NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans
Abstract
1. Introduction
- (1)
- potentially deleterious effects of commercially available nanoTiO2 sunscreen by measuring markers of oxidative stress and/or inflammation in plasma, urine, and EBC;
- (2)
- beneficial preventive effects of commercial nanoTiO2 sunscreen in limiting/blocking oxidative stress and inflammatory markers in plasma, urine, and EBC, triggered by UV radiation from a commercial tanning bed;
- (3)
- potential absorption of titania and TiO2 nanoparticles by their presence in biological fluids—plasma and urine—prior to, during, and one week after sunscreen use;
- (4)
- potential inhalational absorption of titania and TiO2 nanoparticles (as powder contamination from the dry sunscreen on skin) by analysing the EBC in the collected samples;
- (5)
- spirometry and FeNO to evaluate potential respiratory disorders.
2. Materials and Methods
2.1. Subjects and Biological Sample Collection
2.2. Sunscreen
2.2.1. Label Information for the Commercial Sunscreen
2.2.2. X-ray Powder Diffraction (XRD)
2.2.3. Microscopy Techniques
2.3. UV Exposure
2.4. Exhaled Breath Condensate (EBC) Sample Collection and Analysis
2.5. Analysis of Markers of Oxidative Stress and Inflammation
2.6. TiO2 Particle Analysis in Plasma, Urine, and EBC
2.6.1. Quantitative Determination of TiO2 by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.6.2. Transmission Electron Microscopy (TEM) Particle Analysis
2.7. FeNO Measurement
2.8. Spirometry
2.9. Statistical Analysis
3. Results
3.1. Subjects
3.2. Sunscreen Use and Analysis
3.3. UV Exposure
3.4. EBC Alpha-Amylase Concentration and pH
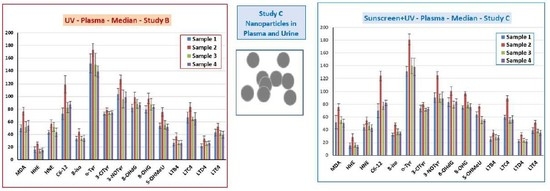
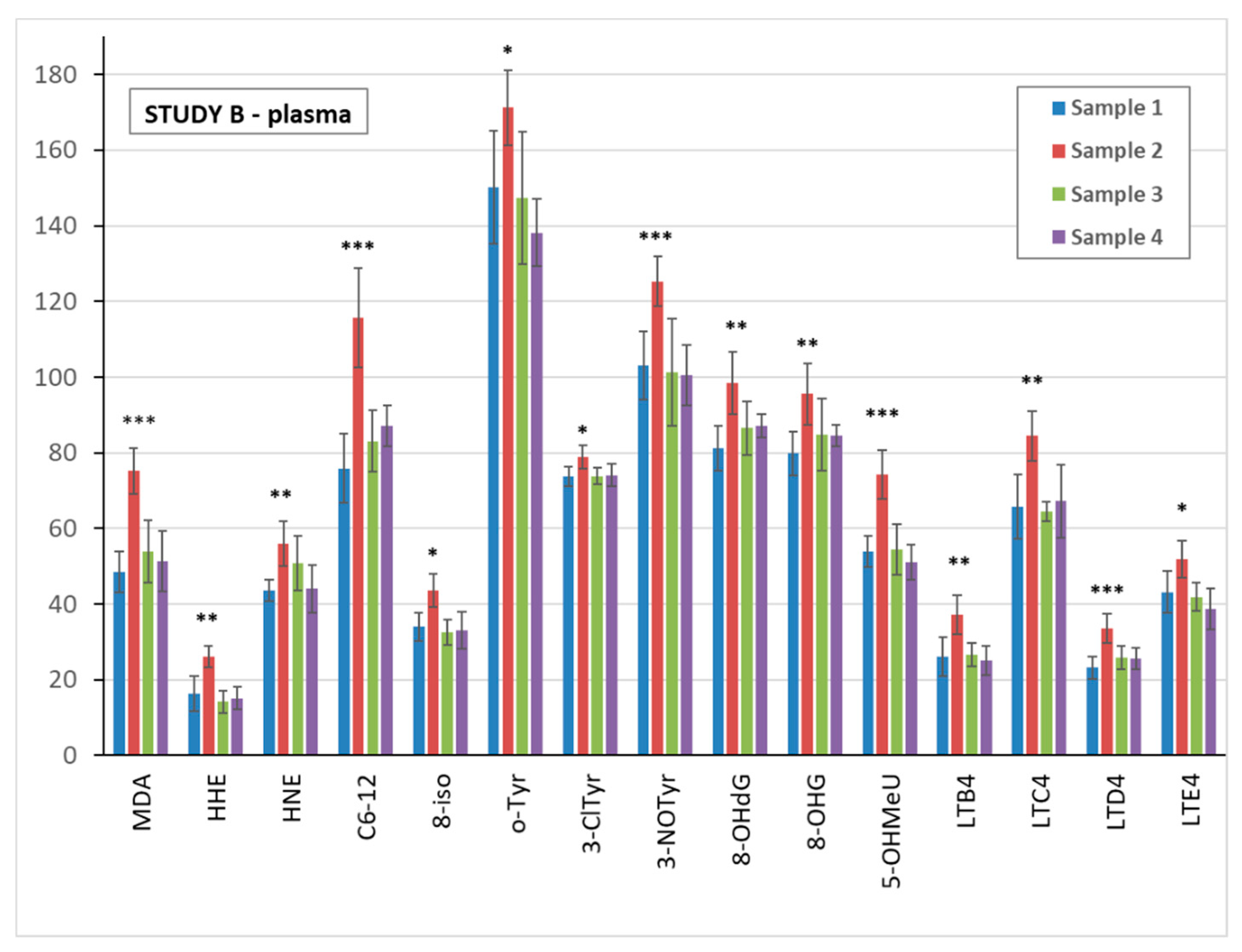
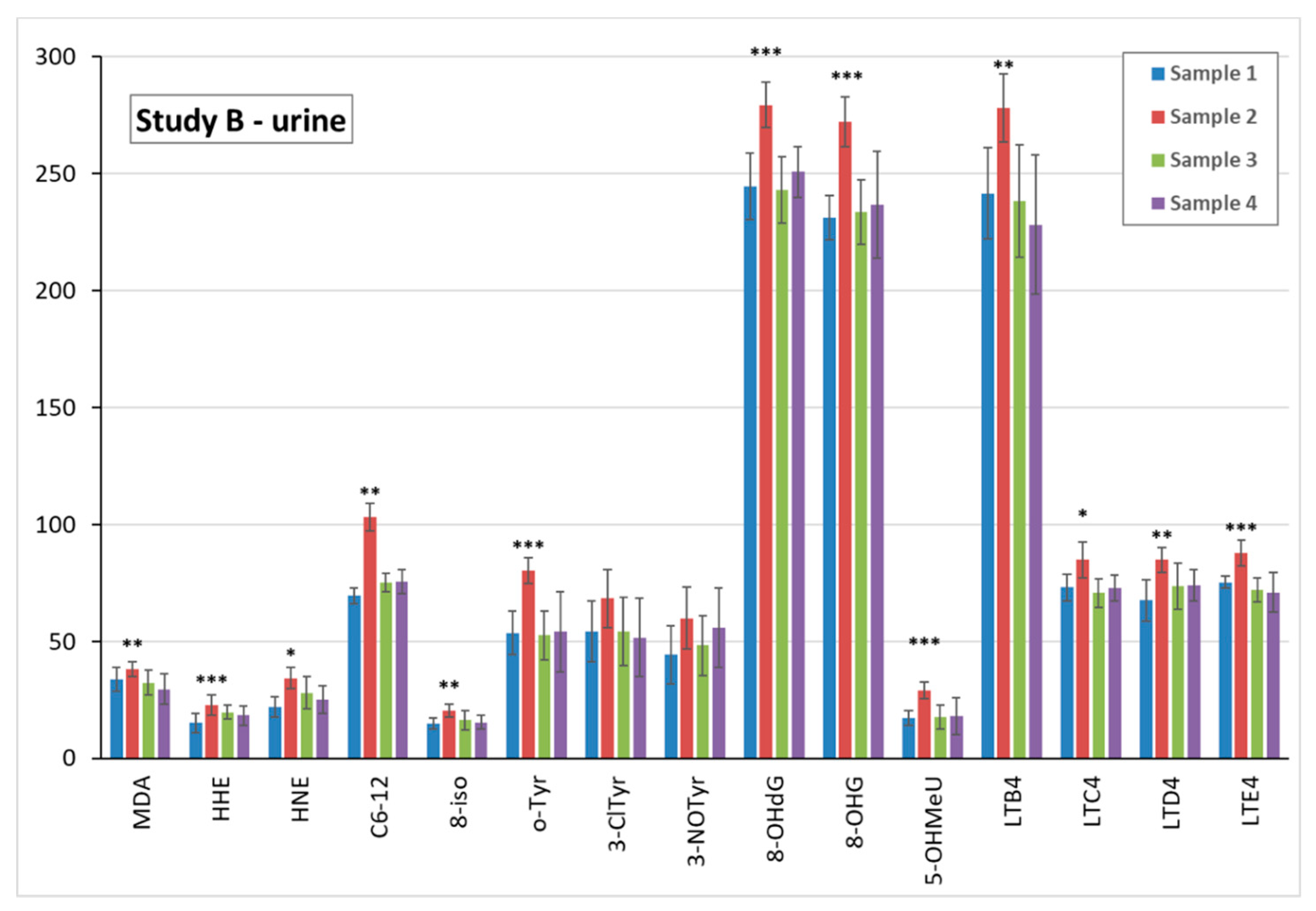
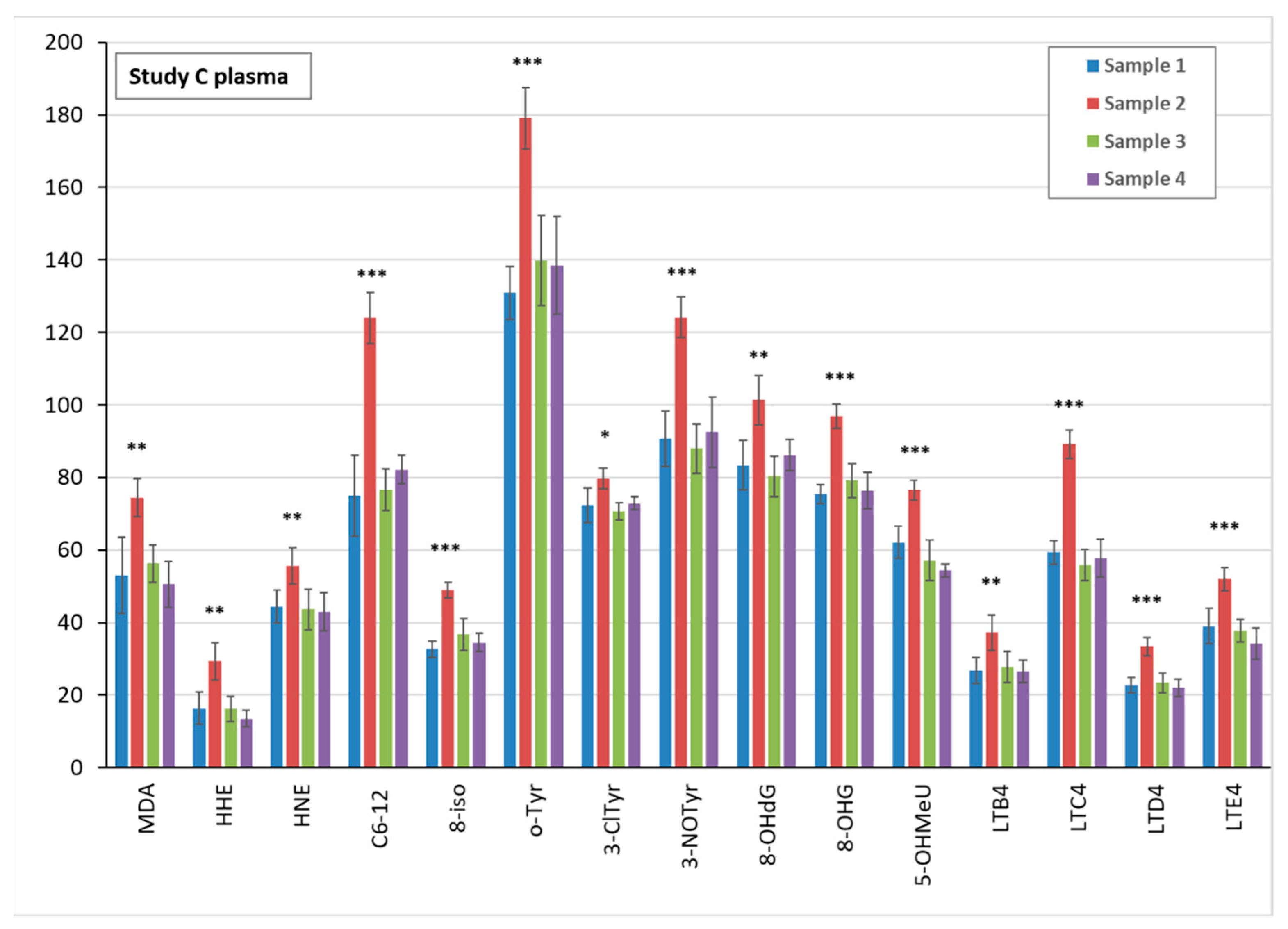
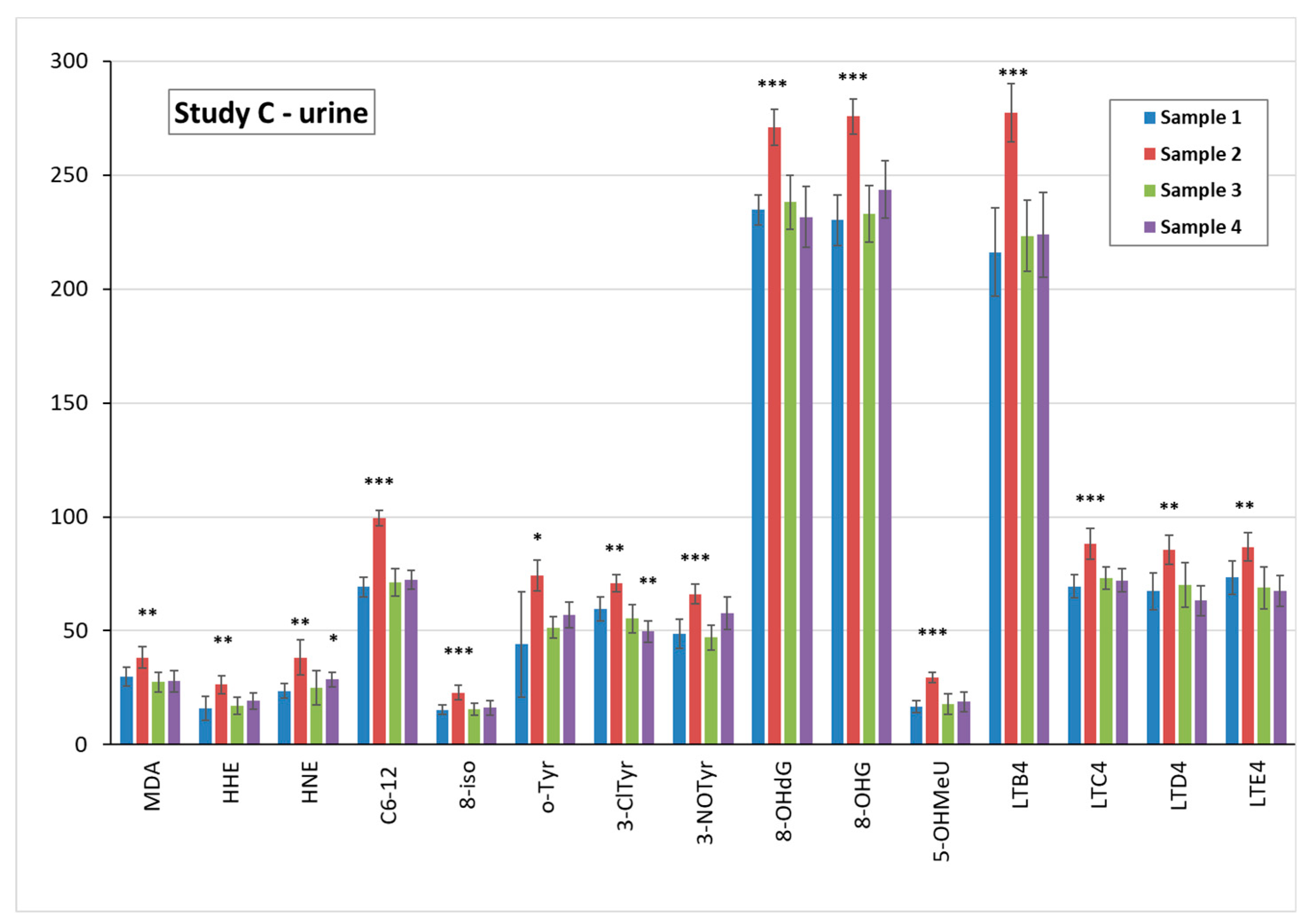
3.5. Markers of Oxidative Stress and Inflammation in Plasma, Urine, and EBC
3.6. TiO2 Concentration and Particle Analysis in Plasma, Spot Urine, and EBC
3.6.1. TiO2 Concentration in Biological Samples
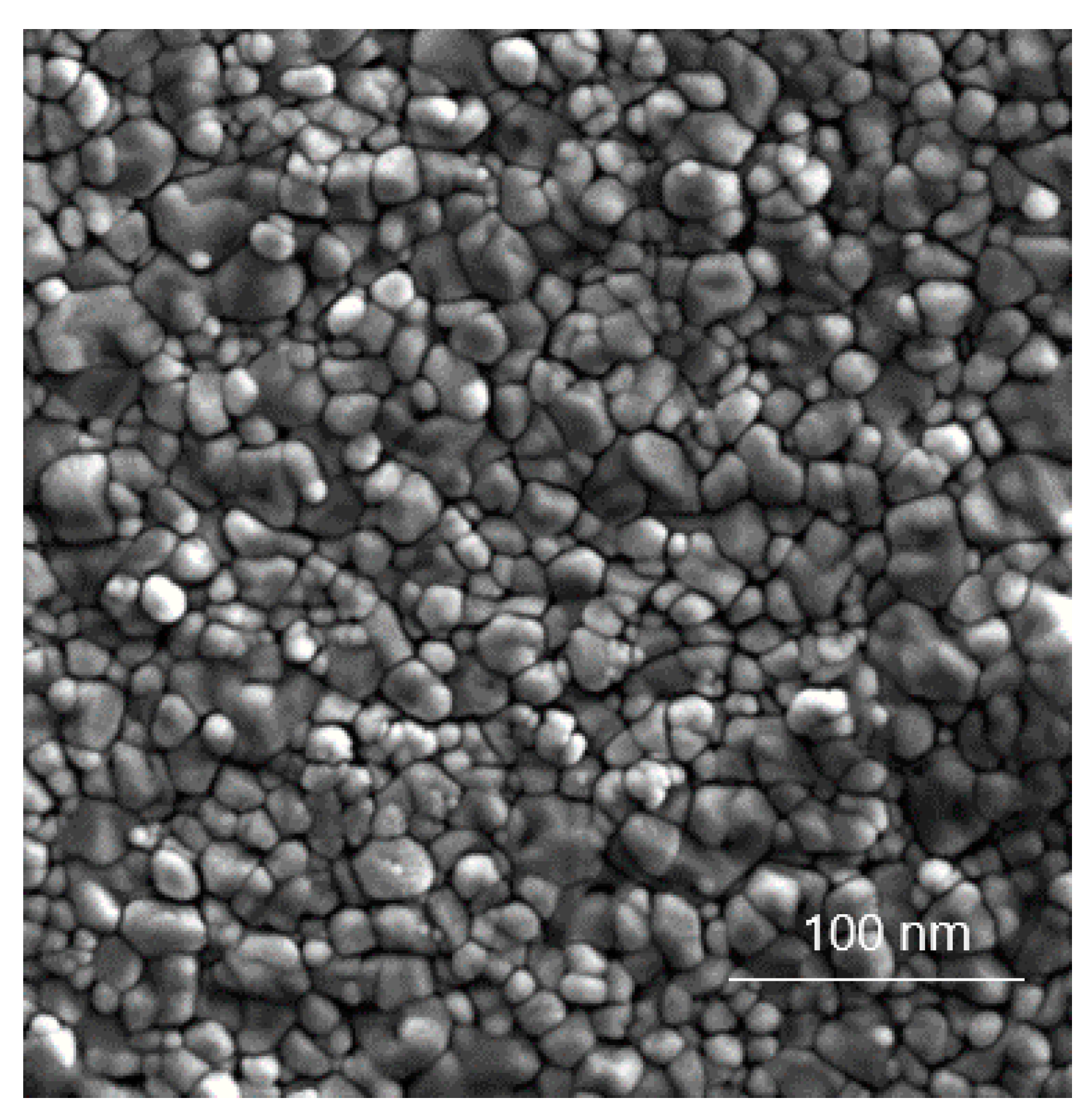
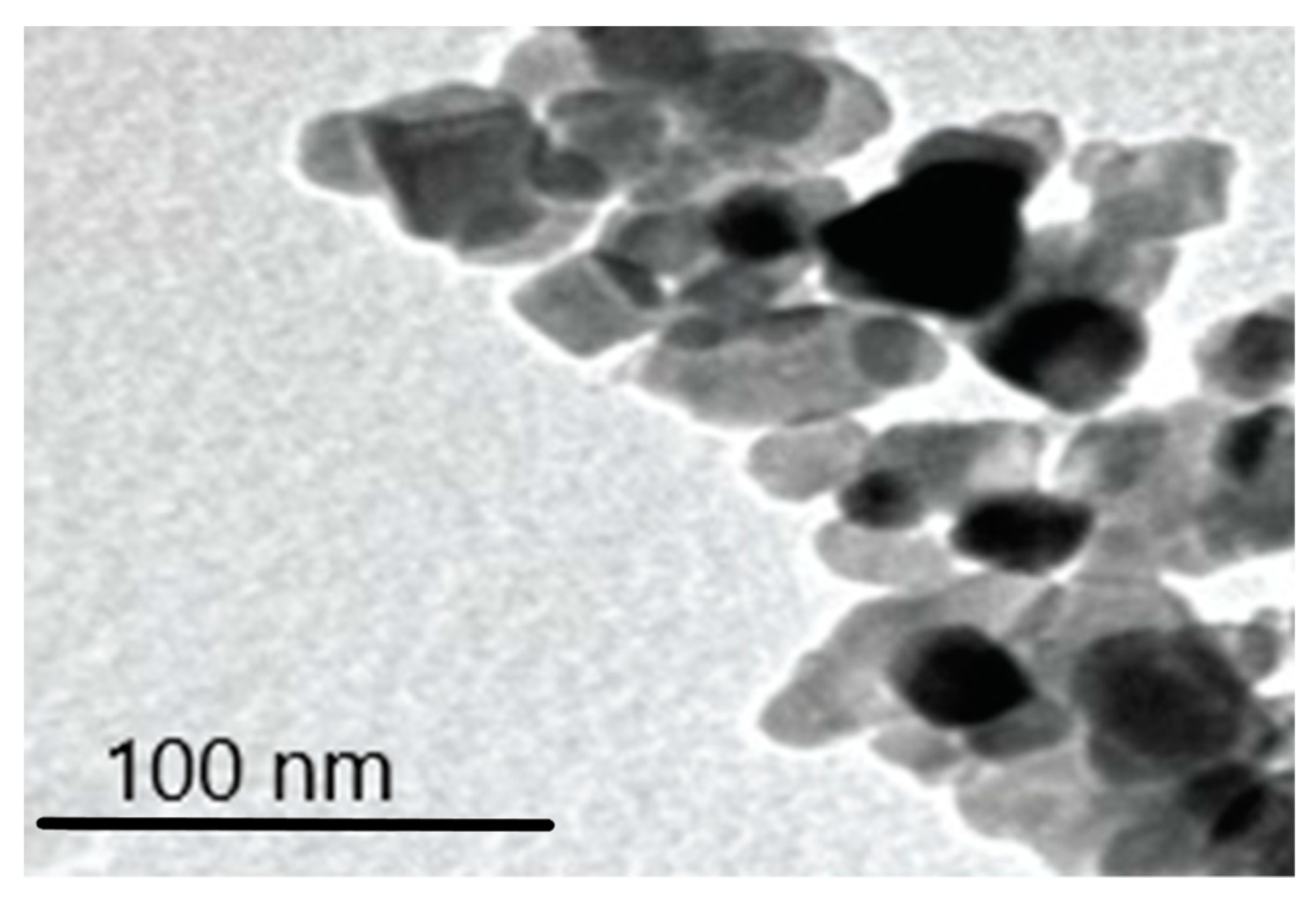
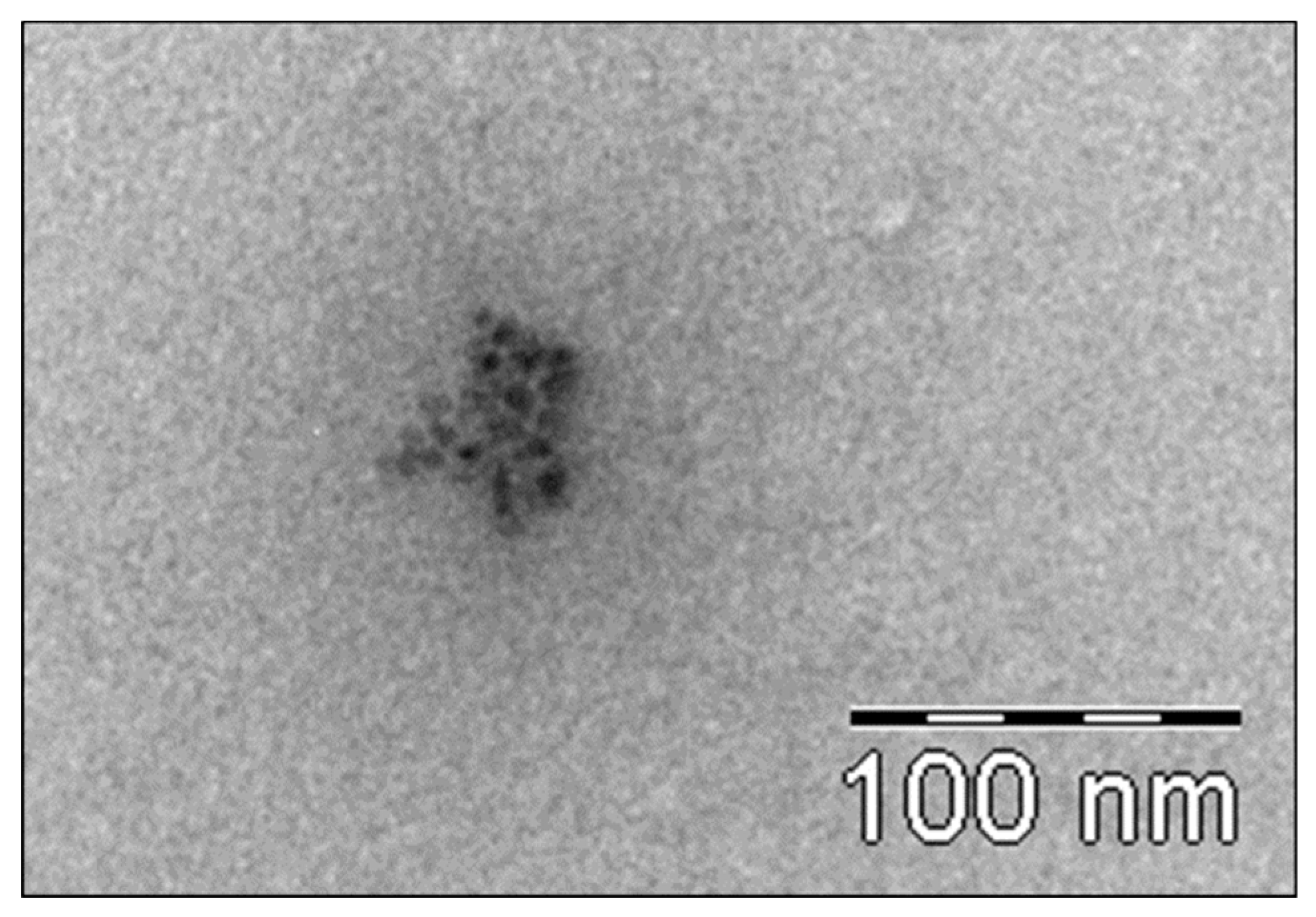
3.6.2. Transmission Electron Microscopy (TEM) Analysis of TiO2 Particles in Plasma, Urine, and EBC
3.7. FeNO
3.8. Spirometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsen, C.M.; Wilson, L.F.; Green, A.C.; Bain, C.J.; Fritschi, L.; Neale, R.E.; Whiteman, D.C. Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust. N. Z. J. Public Health 2015, 39, 471–476. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.C. The safety of nanoparticles in sunscreens: An update for general practice. Aust. Fam. Physician 2016, 45, 397–399. [Google Scholar] [PubMed]
- Westerdahl, J.; Ingvar, C.; Mâsbäck, A.; Olsson, H. Sunscreen use and malignant melanoma. Int. J. Cancer 2000, 871, 45–50. [Google Scholar] [CrossRef]
- Vainio, H.; Bianchini, F. Cancer-preventive effects of sunscreens are uncertain. Scand. J. Work Environ. Health 2000, 26, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Eide, M.J.; Weinstock, M.A. Public health challenges in sun protection. Dermatol. Clin. 2006, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Peira, E.; Corazzari, I.; Fenoglio, I.; Trotta, M.; Fubini, B. Crystalline phase modulates the potency of nanometric TiO2 to adhere to and perturb the stratum corneum of porcine skin under indoor light. Chem. Res. Toxicol. 2013, 26, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Kezic, S.; Crosera, M.; Svetličić, V.; Šegota, S.; Maina, G.; Romano, C.; Larese, F.; Adami, G. In vitro percutaneous penetration and characterization of silver from silver-containing textiles. Int. J. Nanomed. 2015, 10, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Agents Classified by the IARC Monographs, Volumes 1–123. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf (accessed on 15 January 2019).
- Tran, D.T.; Salmon, R. Potential photocarcinogenic effects of nanoparticle sunscreens. Australas. J. Dermatol. 2011, 52, 1–6. [Google Scholar] [CrossRef]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Barnard, I.R.M.; Tierney, P.; Campbell, C.L.; McMillan, L.; Moseley, H.; Eadie, E.; Brown, C.T.A.; Wood, K. Quantifying Direct DNA Damage in the Basal Layer of Skin Exposed to UV Radiation from Sunbeds. Photochem. Photobiol. 2018, 94, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Bloch, W.; Kopp, K.; Hainzl, A.; Rettberg, P.; Wlaschek, M.; Horneck, G.; Scharffetter-Kochanek, K. 8-Isoprostane is a dose-related biomarker for photo-oxidative ultraviolet (UV) B damage in vivo: A pilot study with personal UV dosimetry. Br. J. Dermatol. 2006, 154, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Bradna, P.; Ondrackova, L.; Zdimal, V.; Navratil, T.; Pelclova, D. Detection of nanoparticles released at finishing of dental composite materials. Monatsh. Chem. 2017, 148, 531–537. [Google Scholar] [CrossRef]
- Naess, E.M.; Hofgaard, A.; Skaug, V.; Gulbrandsen, M.; Danielsen, T.E.; Grahnstedt, S.; Skogstad, A.; Holm, J.Ø. Titanium dioxide nanoparticles in sunscreen penetrate the skin into viable layers of the epidermis: A clinical approach. Photodermatol. Photoimmunol. Photomed. 2016, 32, 48–51. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- IARC Vol. 93/2010. Available online: https://monographs.iarc.fr/list-of-classifications-volumes/ (accessed on 27 April 2019).
- Moriyama, A.; Yamada, I.; Takahashi, J.; Iwahashi, H. Oxidative stress caused by TiO2 nanoparticles under UV irradiation is due to UV irradiation not through nanoparticles. Chem. Biol. Interact. 2018, 294, 144–150. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.J.; Bowman, D.M.; Pinheiro, T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch. Toxicol. 2015, 89, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. 2017. Available online: https://www.tga.gov.au/literature-review-safety-titanium-dioxide-and-zinc-oxide-nanoparticles-sunscreens (accessed on 27 April 2019).
- Tan, M.H.; Commens, C.A.; Burnett, L.; Snitch, P.J. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas. J. Dermatol. 1996, 37, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Tsai, C.S.; Pelclova, D.; Schubauer-Berigan, M.K.; Schulte, P.A. Assessing the first wave of epidemiological studies of nanomaterial workers. J. Nanopart. Res. 2015, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Wu, W.T.; Liao, H.Y.; Chen, C.Y.; Tsai, C.Y.; Jung, W.T.; Lee, H.L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Chung, Y.T.; Lai, C.H.; Wang, S.L.; Chiang, H.C.; Li, L.A.; Tsou, T.C.; Li, W.F.; Lee, H.L.; Wu, W.T.; et al. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology 2014, 8 (Suppl. 1), 100–110. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Fenclova, Z.; Vlckova, S.; Komarc, M.; Navratil, T.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Leukotrienes in exhaled breath condensate and fractional exhaled nitric oxide in workers exposed to TiO2 nanoparticles. J. Breath Res. 2016, 10, 036004. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Komarc, M.; Vlckova, S.; Fenclova, Z.; Ondracek, J.; Schwarz, J.; Kostejn, M.; Kacer, P.; Dvorackova, S.; et al. Deep Airway Inflammation and Respiratory Disorders in Nanocomposite Workers. Nanomaterials 2018, 8, 731. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Fenclova, Z.; Vlckova, S.; Syslova, K.; Navratil, T.; Schwarz, J.; Zikova, N.; Barosova, H.; et al. Oxidative stress markers are elevated in exhaled breath condensate of workers exposed to nanoparticles during iron oxide pigment production. J. Breath Res. 2016, 10, 016004. [Google Scholar] [CrossRef]
- Zhang, R.; Dai, Y.; Zhang, X.; Niu, Y.; Meng, T.; Li, Y.; Duan, H.; Bin, P.; Ye, M.; Jia, X.; et al. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part. Fibre Toxicol. 2014, 11, 73. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, Y.C.; Shin, J.H.; Lee, J.H.; Lee, Y.; Park, S.Y.; Baek, J.E.; Park, J.D.; Ahn, K.; Yu, I.J. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 2015, 9, 802–811. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occup. Environ. Med. 2016, 73, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Zikova, N.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Makes, O.; Syslova, K.; et al. Markers of lipid oxidative damage in the exhaled breath condensate of nanoTiO2 production workers. Nanotoxicology 2017, 11, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y.; et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Schwarz, J.; Dvorackova, S.; Komarc, M.; Ondracek, J.; Kostejn, M.; Kacer, P.; Vlckova, S.; Fenclova, Z.; et al. Markers of oxidative stress in the exhaled breath condensate of workers handling nanocomposites. Nanomaterials 2018, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Archer, S.; Fagan, K.; Hassoun, P.M.; Hill, N.S.; Humbert, M.; Kawut, S.M.; Krowka, M.; et al. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Syslova, K.; Böhmova, A.; Mikoska, M.; Kuzma, M.; Pelclova, D.; Kacer, P. Multimarker screening of oxidative stress in aging. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef]
- Peters-Golden, M. Expanding roles for leukotrienes in airway inflammation. Curr. Allergy Asthma Rep. 2008, 8, 367–373. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Vlckova, S.; Fenclova, Z.; Navratil, T.; Komarc, M.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of nucleic acids and proteins oxidation among office workers exposed to air pollutants including (nano)TiO2 particles. Neuro Endocrinol. Lett. 2016, 37 (Suppl. 1), 3–16. [Google Scholar]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Zikova, N.; Schwarz, J.; Makes, O.; Navratil, T.; et al. Markers of lipid oxidative damage among office workers exposed intermittently to air pollutants including nanoTiO2 particles. Rev. Environ. Health 2017, 32, 193–200. [Google Scholar] [CrossRef]
- Tiano, L.; Armeni, T.; Venditti, E.; Barucca, G.; Mincarelli, L.; Damiani, E. Modified TiO2 particles differentially affect human skin fibroblasts exposed to UVA light. Free Radic. Biol. Med. 2010, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.M.; Biller, J.; Foss, B.; Hoagland, K.; Dunning, M.B.; Castillo, D.; Bosbous, M.; Sun, F.; Shaker, R. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am. J. Respir. Crit. Care Med. 2003, 168, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Klusackova, P.; Lebedova, J.; Kacer, P.; Kuzma, M.; Brabec, M.; Pelclova, D.; Fenclova, Z.; Navratil, T. Leukotrienes and 8-isoprostane in exhaled breath condensate in bronchoprovocation tests with occupational allergens. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 281–292. [Google Scholar] [CrossRef]
- Syslova, K.; Kacer, P.; Kuzma, M.; Klusackova, P.; Fenclova, Z.; Lebedova, J.; Pelclova, D. Determination of 8-iso-prostaglandin F(2α) in exhaled breath condensate using combination of immunoseparation and LC-ESI-MS/MS. J. Chromatogr. B 2008, 867, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Syslova, K.; Kacer, P.; Kuzma, M.; Pankracova, A.; Fenclova, Z.; Vlckova, S.; Lebedova, J.; Pelclova, D. LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J. Breath Res. 2010, 4, 017104. [Google Scholar] [CrossRef] [PubMed]
- Neprasova, M.; Maixnerova, D.; Novak, J.; Reily, C.; Julian, B.A.; Boron, J.; Novotny, P.; Suchanek, M.; Tesar, V.; Kacer, P. Toward noninvasive diagnosis of IgA nephropathy: A pilot urinary metabolomic and proteomic study. Dis. Markers 2016, 2016, 3650909. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 51. [Google Scholar] [CrossRef]
- Pele, L.C.; Thoree, V.; Bruggraber, S.F.; Koller, D.; Thompson, R.P.; Lomer, M.C.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015, 12, 26. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef]
- Gulson, B.; Wong, H.; Korsch, M.; Gomez, L.; Casey, P.; McCall, M.; McCulloch, M.; Trotter, J.; Stauber, J.; Greenoak, G. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci. Total Environ. 2012, 420, 313–318. [Google Scholar] [CrossRef]
- Giacomoni, P.U.; Mammone, T.; Teri, M.J. Gender-linked differences in human skin. Dermatol. Sci. 2009, 55, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Barosova, H.; Kukutschova, J.; Zdimal, V.; Navratil, T.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Zikova, N.; Kacer, P.; et al. Raman microspectroscopy of exhaled breath condensate and urine in workers exposed to fine and nanoTiO2 particles: A cross-sectional study. J. Breath Res. 2015, 9, 036008. [Google Scholar] [CrossRef] [PubMed]
- Nizamov, S.; Scherbahn, V.; Mirsky, V.M. Detection and quantification of single engineered nanoparticles in complex samples using template matching in wide-field surface plasmon microscopy. Anal. Chem. 2016, 88, 10206–10214. [Google Scholar] [CrossRef] [PubMed]
- Lischkova, L.; Pelclova, D.; Hlusicka, J.; Navratil, T.; Vlckova, S.; Fenclova, Z.; Dvorackova, S.; Popov, A.; Michalcova, A.; Marek, I.; et al. Detection and identification of engineered nanoparticles in exhaled breath condensate, blood serum, and urine of occupationally exposed subjects. Monatsh. Chem. 2019, 150, 511–523. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Celleno, L.; Chaudhry, Q.M.; Coenraads, P.J.; Dusinska, M.; Gaffet, E.; Galli, C.L.; Panteri, W.; Rousselle, C.; et al. SCCS OPINION ON Titanium Dioxide (Nano Form) as UV-Filter in Sprays—SCCS/1583/17; European Union: Brussels, Belgium, 2018; Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/index_en.htm (accessed on 27 April 2019).
- Pelcl, T.; Skrha, J.; Soupal, J.; Flekac, M.; Kacer, P.; Skrha, J.; Navratil, T.; Prazny, M. Lipid peroxidation and impaired vascular function in patients with type 1 diabetes mellitus. Monatsh. Chem. 2019, 150, 525–529. [Google Scholar] [CrossRef]
- Pelclova, D.; Fenclova, Z.; Kacer, P.; Kuzma, M.; Navratil, T.; Lebedova, J. 8-isoprostane and leukotrienes in exhaled breath condensate in Czech subjects with silicosis. Ind. Health 2007, 45, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Fenclova, Z.; Kacer, P.; Kuzma, M.; Navratil, T.; Lebedova, J. 2008 Increased 8-isoprostane, a marker of oxidative stress in exhaled breath condensate in subjects with asbestos exposure. Ind. Health 2008, 46, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Navratil, T.; Vlckova, S.; Fenclova, Z.; Pelcl, T.; Kacerova, T.; Kacer, P. Exhaled breath condensate biomarkers reflect systemic changes in patients with chronic dioxin intoxication. Monatsh. Chem. 2018, 149, 1579–1586. [Google Scholar] [CrossRef]
- Chézeau, L.; Sébillaud, S.; Safar, R.; Seidel, C.; Dembélé, D.; Lorcin, M.; Langlais, C.; Grossmann, S.; Nunge, H.; Michaux, S.; et al. Short- and long-term gene expression profiles induced by inhaled TiO2 nanostructured aerosol in rat lung. Toxicol. Appl. Pharmacol. 2018, 356, 54–64. [Google Scholar] [CrossRef]
- Rollerova, E.; Tulinska, J.; Liskova, A.; Kuricova, M.; Kovriznych, J.; Mlynarcikova, A.; Kiss, A.; Scsukova, S. Titanium dioxide nanoparticles: Some aspects of toxicity/focus on the development. Endocr. Regul. 2015, 49, 97–112. [Google Scholar] [CrossRef]

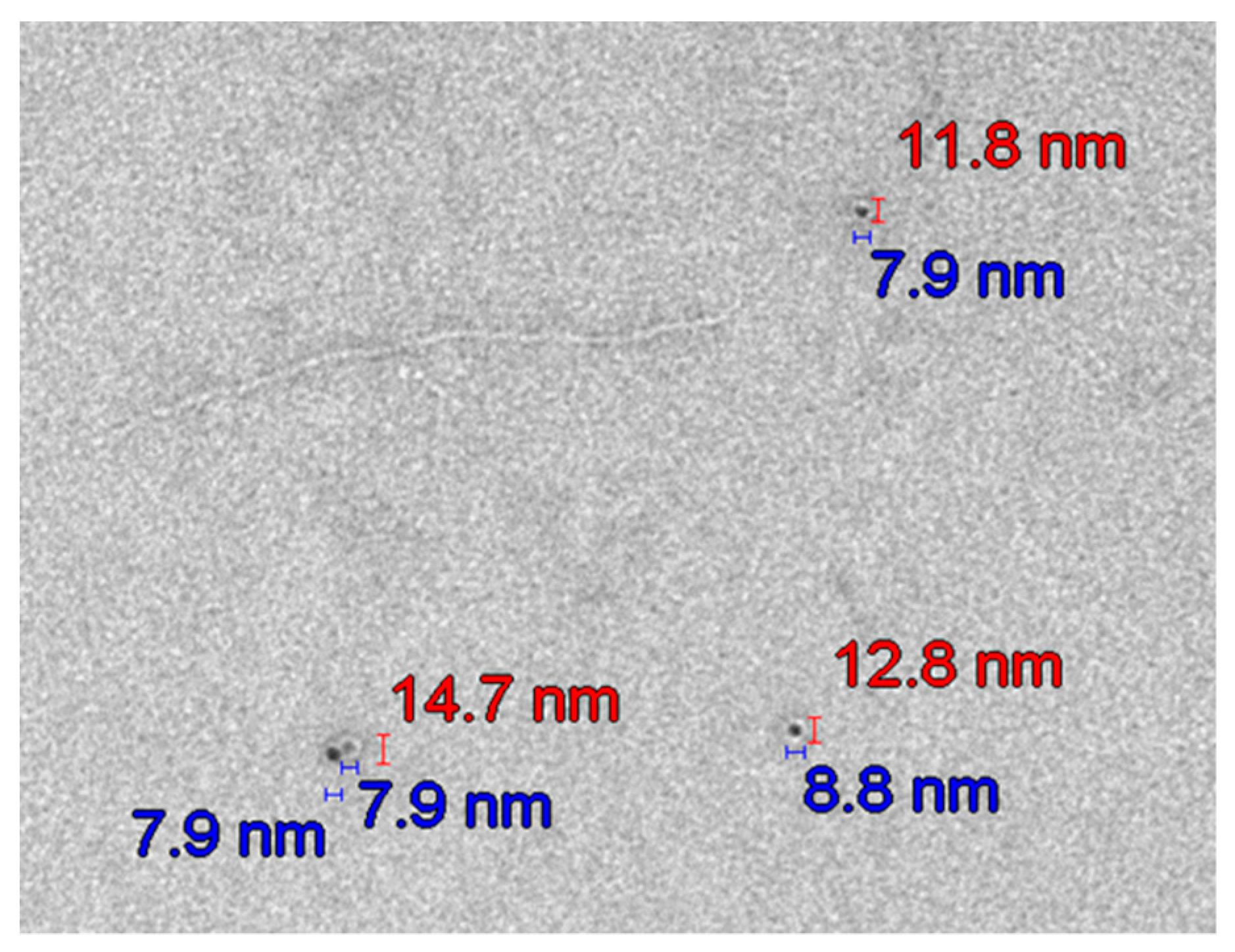
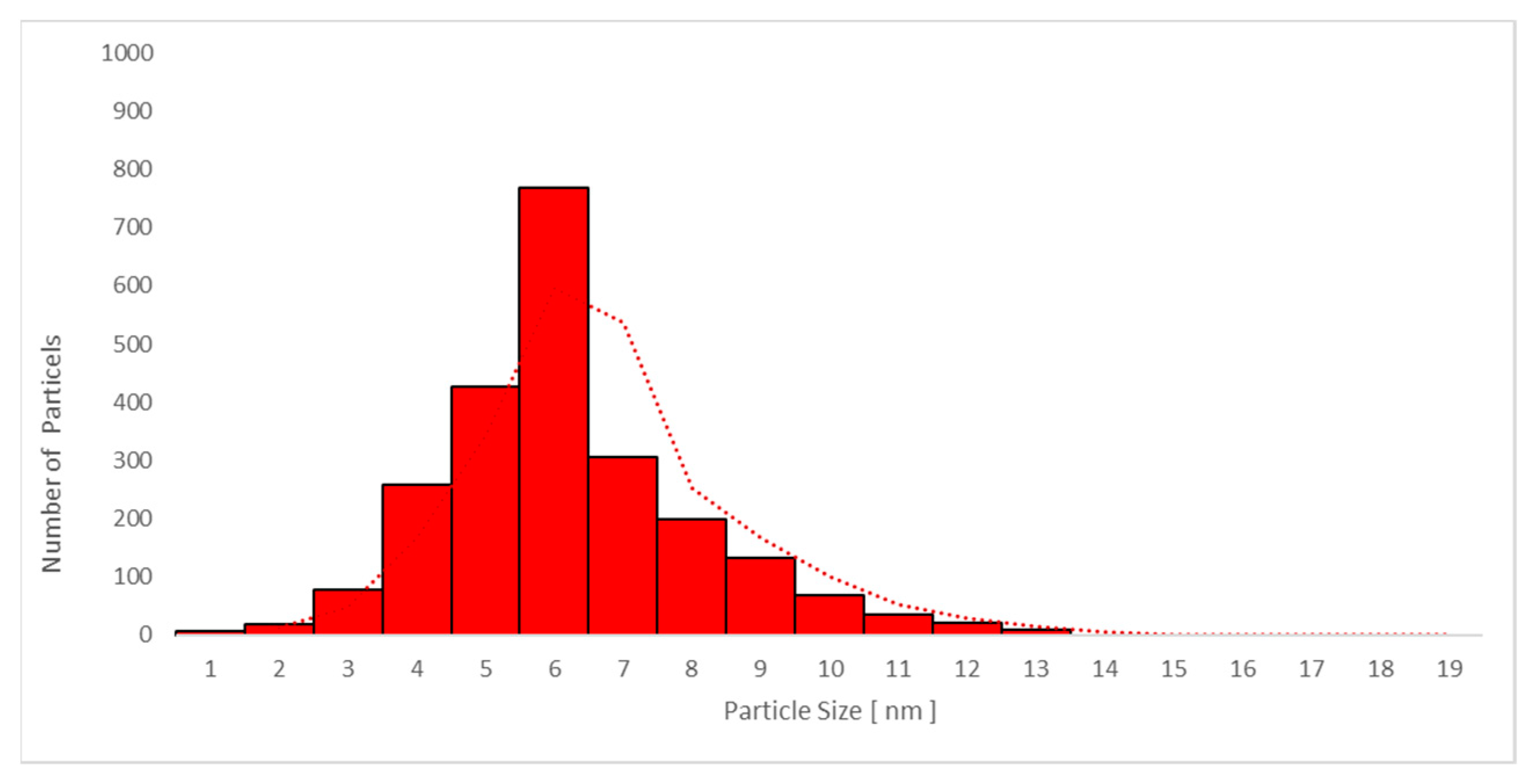
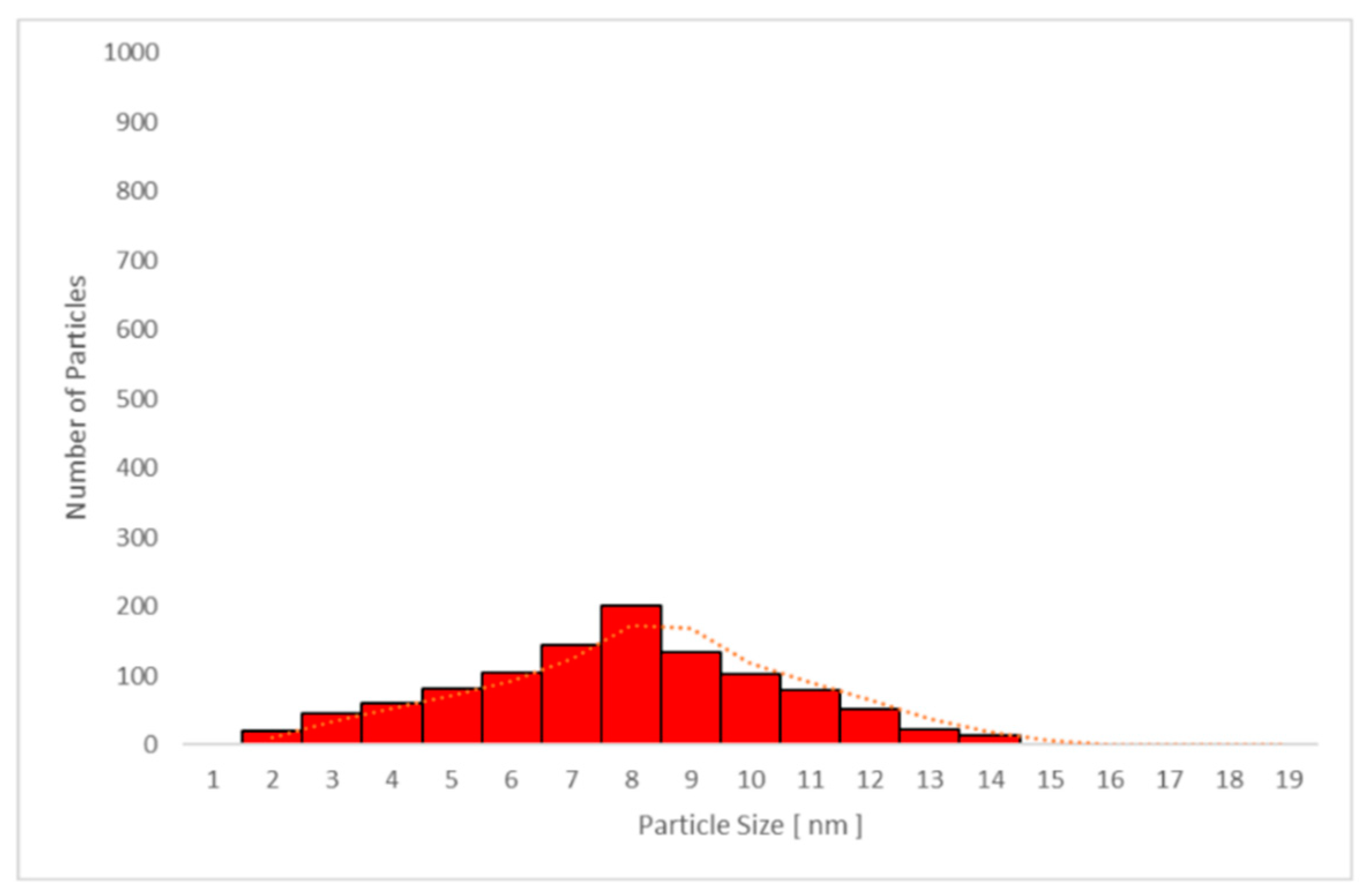
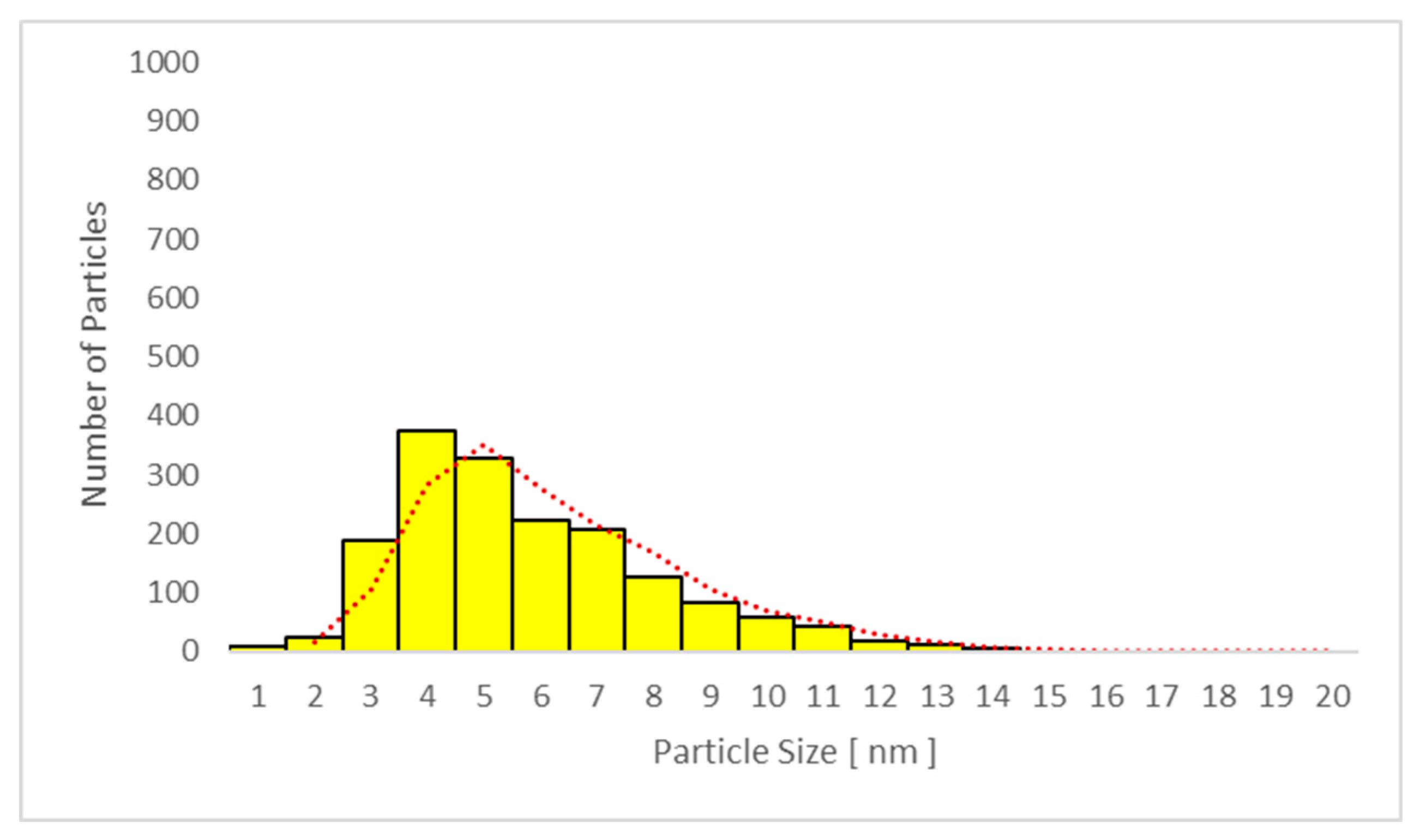
| Day 1 | Day 3 | Day 4 | Day 11 | |||
|---|---|---|---|---|---|---|
| Sample | Sample 1 Pre-test (time) | Tests (time) | Sample 2 (time) | Tests (time) | Sample 3 (time) | Sample 4 Post-test (time) |
| Study A Sunscreen only (December 2014) | 8:00 | 9:00 Sunscreen continuously | 14:00 | Sunscreen continuously | 8:00 (8:30 Sunscreen washed off) | 8:00 |
| Study B UV only (June 2015) | 8:00 | 11:30 UV 1 (8 min) | 14:00 | 11:30 UV 2 (8 min) | 8:00 | 8:00 |
| Study C Sunscreen + UV (March 2015) | 8:00 | 9:00 Sunscreen continuously | 14:00 | Sunscreen continuously | 8:00 (8:30 Sunscreen washed off) | 8:00 |
| 11:30 UV 1 (8 min) | 11:30 UV 2 (8 min) | |||||
| Sample No/Group | TiO2 Plasma (ng/mL) | TiO2 Urine (ng/mL) | TiO2 EBC (ng/mL) |
|---|---|---|---|
| 1 All subjects | <LOD | <LOD | <LOD |
| 2 Men | <LOD | <LOD | <LOD |
| 2 Women | 9 | 5.8 | <LOD |
| 3 Men | 6.8 | 6.9 | <LOD |
| 3 Women | 12.8 | 9.3 | <LOD |
| 4 Men | 5.1 | 4.5 | <LOD |
| 4 Women | 10.9 | 7.2 | <LOD |
| All samples Mean ± SD | 8.9 ± 2.8 | 6.7 ± 1.6 | <LOD |
| Sample No/Group | TiO2 Plasma (ng/mL) | TiO2 Urine (ng/mL) | TiO2 EBC(ng/mL) |
|---|---|---|---|
| All subjects | <LOD | <LOD | <LOD |
| 2 Men | <LOD | <LOD | <LOD |
| 2 Women | 11.6 | 5.5 | <LOD |
| 3 Men | 6.7 | 7.2 | <LOD |
| 3 Women | 14.1 | 9.3 | <LOD |
| 4 Men | 4.7 | 4.4 | <LOD |
| 4 Women | 10.7 | 6.8 | <LOD |
| All samples Mean ± SD | 9.6 ± 3.4 | 6.6 ± 1.7 | <LOD |
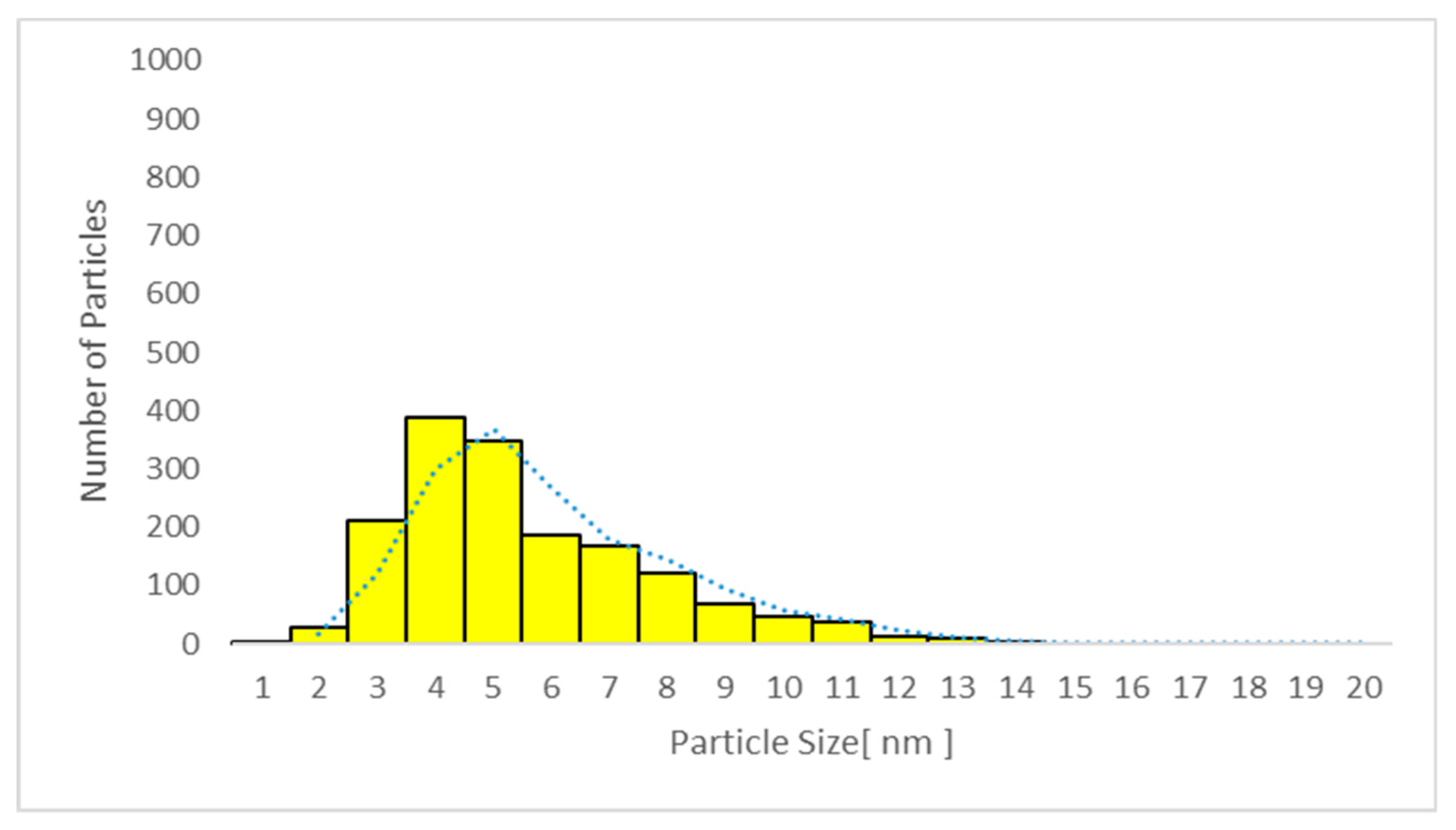
| Sample No/Group | Average Particle Size TiO2 Plasma (nm) | Average Particle Size TiO2 Urine (nm) | Average Particle Size TiO2 EBC (nm) |
|---|---|---|---|
| 1 All subjects | <LOD | <LOD | <LOD |
| 2 Men | <LOD | <LOD | <LOD |
| 2 Women | 6.5 | 5.4 | <LOD |
| 3 Men | 7.2 | 5.2 | <LOD |
| 3 Women | 7.5 | 6.1 | <LOD |
| 4 Men | 7.6 | 5.6 | <LOD |
| 4 Women | 8.4 | 6.7 | <LOD |
| All samples Mean ± SD | 7.4 ± 0.6 | 5.8 ± 0.5 | <LOD |
| Sample No/Group | TiO2 Plasma (nm) | TiO2 Urine (nm) | TiO2 EBC (nm) |
|---|---|---|---|
| 1 All subjects | <LOD | <LOD | <LOD |
| 2 Men | <LOD | <LOD | <LOD |
| 2 Women | 6.1 | 5.2 | <LOD |
| 3 Men | 6.8 | 5.0 | <LOD |
| 3 Women | 7.2 | 5.8 | <LOD |
| 4 Men | 7.4 | 5.4 | <LOD |
| 4 Women | 8.2 | 6.4 | <LOD |
| All samples Mean ± SD | 7.1 ± 0.7 | 5.6 ± 0.5 | <LOD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials 2019, 9, 888. https://doi.org/10.3390/nano9060888
Pelclova D, Navratil T, Kacerova T, Zamostna B, Fenclova Z, Vlckova S, Kacer P. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials. 2019; 9(6):888. https://doi.org/10.3390/nano9060888
Chicago/Turabian StylePelclova, Daniela, Tomas Navratil, Tereza Kacerova, Blanka Zamostna, Zdenka Fenclova, Stepanka Vlckova, and Petr Kacer. 2019. "NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans" Nanomaterials 9, no. 6: 888. https://doi.org/10.3390/nano9060888
APA StylePelclova, D., Navratil, T., Kacerova, T., Zamostna, B., Fenclova, Z., Vlckova, S., & Kacer, P. (2019). NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans. Nanomaterials, 9(6), 888. https://doi.org/10.3390/nano9060888