH Polymorphs. J. Phys. Chem. A 2016, 120, 6249–6257. [Google Scholar] [CrossRef] [PubMed]
- Ennas, G.; Marongiu, G.; Musinu, A.; Falqui, A.; Ballirano, P.; Caminiti, R. Characterization of Nanocrystalline γ–Fe2O3 Prepared by Wet Chemical Method. J. Mater. Res. 1999, 14, 1570–1575. [Google Scholar] [CrossRef]
- Naono, H.; Nakai, K. Thermal decomposition of γ-FeOOH fine particles. J. Colloid Interface Sci. 1989, 128, 146–156. [Google Scholar] [CrossRef]
- Kachi, S.; Momiyama, K.; Shimizu, S. An electron diffraction study and a theory of the transformation from γ-Fe2O3 to α-Fe2O3. J. Phys. Soc. Jpn. 1963, 18, 106–116. [Google Scholar] [CrossRef]
- Giovanoli, R.; Brütsch, R. Dehydration of γ-FeOOH: Direct Observation of the Mechanism. Chimia 1974, 28, 188–191. [Google Scholar]
- De Bakker, P.M.A.; De Grave, E.; Vandenberghe, R.E.; Bowen, L.H.; Pollard, R.J.; Persoons, R.M. Mössbauer study of the thermal decomposition of lepidocrocite and characterization of the decomposition products. Phys. Chem. Miner. 1991, 18, 131–143. [Google Scholar] [CrossRef]
- Fang, J.; Kumbhar, A.; Zhou, W.L.; Stokes, K.L. Nanoneedles of maghemite iron oxide prepared from a wet chemical route. Mater. Res. Bull. 2003, 38, 461–467. [Google Scholar] [CrossRef]
- Takada, T.; Kiyama, M.; Shimizu, S. Morphological and Crystallographic Studies on the Oriented Transformation in γ–FeOOH and its Decomposed Oxides. Bull. Inst. Chem. Res. Kyoto Univ. 1965, 42, 505–510. [Google Scholar]
- Naono, H.; Fujiwara, R.; Sugioka, H.; Sumiya, K.; Yanazawa, H. Micropore formation due to thermal decomposition of acicular microcrystals of β-FeOOH. J. Colloid Interface Sci. 1982, 87, 317–332. [Google Scholar] [CrossRef]
- Naono, H.; Fujiwara, R. Micropore formation due to thermal decomposition of acicular microcrystals of α-FeOOH. J. Colloid Interface Sci. 1980, 73, 406–415. [Google Scholar] [CrossRef]
- Kozin, P.A.; Salazar-Alvarez, G.; Boily, J.F. Oriented aggregation of lepidocrocite and impact on surface charge development. Langmuir ACS J. Surf. Colloids 2014, 30, 9017–9021. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Song, X.; Boily, J.F. Structural controls on OH site availability and reactivity at iron oxyhydroxide particle surfaces. Phys. Chem. Chem. Phys. 2012, 14, 2579. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Li, Z. Characterization of Different Shaped Nanocrystallites using X-ray Diffraction Line Profiles. Part. Part. Syst. Charact. 2011, 28, 19–24. [Google Scholar] [CrossRef]
- Mitov, I.; Paneva, D.; Kunev, B. Comparative study of the thermal decomposition of iron oxyhydroxides. Thermochim. Acta 2002, 386, 179–188. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ma, J.; Hu, F.; Li, M.C. Synthesis and Characterization of Mesoporous Alumina via a Reverse Precipitation Method. J. Mater. Sci. Technol. 2012, 28, 572–576. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trushkina, Y.; Tai, C.-W.; Salazar-Alvarez, G. Fabrication of Maghemite Nanoparticles with High Surface Area. Nanomaterials 2019, 9, 1004. https://doi.org/10.3390/nano9071004
Trushkina Y, Tai C-W, Salazar-Alvarez G. Fabrication of Maghemite Nanoparticles with High Surface Area. Nanomaterials. 2019; 9(7):1004. https://doi.org/10.3390/nano9071004
Chicago/Turabian StyleTrushkina, Yulia, Cheuk-Wai Tai, and German Salazar-Alvarez. 2019. "Fabrication of Maghemite Nanoparticles with High Surface Area" Nanomaterials 9, no. 7: 1004. https://doi.org/10.3390/nano9071004
APA StyleTrushkina, Y., Tai, C.-W., & Salazar-Alvarez, G. (2019). Fabrication of Maghemite Nanoparticles with High Surface Area. Nanomaterials, 9(7), 1004. https://doi.org/10.3390/nano9071004