Fabrication of Troponin I Biosensor Composed of Multi-Functional DNA Structure/Au Nanocrystal Using Electrochemical and Localized Surface Plasmon Resonance Dual-Detection Method
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Construction of cTnI Plasmid, and Expression and Purification of cTnI
2.3. Fabrication of AuNC on ITO Substrate
2.4. Assembly of Multi-Functional DNA 3WJ
2.5. Fabrication and Investigation of Multi-Functional DNA 3WJ/AuNC Heterolayer
2.6. Optical Analysis of cTnI Detection by Localized Surface Plasmon Resonance Method
2.7. Electrochemical Analysis of cTnI Detection by EIS
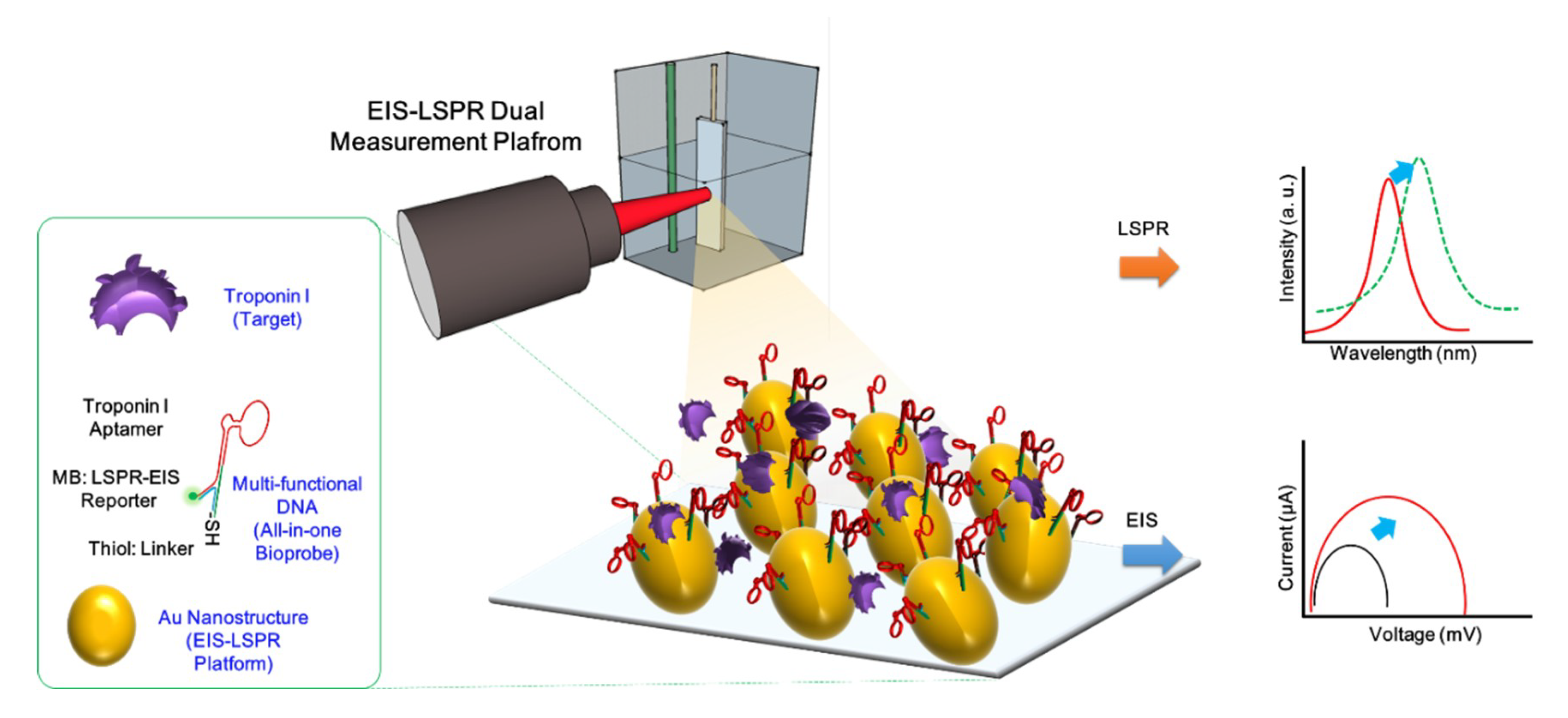
3. Results and Discussion
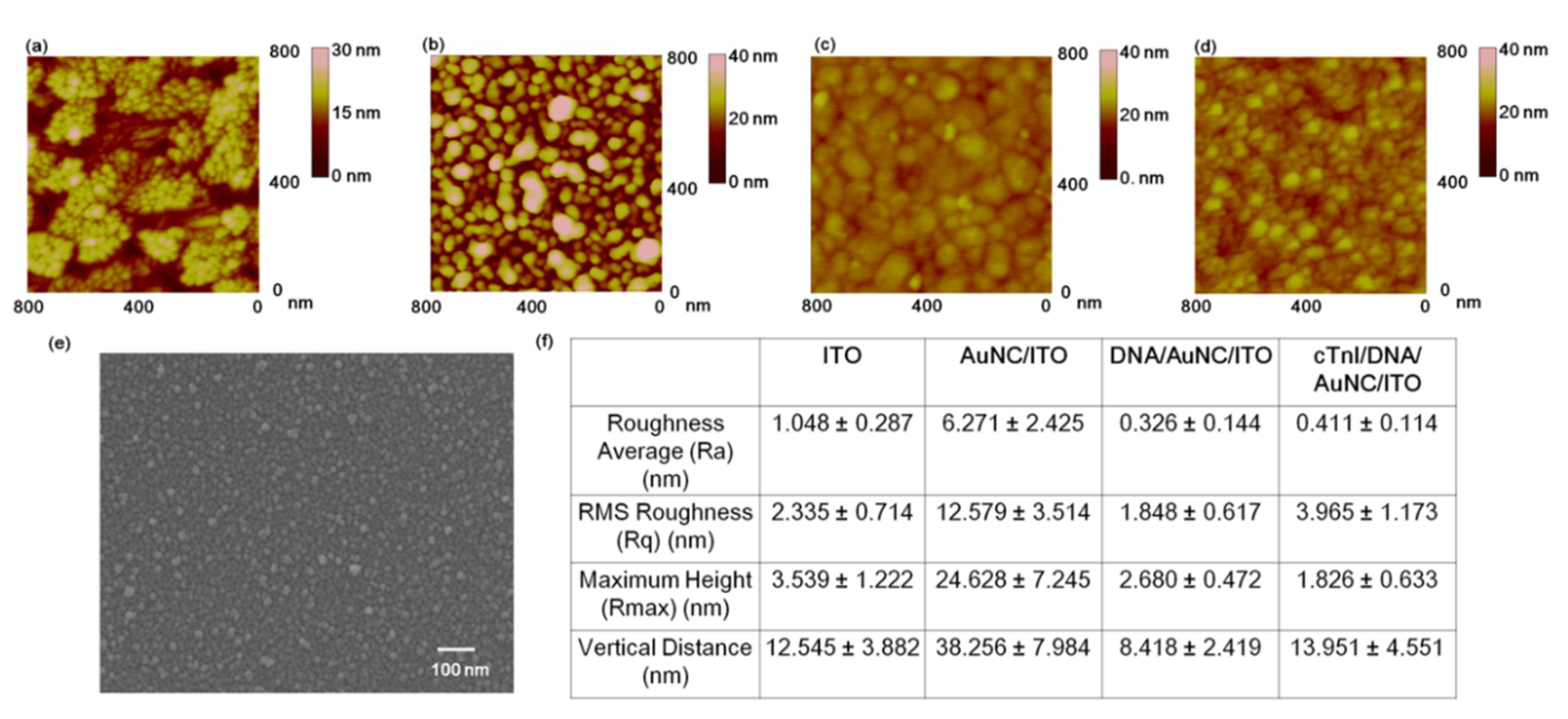
3.1. Investigation of Fabricated cTnI/Multi-Functional DNA on AuNC-Modified ITO Substrate
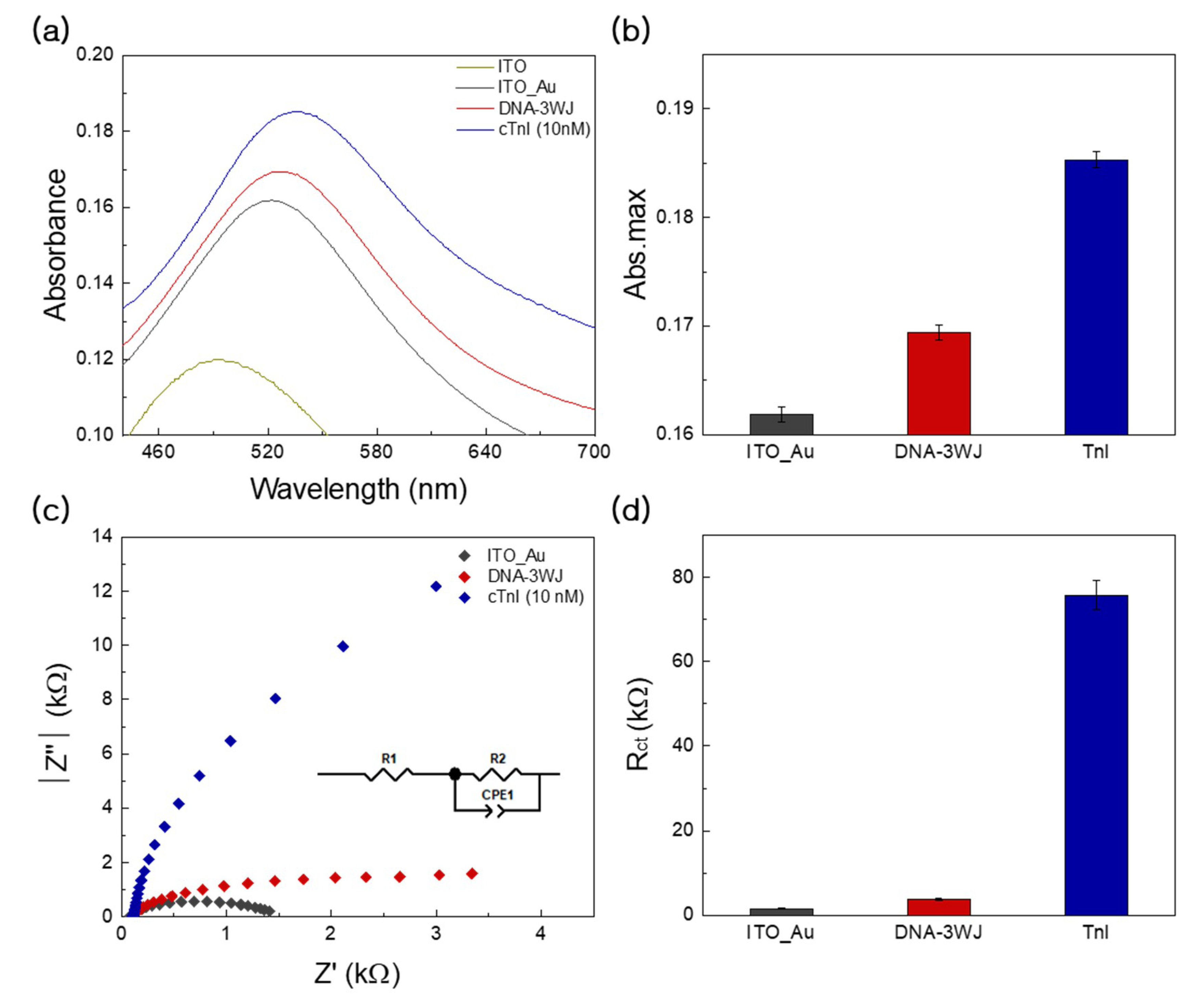
3.2. Optical and Electrochemical Properties of Fabricated Biosensor
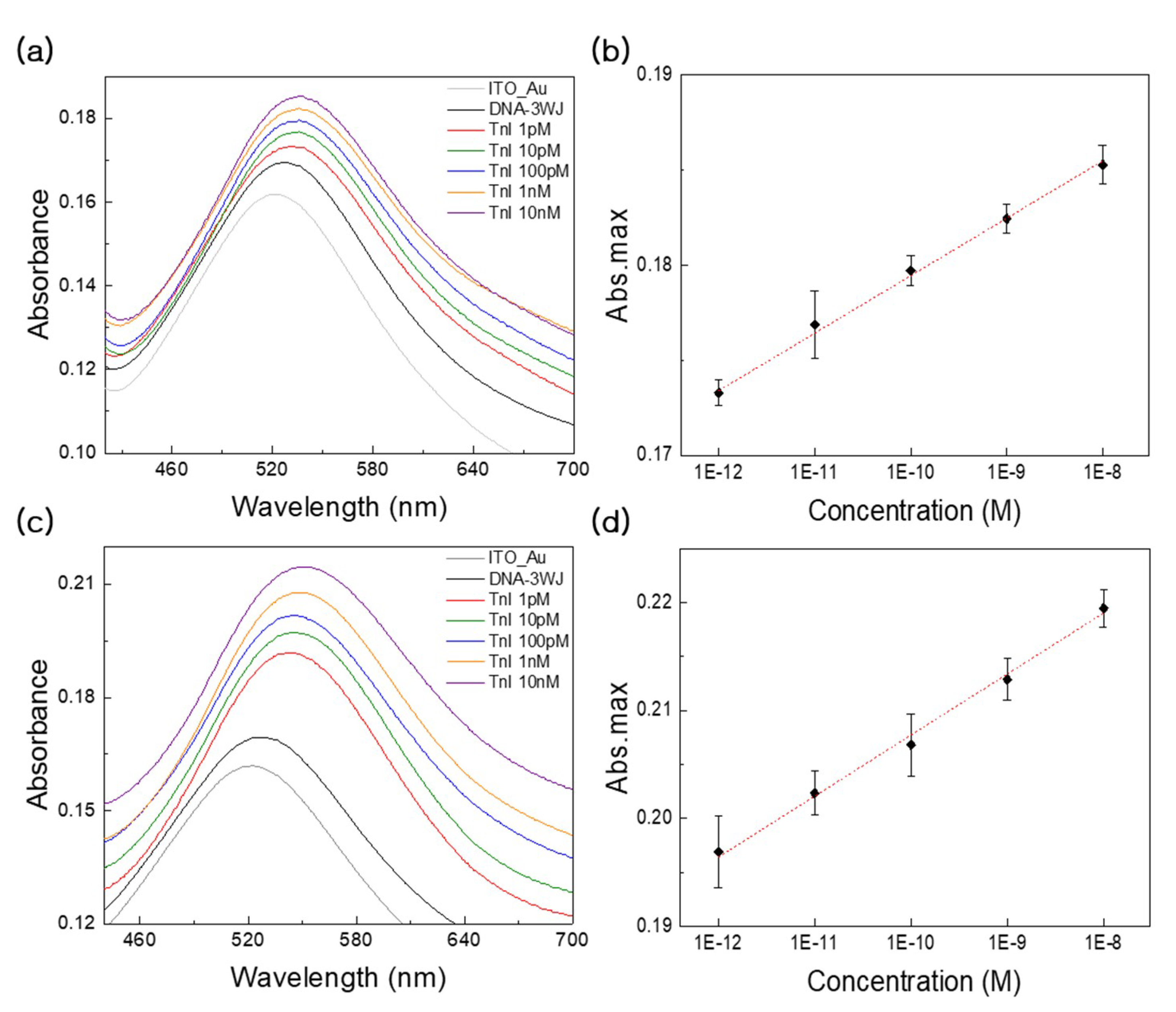
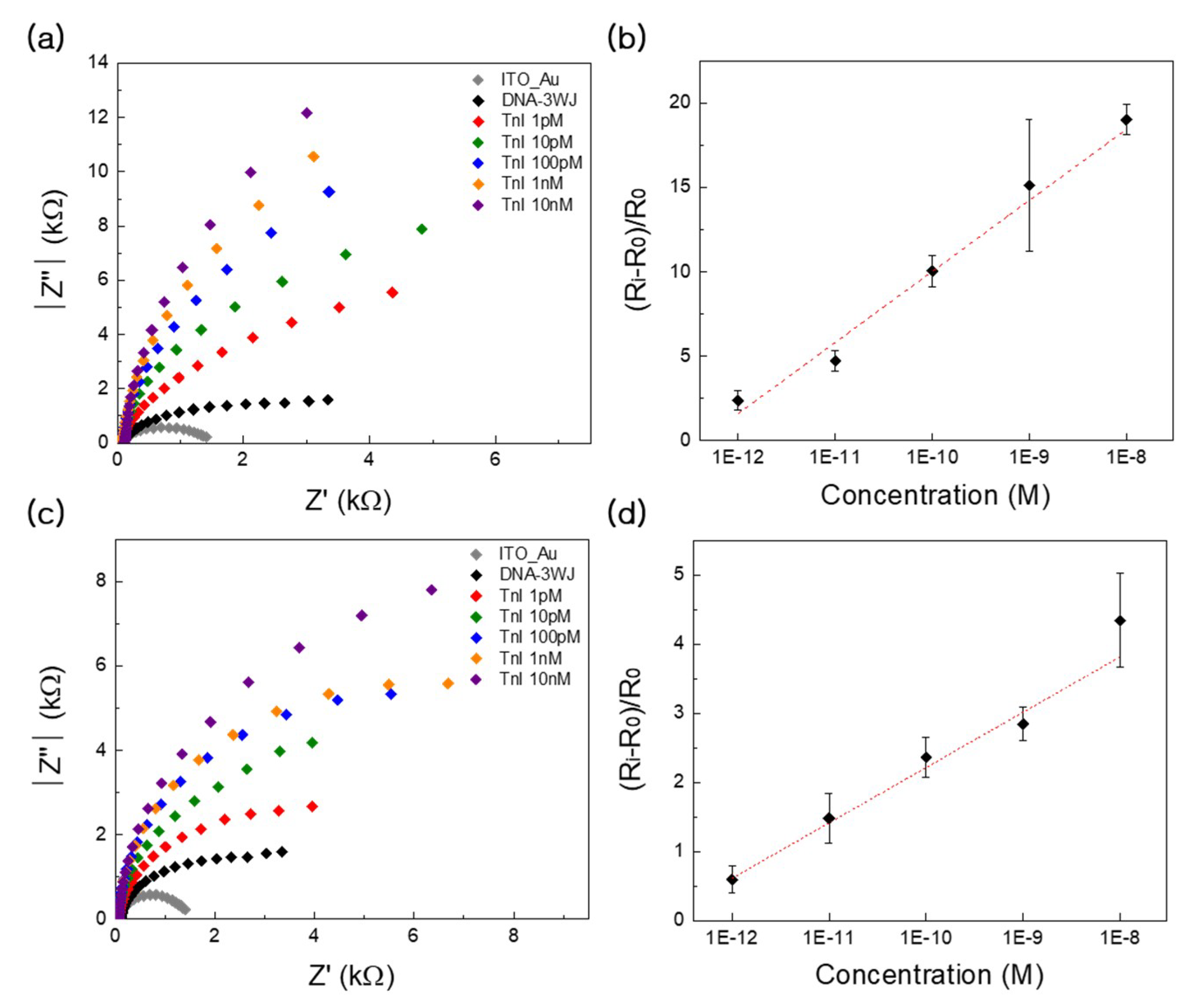
3.3. Detection Performance and Clinical Test Using LSPR
3.4. Detection Performance and Clinical Testing Using EIS
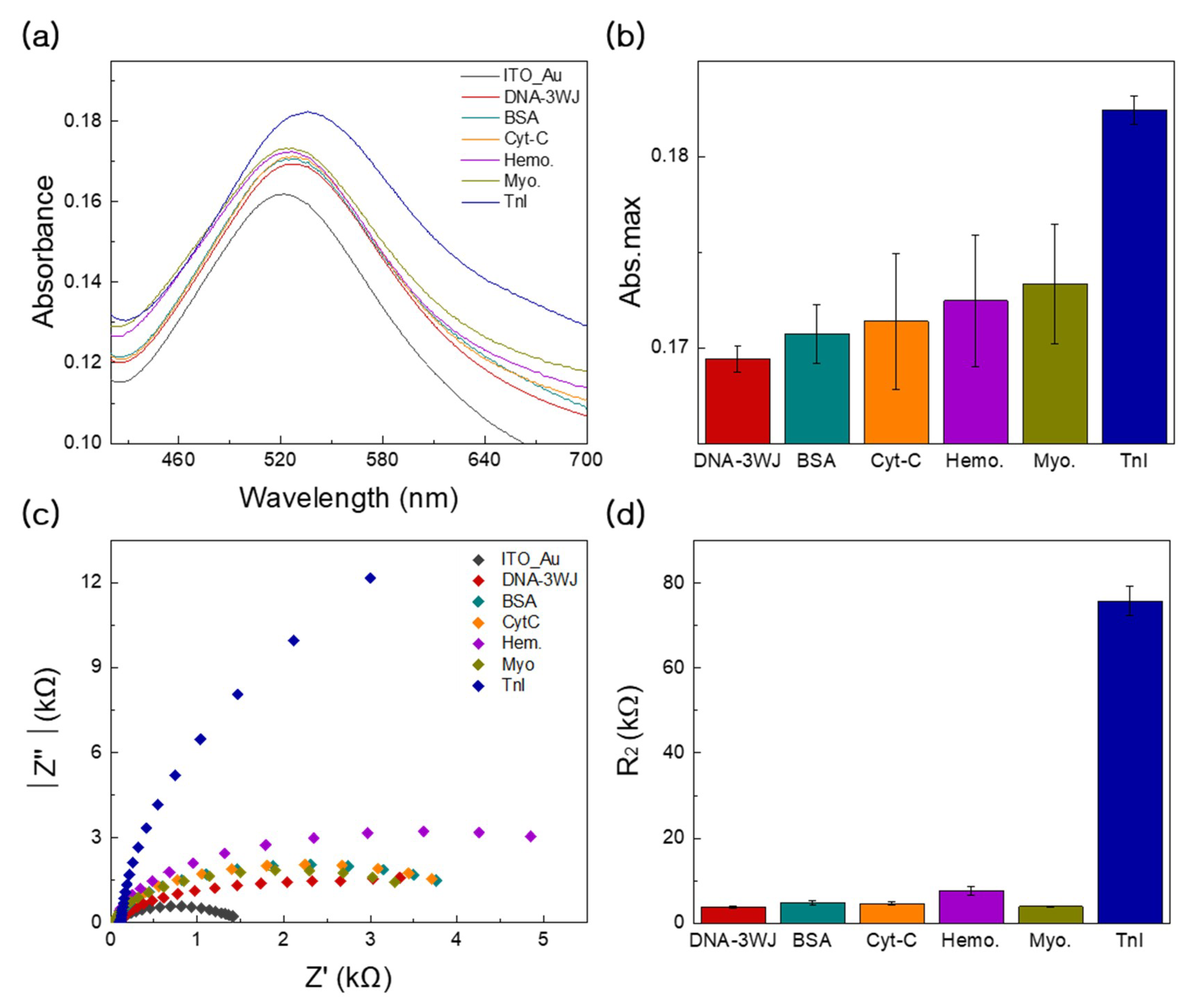
3.5. Specificity Tests Using EC and LSPR Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sutcliffe, P.; Connock, M.; Gurung, T.; Freeman, K.; Johnson, S.; Ngianga-Bakwin, K.; Grove, A.; Gurung, B.; Stranges, S.; Clarke, A. Aspirin in primary prevention of cardiovascular disease and cancer: A systematic review of the balance of evidence from reviews of randomized trials. PLoS ONE 2013, 8, e81970. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Fakanya, W.M.; Tothill, I.E. Cardiovascular disease detection using bio-sensing techniques. Talanta 2014, 128, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Wei, J.; Wenger, N.K. Ischemic heart disease in women: A focus on risk factors. Trends Cardiovasc. Med. 2015, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Valensi, P.; Lorgis, L.; Cottin, Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: A review of the literature. Arch. Cardiovasc. Dis. 2011, 104, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.S.; Jones, N.; Moorjani, N. Mechanical complications of acute myocardial infarction. Cardiol. Clin. 2013, 31, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Evolution of myocardial infarction and its biomarkers: A historical perspective. Heart Views 2016, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bodor, G.S. Biochemical markers of myocardial damage. Ejifcc 2016, 27, 95–111. [Google Scholar] [PubMed]
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction. Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171–172, 62–76. [Google Scholar] [CrossRef]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.K.; Lee, J.; Shin, J.H.; Ban, C. Electrochemical aptasensor of cardiac troponin I for the early diagnosis of acute myocardial infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, S.; Peng, Z.; Othman, A.M.; Leblanc, R. Recent development of cardiac troponin I detection. ACS Sens. 2016, 1, 106–114. [Google Scholar] [CrossRef]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, K.S.; Meyer, N.L.; Yuan, J.; Hirst, L.S.; Chase, P.B.; Xiong, P. Functionalized SnO2 nanobelt field-effect transistor sensors for label-free detection of cardiac troponin. Biosens. Bioelectron. 2011, 26, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, Y.; Park, S.Y.; Hong, K.; Kim, Y.; Park, C.; Chung, Y.H.; Lee, M.H.; Min, J. Fabrication of electrochemical biosensor composed of multi-functional DNA structure/Au nanospike on micro-gap/PCB system for detecting troponin I in human serum. Colloids Surf. B Biointerfaces 2019, 175, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, S.H. Quenching effect on gold nano-patterned cardiac troponin I chip by total internal reflection fluorescence microscopy. Talanta 2013, 104, 32–38. [Google Scholar] [CrossRef]
- Kar, P.; Pandey, A.; Greer, J.J.; Shankar, K. Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays. Lab Chip 2012, 12, 821–828. [Google Scholar] [CrossRef]
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In vitro and in vivo SERS biosensing for disease diagnosis. Biosensors 2018, 8, 46. [Google Scholar] [CrossRef]
- Cho, I.H.; Paek, E.H.; Kim, Y.K.; Kim, J.H.; Paek, S.H. Chemiluminometric enzyme-linked immunosorbent assays (ELISA)-on-a-chip biosensor based on cross-flow chromatography. Anal. Chim. Acta 2009, 632, 247–255. [Google Scholar] [CrossRef]
- Ashaduzzaman, M.; Deshpande, S.R.; Murugan, N.A.; Mishra, Y.K.; Turner, A.P.F.; Tiwari, A. On/off-switchable LSPR nano-immunoassay for troponin-T. Sci. Rep. 2017, 7, 44027. [Google Scholar] [CrossRef]
- Guo, Z.R.; Gu, C.R.; Fan, X.; Bian, Z.P.; Yang, D.; Gu, N.; Jhang, J.N. Fabrication of anti-human cardiac troponin I immunogold nanorods for sensing acute myocardial damage. Nanoscale Res. Lett. 2009, 4, 1428. [Google Scholar] [CrossRef]
- Shu, D.; Shu, Y.; Haque, F.; Abdelmawla, S.; Guo, P. Thermodynamically stable RNA three-way junctions for constructing multifuntional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011, 6, 658. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lin, Y.; Le, X.C. Binding-induced formation of DNA three-way junctions and its application to protein detection and DNA strand displacement. Anal. Chem. 2013, 85, 10835–10841. [Google Scholar] [CrossRef] [PubMed]
- Jesus, V.P.S.; Raniero, L.; Lemes, G.M.; Bhattacharjee, T.T.; Júnior, P.C.C.; Castilho, M.L. Nanoparticles of methylene blue enhance photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 23, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-enhanced fluorescence biosensors: A review. Plsamonics 2014, 9, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, B.C.; Oh, B.K.; Choi, J.W. Highly sensitive localized surface plasmon resonance immunosensor for label-free detection of HIV-1. Nanomedicine 2013, 9, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, S.Y.; Jang, H.; Kim, G.H.; Lee, Y.; Park, C.; Mohammadniaei, M.; Lee, M.H.; Min, J. Fabrication of electrochemical biosensor consisted of multi-functional DNA structure/porous Au nanoparticle for avian influenza virus (H5N1) in human serum. Mater. Sci. Eng. C 2019, 99, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, S.U.; Min, J.; Choi, J.W. Multilevel biomemory device consisting of recombinant azurin/cytochrome c. Adv. Mater. 2010, 22, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Kongsuphol, P.; Wong, C.C.; Polla, L.J.; Park, M.K. Label free biosensor for sensitive human influenza virus hemagglutinin specific antibody detection using coiled-coil peptide modified microelectrode array based platform. Sens. Actuators B 2014, 194, 127–133. [Google Scholar] [CrossRef]
- Zhang, Q.; Prabhu, A.; San, A.; Al-Sharab, J.F.; Levon, K. A polyaniline based ultrasensitive potentiometric immunosensor for cardiac troponin complex detection. Biosens. Bioelectron. 2015, 72, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lu, M.; Wang, W.; Bian, Z.P.; Zhang, J.R.; Zhu, J.J. Electrochemical immunosensor for simultaneous detection of dual cardiac markers based on a poly (dimethylsiloxane)-gold nanoparticles composite microfluidic chip: A proof of principle. Clin. Chem. 2010, 56, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.S.; Choi, Y.H.; Koh, K.; Kim, J.H.; Lee, J.J.; Lee, M. Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochem. Sci. 2011, 6, 1906–1916. [Google Scholar]
- Alsaif, M.M.Y.A.; Lathanm, K.; Field, M.R.; Yao, D.D.; Medehkar, N.V.; Beane, G.A.; Kaner, R.B.; Russo, S.P.; Ou, J.Z.; Kanlantar-Zadeh, K. Tunable plasmon Resonances in two-dimensional molybdenum oxide nanoflakes. Adv. Mater. 2014, 26, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ou, J.Z.; Chrimes, A.F.; Carey, B.J.; Daeneke, T.; Alsaif, M.M.Y.A.; Mortazavi, M.; Zhuiykov, S.; Medhekar, N.; Bhaskaran, M.; et al. Plasmon resonances of highly doped two-dimensional MoS2. Nano Lett. 2015, 15, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Mohammadniaei, M.; Lee, T.; Bharate, B.G.; Yoon, J.; Choi, H.K.; Park, S.; Kim, J.; Kim, J.; Choi, J.-W. Bifunctional Au@Bi2Se3 core–shell nanoparticle for synergetic therapy by SERS-traceable antagomiR delivery and photothermal treatment. Small 2018, 14, 1802934. [Google Scholar] [CrossRef] [PubMed]
| Material | Detection Method | Detection Limit | Ref. |
|---|---|---|---|
| Antibody | CV/EIS | 5 pg/mL | [29] |
| Antibody/AuNR | SPR | 10 ng/mL | [20] |
| Antibody | Fluorescence | 35 aM | [15] |
| Antibody | Fluorescence | 0.1 pg/mL | [16] |
| Antibody | SWV | 4 pg/mL | [30] |
| Antibody/GNP | CV | 1 ng/mL | [31] |
| Si-nanowire/Antibody | FET | 5 pg/mL | [12] |
| SnO2-nanowire/Antibody | FET | 2 ng/mL | [13] |
| DNA 3WJ/AuNS | CV | 24 pg/mL (1 pM) | [14] |
| MF-DNA/AuNC | EIS/LSPR | 110 fM (Buffer, LSPR) 840 fM (Serum, LSPR) 497 fM (Buffer, EIS) 829 fM (Serum, EIS) | Present Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Kim, J.; Nam, I.; Lee, Y.; Kim, H.E.; Sohn, H.; Kim, S.-E.; Yoon, J.; Seo, S.W.; Lee, M.-H.; et al. Fabrication of Troponin I Biosensor Composed of Multi-Functional DNA Structure/Au Nanocrystal Using Electrochemical and Localized Surface Plasmon Resonance Dual-Detection Method. Nanomaterials 2019, 9, 1000. https://doi.org/10.3390/nano9071000
Lee T, Kim J, Nam I, Lee Y, Kim HE, Sohn H, Kim S-E, Yoon J, Seo SW, Lee M-H, et al. Fabrication of Troponin I Biosensor Composed of Multi-Functional DNA Structure/Au Nanocrystal Using Electrochemical and Localized Surface Plasmon Resonance Dual-Detection Method. Nanomaterials. 2019; 9(7):1000. https://doi.org/10.3390/nano9071000
Chicago/Turabian StyleLee, Taek, Jinmyeong Kim, Inho Nam, Yeonju Lee, Ha Eun Kim, Hiesang Sohn, Seong-Eun Kim, Jinho Yoon, Sang Woo Seo, Min-Ho Lee, and et al. 2019. "Fabrication of Troponin I Biosensor Composed of Multi-Functional DNA Structure/Au Nanocrystal Using Electrochemical and Localized Surface Plasmon Resonance Dual-Detection Method" Nanomaterials 9, no. 7: 1000. https://doi.org/10.3390/nano9071000
APA StyleLee, T., Kim, J., Nam, I., Lee, Y., Kim, H. E., Sohn, H., Kim, S.-E., Yoon, J., Seo, S. W., Lee, M.-H., & Park, C. (2019). Fabrication of Troponin I Biosensor Composed of Multi-Functional DNA Structure/Au Nanocrystal Using Electrochemical and Localized Surface Plasmon Resonance Dual-Detection Method. Nanomaterials, 9(7), 1000. https://doi.org/10.3390/nano9071000







