Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach
Abstract
1. Introduction
2. Thermosensitive DDS
2.1. In Situ Thermosensitive Hydrogels
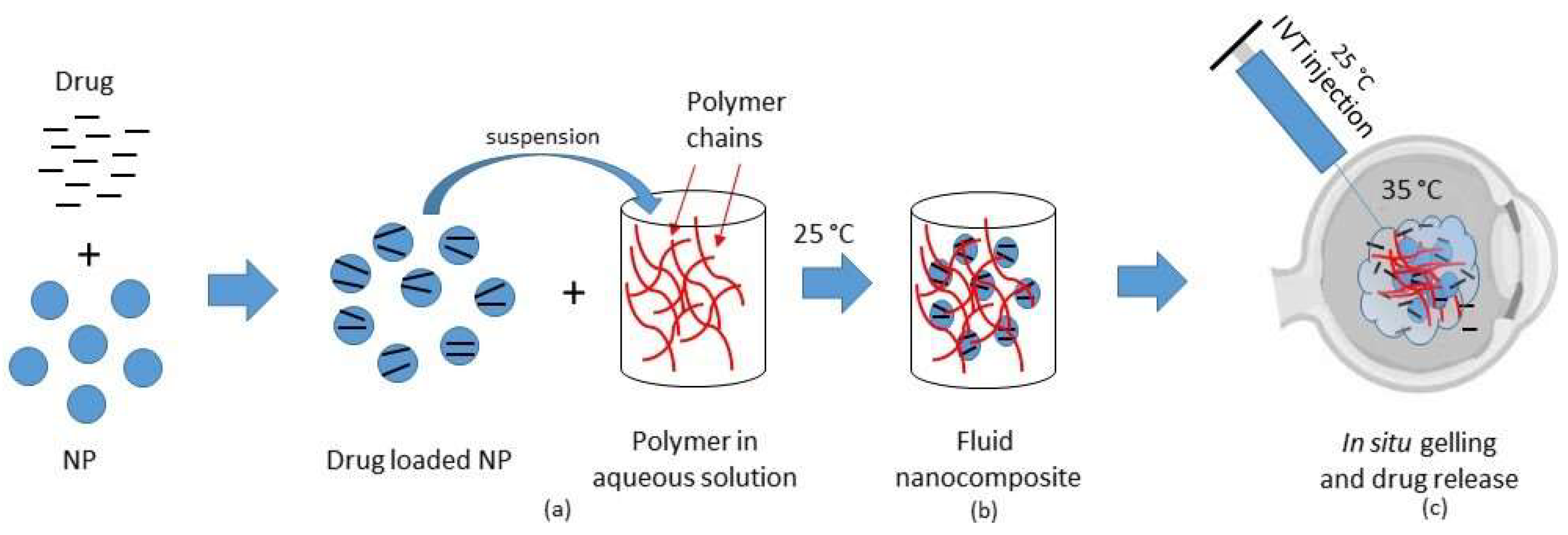
2.2. Thermosensitive Composites
2.3. Thermosensitive Devices
3. In Vitro and In Silico Models to Test Intraocular DDS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, S.S.; Denham, L.V.; Elison, J.R.; Bhattacharjee, P.S.; Huq, T.; Clement, C.; Hill, J.M. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev. Ophthalmol. 2010, 5, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Hughes, P.M.; Robinson, M.R. Recent advances in drug delivery systems for treating ocular complications of systemic diseases. Curr. Opin. Ophthalmol. 2009, 20, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- Oelker, A.M.; Grinstaff, M.W. Ophthalmic adhesives: A materials chemistry perspective. J. Mater. Chem. 2008, 18, 2521–2536. [Google Scholar] [CrossRef]
- Swindle, K.E.; Ravi, N. Recent advances in polymeric vitreous substitutes. Expert Rev. Ophthalmol. 2007, 2, 255–265. [Google Scholar] [CrossRef]
- Wang, K.; Han, Z. Injectable hydrogels for ophthalmic applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef]
- Kozak, I.; Cheng, L.; Freeman, W.R. Lidocaine gel anesthesia for intravitreal drug administration. Retina 2005, 25, 994–998. [Google Scholar] [CrossRef]
- Shah, H.R.; Reichel, E.; Busbee, B.G. A novel lidocaine hydrochloride ophthalmic gel for topical ocular anesthesia. Local Reg. Anesth. 2010, 3, 57–63. [Google Scholar]
- Ho, V.Y.; Shah, G.K.; Liu, E.M. Resure sealant for pars plana vitrectomy wound closure. OSLI Retina 2015, 46, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Gause, S.; Chauhan, A. Review of ophthalmic drug delivery by contact lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic Vis. Res. 2011, 6, 317–329. [Google Scholar] [PubMed]
- Alhalafi, A.M. Applications of polymers in intraocular drug delivery systems. Oman J. Ophthalmol. 2017, 10, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Vanrell, R.; Refojo, M.F. Biodegradable microspheres for vitreoretinal drug delivery. Adv. Drug Deliv. Rev. 2001, 52, 5–16. [Google Scholar] [CrossRef]
- Saliba, J.B.; Faraco, A.A.G.; Yoshida, M.I.; de Vasconcelos, W.L.; da Silva-Cunha, A.; Mansur, H.S. Development and characterization of an intraocular biodegradable polymer system containing cyclosporine-a for the treatment of posterior uveitis. Mater. Res. 2008, 11, 207–211. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone acetonide implant (retisert) for noninfectious posterior uveitis. Thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Control. Release 2014, 196, 208–221. [Google Scholar] [CrossRef]
- Joseph, M.; Trinh, H.M.; Cholkar, K.; Pal, D.; Mitra, A.K. Recent perspectives on the delivery of biologics to back of the eye. Expert Opin. Drug Deliv. 2017, 14, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Zirpel, J.J.; Gerhardt, C.; Pfister, I.B. The fate of eyes with wet amd beyond four years of anti-vegf therapy. Graefe Arch. Clin. Exp. Ophthalmol. 2018, 256, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.A.; Evans, J.B.; Bielory, L. Wet amd market. Nat. Rev. Drug Discov. 2012, 11, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Cundy, O.; Shah, M.; Downes, S.M. Intravitreal aflibercept: Its role in treatment of neovascular age-related macular degeneration. Expert Rev. Ophthalmol. 2018, 13, 75–86. [Google Scholar] [CrossRef]
- Jo, N.; Mailhos, C.; Ju, M.; Cheung, E.; Bradley, J.; Nishijima, K.; Robinson, G.S.; Adamis, A.P.; Shima, D.T. Inhibition of platelet-derived growth factor b signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am. J. Pathol. 2006, 168, 2036–2053. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Mrejen, S.; Gallego-Pinazo, R. An update on the pharmacotherapy of neovascular age-related macular degeneration. Expert Opin. Pharmacother. 2013, 14, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T., Jr. Risks of intravitreous injection: A comprehensive review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Hsu, J. Drug delivery methods for posterior segment disease. Curr. Opin. Ophthalmol. 2007, 18, 235–239. [Google Scholar] [CrossRef]
- Choonara, Y.E.; Pillay, V.; Danckwerts, M.P.; Carmichael, T.R.; Du Toit, L.C. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J. Pharm. Sci. 2010, 99, 2219–2239. [Google Scholar] [CrossRef]
- Kompella, U.B.; Amrite, A.C.; Pacha Ravi, R.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Ret. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef]
- Moinard-Checot, D.; Chevalier, Y.; Briançon, S.; Fessi, H.; Guinebretière, S. Nanoparticles for drug delivery: Review of the formulation and process difficulties illustrated by the emulsion-diffusion process. J. Nanosci. Nanotechnol. 2006, 6, 2664–2681. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Ocular drug delivery: Nanomedicine applications. Nanomedicine 2007, 2, 11–21. [Google Scholar] [CrossRef]
- Booth, B.A.; Denham, L.V.; Bouhanik, S.; Jacob, J.T.; Hill, J.M. Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: Present and future applications. Drugs Aging 2007, 24, 581–602. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef]
- Irache, J.M.; Merodio, M.; Arnedo, A.; Camapanero, M.A.; Mirshahi, M.; Espuelas, S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev. Med. Chem. 2005, 5, 293–305. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular drug delivery—New strategies for targeting anterior and posterior segments of the eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef]
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.; Fresta, M. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of peg coating on ocular drug bioavailability. Pharm. Res. 2003, 20, 584–590. [Google Scholar] [CrossRef]
- Das, S.K.; Tucker, I.G.; Hill, D.J.; Ganguly, N. Evaluation of poly(isobutylcyanoacrylate) nanoparticles for mucoadhesive ocular drug delivery. I. Effect of formulation variables on physicochemical characteristics of nanoparticles. Pharm. Res. 1995, 12, 534–540. [Google Scholar] [CrossRef]
- Vega, E.; Egea, M.A.; Valls, O.; Espina, M.; Garcia, M.L. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J. Pharm. Sci. 2006, 95, 2393–2405. [Google Scholar] [CrossRef]
- Calvo, P.; Sánchez, A.; Martínez, J.; López, M.I.; Calonge, M.; Pastor, J.C.; Alonso, M.J. Polyester nanocapsules as new topical ocular delivery systems for cyclosporin a. Pharm. Res. 1996, 13, 311–315. [Google Scholar] [CrossRef]
- Rawas-Qalaji, M.; Williams, C.A. Advances in ocular drug delivery. Curr. Eye Res. 2012, 37, 345–356. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Chen, P.C.; Kohane, D.S.; Park, Y.J.; Bartlett, R.H.; Langer, R.; Yang, V.C. Injectable microparticle-gel system for prolonged and localized lidocaine release. Ii. In vivo anesthetic effects. J. Biomed. Mater. Res. A 2004, 70, 459–466. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Shastri, D.H.; Patel, L.D.; Parikh, R.K. Studies on in situ hydrogel: A smart way for safe and sustained ocular drug delivery. J. Young Pharm. 2010, 2, 116–120. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Del Fante, C.; Perotti, C.; Caramella, C. Thermosensitive eyedrops containing platelet lysate for the treatment of corneal ulcers. Int. J. Pharm. 2012, 426, 1–6. [Google Scholar] [CrossRef]
- Cho, I.S.; Park, C.G.; Huh, B.K.; Cho, M.O.; Khatun, Z.; Li, Z.; Kang, S.W.; Choy, Y.B.; Huh, K.M. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016, 39, 124–132. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly(n-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Lima, L.H.; Morales, Y.; Cabral, T. Ocular biocompatibility of poly-n-isopropylacrylamide (pnipam). J. Ophthalmol. 2016, 2016, 5356371. [Google Scholar] [CrossRef]
- Dhara, D.; Chatterji, P.R. Phase transition in linear and cross-linked poly(n-isopropylacrylamide) in water: Effect of various types of additives. J. Macromol. Sci. Polymer Rev. 2000, 40, 51–68. [Google Scholar] [CrossRef]
- Kang Derwent, J.J.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–213; discussion 213–204. [Google Scholar]
- Drapala, P.W.; Brey, E.M.; Mieler, W.F.; Venerus, D.C.; Kang Derwent, J.J.; Perez-Luna, V.H. Role of thermo-responsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(n-isopropylacrylamide) hydrogels. J. Biomater. Sci. Polym. Ed. 2011, 22, 59–75. [Google Scholar] [CrossRef]
- Fitzpatrick, S.D.; Jafar Mazumder, M.A.; Muirhead, B.; Sheardown, H. Development of injectable, resorbable drug-releasing copolymer scaffolds for minimally invasive sustained ophthalmic therapeutics. Acta Biomater. 2012, 8, 2517–2528. [Google Scholar] [CrossRef]
- Prosperi-Porta, G.; Muirhead, B.; Sheardown, H. Tunable release of ophthalmic therapeutics from injectable, resorbable, thermoresponsive copolymer scaffolds. J. Biomed. Mater. Res. B 2017, 105, 53–62. [Google Scholar] [CrossRef]
- Mazumder, M.A.; Fitzpatrick, S.D.; Muirhead, B.; Sheardown, H. Cell-adhesive thermogelling pnipaam/hyaluronic acid cell delivery hydrogels for potential application as minimally invasive retinal therapeutics. J. Biomed. Mater. Res. A 2012, 100, 1877–1887. [Google Scholar] [CrossRef]
- Park, D.; Shah, V.; Rauck, B.M.; Friberg, T.R.; Wang, Y. An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration. Macromol. Biosci. 2013, 13, 464–469. [Google Scholar] [CrossRef]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Invest. Ophthalmol. Vis. Sci. 2014, 55, 469–476. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhong, Z.Y.; Jiang, X.; Hennink, W.E.; Dijkstra, P.J.; Feijen, J. Peg-plla and peg-pdla multiblock copolymers: Synthesis and in situ hydrogel formation by stereocomplexation. J. Control. Release 2006, 116, e17-19. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Ren, F.; Gao, S. Plga-peg-plga hydrogel for ocular drug delivery of dexamethasone acetate. Drug Dev. Ind. Pharm. 2010, 36, 1131–1138. [Google Scholar] [CrossRef]
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An injectable thermosensitive polymeric hydrogel for sustained release of avastin to treat posterior segment disease. Int. J. Pharm. 2015, 490, 375–383. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, W.; Luan, J.; Yang, D.; Wei, G.; Yu, L.; Lu, W.; Ding, J. Sustained intravitreal delivery of dexamethasone using an injectable and biodegradable thermogel. Acta Biomater. 2015, 23, 271–281. [Google Scholar] [CrossRef]
- Patel, S.P.; Vaishya, R.; Yang, X.; Pal, D.; Mitra, A.K. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein Pept. Lett. 2014, 21, 1185–1200. [Google Scholar] [CrossRef]
- Park, S.Y.; Chung, H.J.; Lee, Y.; Park, T.G. Injectable and sustained delivery of human growth hormone using chemically modified pluronic copolymer hydrogels. Biotechnol. J. 2008, 3, 669–675. [Google Scholar] [CrossRef]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Bhoyar, B.S.; Agnihotrh, V.V.; Bodhankar, M.M. A noval thermoreversible phase transition system with flux enhancers for opthalmic application. Int. J. Pharm. Pharm. Sci. 2011, 3, 367–370. [Google Scholar]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef]
- Chung, H.J.; Go, D.H.; Bae, J.W.; Jung, I.K.; Lee, J.W.; Park, K.D. Synthesis and characterization of pluronic® grafted chitosan copolymer as a novel injectable biomaterial. Curr. Appl. Phys. 2005, 5, 485–488. [Google Scholar] [CrossRef]
- Chen, J.P.; Cheng, T.H. Thermo-responsive chitosan-graft-poly(n-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Li, Y.; Du, Y. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing nanoparticles for drug delivery. Polym. Bull. 2010, 64, 791–804. [Google Scholar] [CrossRef]
- Ta, H.T.; Han, H.; Larson, I.; Dass, C.R.; Dunstan, D.E. Chitosan-dibasic orthophosphate hydrogel: A potential drug delivery system. Int. J. Pharm. 2009, 371, 134–141. [Google Scholar] [CrossRef]
- Hu, C.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Biomed. Mater. Eng. 2014, 24, 1941–1950. [Google Scholar]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Scalarone, D.; Brunella, V.; Ugazio, E.; Sapino, S.; Berlier, G. Thermoresponsive copolymer-grafted sba-15 porous silica particles for temperature-triggered topical delivery systems. Express Polym. Lett. 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Lin, T.C.; Hung, K.H.; Peng, C.H.; Liu, J.H.; Woung, L.C.; Tsai, C.Y.; Chen, S.J.; Chen, Y.T.; Hsu, C.C. Nanotechnology-based drug delivery treatments and specific targeting therapy for age-related macular degeneration. J. Chin. Med. Assoc. 2015, 78, 635–641. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Hung, W.T.; Christenson, L.K.; Mitra, A.K. Composite nanoformulation therapeutics for long-term ocular delivery of macromolecules. Mol. Pharm. 2016, 13, 2912–2922. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Mandal, A.; Pal, D.; Mitra, A.K. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert Opin. Drug Deliv. 2017, 14, 1145–1162. [Google Scholar] [CrossRef]
- Tan, G.; Yu, S.; Li, J.; Pan, W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int. J. Biol. Macromol. 2017, 103, 941–947. [Google Scholar] [CrossRef]
- Fedorchak, M.V.; Conner, I.P.; Medina, C.A.; Wingard, J.B.; Schuman, J.S.; Little, S.R. 28-day intraocular pressure reduction with a single dose of brimonidine tartrate-loaded microspheres. Exp. Eye Res. 2014, 125, 210–216. [Google Scholar] [CrossRef]
- Fabiano, A.; Bizzarri, R.; Zambito, Y. Thermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracil. Int. J. Nanomed. 2017, 12, 633–643. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev. Ind. Pharm. 2010, 36, 1330–1339. [Google Scholar] [CrossRef]
- Gao, S.Q.; Maeda, T.; Okano, K.; Palczewski, K. A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6314–6323. [Google Scholar] [CrossRef]
- Hirani, A.; Grover, A.; Lee, Y.W.; Pathak, Y.; Sutariya, V. Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for age-related macular degeneration. Pharm. Dev. Technol. 2016, 21, 61–67. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Boddu, S.H.S.; Jwala, J.; Chowdhury, M.R.; Mitra, A.K. In vitro evaluation of a targeted and sustained release system for retinoblastoma cells using doxorubicin as a model drug. J. Ocul. Pharmacol. Ther. 2010, 26, 459–468. [Google Scholar] [CrossRef]
- Famili, A.; Kahook, M.Y.; Park, D. A combined micelle and poly(serinol hexamethylene urea)-co-poly(n-isopropylacrylamide) reverse thermal gel as an injectable ocular drug delivery system. Macromol. Biosci. 2014, 14, 1719–1729. [Google Scholar] [CrossRef]
- Agrahari, V.; Patel, S.P.; Dhall, N.; Aulgur, Z.; Thukral, S.; Yang, X.; Conley, R.; Mitra, A.K. Nanoparticles in thermosensitive gel based composite nanosystem for ocular diseases. Drug Deliv. Transl. Res. 2018, 8, 422–435. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated microneedles for drug delivery to the eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Thakur, R.R.; Fallows, S.J.; McMillan, H.L.; Donnelly, R.F.; Jones, D.S. Microneedle-mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. J. Pharm. Pharmacol. 2014, 66, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Tunc, M.; Humayun, M.; Cheng, X.; Ratner, B.D. A reversible thermosensitive adhesive for retinal implants: In vivo experience with plasma-deposited poly(n-isopropyl acrylamide). Retina 2008, 28, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Gooch, N.; Molokhia, S.A.; Condie, R.; Burr, R.M.; Archer, B.; Ambati, B.K.; Wirostko, B. Ocular drug delivery for glaucoma management. Pharmaceutics 2012, 4, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Raghu Raj Singh, T. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.P. Moxifloxacin punctum plug for sustained drug delivery. J. Ocul. Pharmacol. Ther. 2012, 28, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Chauhan, A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.; Woo, S.J.; Park, J.H.; Park, S.; Hwang, D.J.; Park, K.H. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J. Ocul. Pharmacol. Ther. 2013, 29, 612–618. [Google Scholar] [CrossRef]
- Kim, H.; Robinson, M.R.; Lizak, M.J.; Tansey, G.; Lutz, R.J.; Yuan, P.; Wang, N.S.; Csaky, K.G. Controlled drug release from an ocular implant: An evaluation using dynamic three-dimensional magnetic resonance imaging. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2722–2731. [Google Scholar] [CrossRef]
- Li, S.K.; Hao, J.; Liu, H.; Lee, J.H. Mri study of subconjunctival and intravitreal injections. J. Pharm. Sci. 2012, 101, 2353–2363. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.C.; Kieselmann, S. Low-level drug release-rate testing of ocular implants using usp apparatus 4 dissolution and hplc end analysis. Dissolut. Technol. 2010, 17, 12–14. [Google Scholar] [CrossRef]
- Fotaki, N. Flow-through cell apparatus (usp apparatus 4): Operation and features. Dissolut. Technol. 2011, 18, 46–49. [Google Scholar] [CrossRef]
- Pescina, S.; Govoni, P.; Potenza, A.; Padula, C.; Santi, P.; Nicoli, S. Development of a convenient ex vivo model for the study of the transcorneal permeation of drugs: Histological and permeability evaluation. J. Pharm. Sci. 2015, 104, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Santi, P.; Ferrari, G.; Padula, C.; Cavallini, P.; Govoni, P.; Nicoli, S. Ex vivo models to evaluate the role of ocular melanin in trans-scleral drug delivery. Eur. J. Pharm. Sci. 2012, 46, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; D’Addino, I.; Peira, E.; Trotta, M.; Gallarate, M. Solid lipid nanoparticles prepared by coacervation method as vehicles for ocular cyclosporine. J. Drug Deliv. Sci. Technol. 2012, 22, 125–130. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y. Development of an ex vivo method for evaluation of precorneal residence of topical ophthalmic formulations. AAPS Pharm. Sci. Technol. 2009, 10, 796–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Repetto, R.; Stocchino, A.; Cafferata, C. Experimental investigation of vitreous humour motion within a human eye model. Phys. Med. Biol. 2005, 50, 4729–4743. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, A.; Lagazzo, A.; Repetto, R.; Stocchino, A. An experimental model of vitreous motion induced by eye rotations. Eye Vis. 2015, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Nagel, S.; Guthoff, R.; Seidlitz, A.; Weitschies, W. The vitreous model—A new in vitro test method simulating the vitreous body. Biomed. Technol. 2012, 57 (Suppl. 1). [Google Scholar] [CrossRef]
- Loch, C.; Stein, S.; Nagel, S.; Seidlitz, A.; Guthoff, R.; Weitschies, W. Simulation of the conjunctival and choroidal blood flow using a new multi-layer diffusion cell. Biomed. Technol. 2013, 58 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
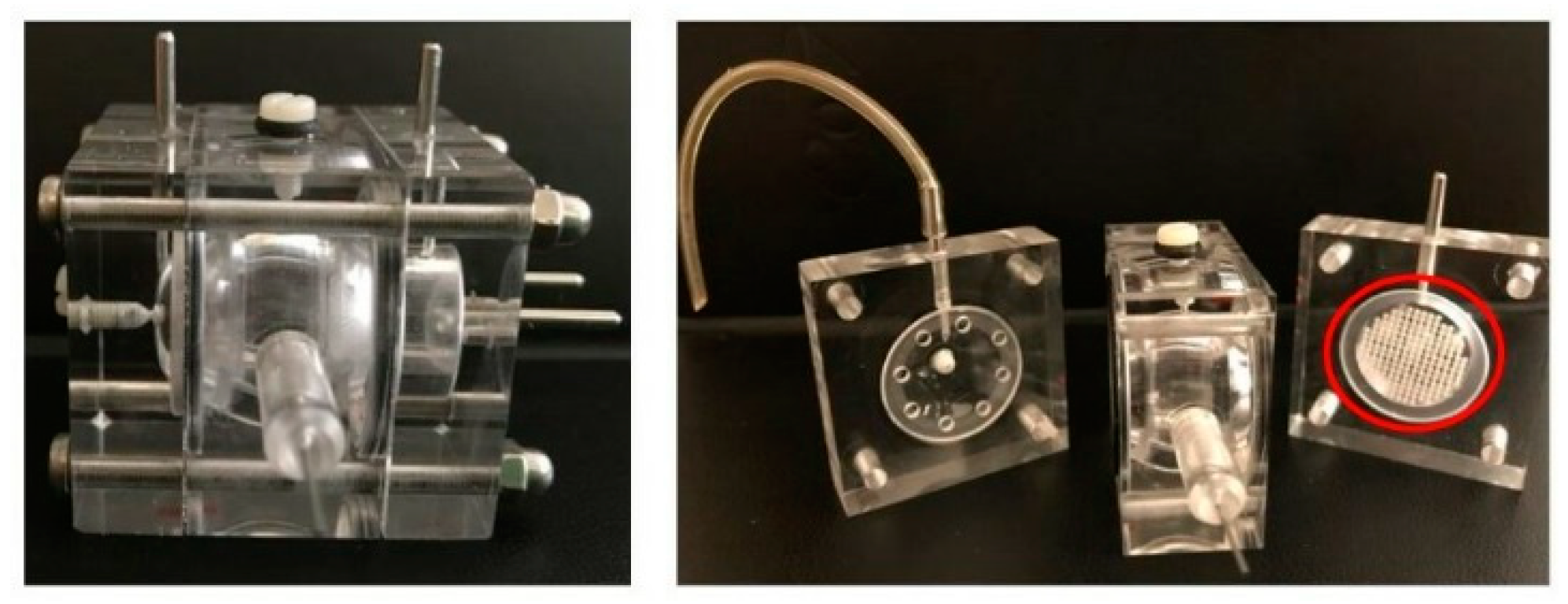
- Awwad, S.; Lockwood, A.; Brocchini, S.; Khaw, P.T. The pk-eye: A novel in vitro ocular flow model for use in preclinical drug development. J. Pharm. Sci. 2015, 104, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Peira, E.; Chirio, D.; Brunella, V.; Guglielmo, S.; Gallarate, M. Thermosensitive drug delivery systems for intravitreal administration of cefuroxime: A novel in vitro eye flow cell. Presented at the 3rd Conference on Pharmaceutics, Bologna, Italy, 25–26 March 2019. [Google Scholar]
- Patel, S.; Müller, G.; Stracke, J.O.; Altenburger, U.; Mahler, H.C.; Jere, D. Evaluation of protein drug stability with vitreous humor in a novel ex-vivo intraocular model. Eur. J. Pharm. Biopharm. 2015, 95, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Stracke, J.O.; Altenburger, U.; Mahler, H.C.; Metzger, P.; Shende, P.; Jere, D. Prediction of intraocular antibody drug stability using ex-vivo ocular model. Eur. J. Pharm. Biopharm. 2017, 112, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, L.; Ranta, V.P.; Moilanen, H.; Urtti, A. Permeability of retinal pigment epithelium: Effects of permeant molecular weight and lipophilicity. Investig. Ophthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Steuer, H.; Jaworski, A.; Stoll, D.; Schlosshauer, B. In vitro model of the outer blood-retina barrier. Brain Res. Protoc. 2004, 13, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Cheng, K.; Lai, Y.; Kisaalita, W.S. Three-dimensional polymer scaffolds for high throughput cell-based assay systems. Biomaterials 2008, 29, 2802–2812. [Google Scholar] [CrossRef]
- Postnikoff, C.K.; Pintwala, R.; Williams, S.; Wright, A.M.; Hileeto, D.; Gorbet, M.B. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS ONE 2014, 9, e96448. [Google Scholar] [CrossRef]
- Stay, M.S.; Xu, J.; Randolph, T.W.; Barocas, V.H. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm. Res. 2003, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Juan, T.; Hubschman, J.P.; Eldredge, J.D. A computational study of the flow through a vitreous cutter. J. Biomech. Eng. 2010, 132, 121005. [Google Scholar] [CrossRef] [PubMed]
- Jooybar, E.; Abdekhodaie, M.J.; Farhadi, F.; Cheng, Y.L. Computational modeling of drug distribution in the posterior segment of the eye: Effects of device variables and positions. Math. Biosci. 2014, 255, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Tervonen, A.; Vainio, I.; Nymark, S.; Hyttinen, J. Prediction of passive drug permeability across the blood-retinal barrier. Pharm. Res. 2014, 31, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Edward, A.; Prausnitz, M.R. Predicted permeability of the cornea to topical drugs. Pharm. Res. 2001, 18, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
| Thermosensitive DDS | Mechanism |
|---|---|
| In situ hydrogels | Formulation is liquid at room temperature (20–25 °C). The contact with body temperature (35–37 °C) leads to the formation of a hydrogel network |
| Composite systems | Microparticles (MP) or nanoparticles (NP) suspended in a thermogelling matrix generally made of biodegradable thermosensitive polymers |
| Devices | Polymeric materials (microneedle, punctal plug, contact lens) that, once inserted in the eye, release the loaded drug by change in temperature (from 25 to 35 °C) |
| Drug Delivery Systems (DDS) | Material/Aim | Molecule Delivered | Observation | References |
|---|---|---|---|---|
| In situ hydrogel | Poly(N-isopropylacrylamide) (PNIPAM) crosslinked with poly(ethylene glycol) diacrylate (PEG-DA) as an intravitreal injectable vehicle. | Proteins, Ig, Bevacizumab, Ranibizumab | Release profiles function of the cross-link density. Release sustained for approximately 3 weeks. | [54] |
| In situ hydrogel | Copolymers of N-isopropylacrylamide (NIPAM), acrylic acid N-hydroxysuccinimide (NAS) and varying concentrations of acryloyloxy dimethyl-c-butyrolactone (DBA) and acrylic acid (AA) for intravitreal injections. | Dexamethasone | Slow-degrading copolymers (over 130 days of incubation in PBS), injectable from a 30-gauge needle, offering slow drug release. | [56,57] |
| In situ hydrogel | Copolymers of NIPAM and acrylic acid N-hydroxysuccinimide (NAS) conjugated with amine-functionalized hyaluronic acid (HA) as a biomaterial scaffolds for bolus injection into the sub-retinal space. | Cells | Temperature-induced scaffold formation for transplanted cells entrapment; optimal compatibility with retinal pigment epithelial. | [58] |
| In situ hydrogel | PEG-poly-serinol hexamethylene (ESHU) as an intraocular drug-delivery vehicle for age-related macular degeneration (AMD). | Bevacizumab | The release of bevacizumab was sustained up to 17 weeks and its concentration was maintained averaging 4.7 times higher than that in eyes receiving bevacizumab bolus injections. Biodegradable. | [59,60] |
| In situ hydrogel | Poly(lactic-co-glycolic acid) (PLGA)-PEG-PLGA triblock as an intravitreal injectable hydrogel for sustained drug release. | Bevacizumab | Hydrogel immediately formed after intravitreal injection; in vitro sustained drug release over a period of up to 14 days. | [63] |
| In situ hydrogel | PLGA-PEG-PLGA triblock as an intravitreal injectable hydrogel for sustained drug release. | Dexamethasone | Drug ocular retention time was prolonged from several hours to more than 1 week after a single intravitreal injection; excellent biocompatibility. | [64] |
| In situ hydrogel | PLA-PCL-PEG pentablock-based injectable hydrogel for the treatment of posterior segment neovascular diseases. | IgG | Significantly longer sustained release of IgG was provided by pentablock (more than 20 days) respect to triblock copolymers. | [65] |
| In situ hydrogel | Poloxamer 407 in combination with other polymers to form a topical in situ gel. | Ciprofloxacin | Improved antimicrobial effect in vitro compared to the market eye drops. Eight hour sustained release of ciprofloxacin. | [68] |
| In situ hydrogel | mPEG-PLGA cross-linked with 2,2-bis (2-oxazoline) aqueous solution as an intravitreal injection carrier. | Bevacizumab | Thermoresponsive, controlled drug release. Intraocular biocompatibility biodegradability and bioactivity of loaded drug. | [74] |
| Nanocarriers inin situ gel | Nanostructured lipid carriers (NLC) in thermoreversible HACC/GP gel for topical ocular delivery. | Dexamethasone | Precorneal sustained release of drug from NLC-HACC/GP gel in vitro. | [80] |
| In situ gelled nanoemulsion | Poloxamer 407 and Poloxamer 188 in nanoemulsion for topical ocular delivery. | Dorzolamide hydrochloride | Increased precorneal residence time and bioavailability. | [83] |
| NP in hydrogel | PEG-PLGA NP in PLGA–PEG–PLGA thermoreversible intraocular injectable gel. | Triamcinolone acetonide | Non-toxic and able to reduce vascular endothelial growth factor (VEGF) levels in ARPE-19 cells; sustained release of the drug over 10 days. | [85] |
| NP in hydrogel | PCL-PLA-PEG-PLA-PCL-based NP in thermosensitive mPEG-PCL-PLA-PCL-PEGm intraocular injectable gel. | IgG-Fab | Minimal burst release with near zero-order release profile from the composite nanoformulation up to 80 days. In vitro cell viability and biocompatibility. | [78] |
| Micelles in hydrogel | PLGA-MP in poly(N-isopropylacrylamide) as an injectable MP-hydrogel drug delivery system. | Ranibizumab and Aflibercept | Controlled and extended intraocular release for approximately 200 days. | [86] |
| Micelles in hydrogel | Poly(d,l-lactide-co-glycolide)-poly(ethylene glycol)-folate (PLGA-PEG-FOL) micelles in PLGA-PEG-PLGA thermoreversible gel for intravitreal administration. | Doxorubicin | Sustained drug release for 2 weeks; increased drug uptake in Y-79 cells overexpressing folate receptors. | [87] |
| Micelles in hydrogel | PEG-PHS-PEG micelles in PNIPAM-PSHU backbone as an injectable ocular DDS. | Triamcinolone acetonide | Sustained long-term drug release and reduced burst release. | [88] |
| Model | Molecules Delivered | Outcomes | References |
|---|---|---|---|
| Dissolution apparatus (USP4) with a 1.5 mL/min flow (8–19 mL chamber) | Model drugs loaded in ocular implant | Adequate sensitivity; correlation with in vivo conditions | [102] |
| Spherical cavity, magnified with respect to the real geometry, carved within a Perspex cylinder and able to rotate | Blank glycerol solution | First attempt to measure the flow field induced by saccadic eye movements on a model of the vitreous chamber | [108] |
| Plexiglas cylinder with an internal spherical cavity, magnified with respect to the real geometry, able to rotate | Model drug particles | Three-dimensional (3D) understanding of the saccade-induced steady component of the vitreous flow | [109] |
| Spherical glass corpus filled with a polyacrylamide gel placed on an altered orbital shaker | Fluorescein sodium solution | Good accordance of the results with the porcine vitreous humor | [110] |
| Multi-layer diffusion cell composed of three layers placed on top of each other, perfused with buffer using a multi-channel pump | Fluorescein sodium solution | Opportunity to simulate the choroidal and conjunctival blood flow in a simplified setup | [111] |
| Two-compartment in vitro eye flow model (pharmacokinetic (PK)-Eye) | Dye (Coomassie Brilliant Blue) | To mime the intraocular aqueous outflow for vitreous clearance times estimation for proteins and poorly soluble drugs (injectable suspensions or implants) | [112] |
| Ex-vivo intravitreal horizontal stability model (ExVit-HS) | Bi-specific monoclonal antibody (mAb) | Valuable tool to evaluate protein and other drugs stability after IVT injection | [115] |
| Isolated bovine retinal pigment epithelium (RPE)-choroid sealed in a vertical diffusion chamber | FITC-dextran | Useful for pharmacokinetic simulation, particulalrly to evaluate the the retinal entry of drugs after transscleral and systemic delivery | [116] |
| Reconstructed corneal epithelium in the shape of the regular human cornea | Benzalkonium chloride | Suitable for biocompatibility experiments | [121] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapino, S.; Chirio, D.; Peira, E.; Abellán Rubio, E.; Brunella, V.; Jadhav, S.A.; Chindamo, G.; Gallarate, M. Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach. Nanomaterials 2019, 9, 884. https://doi.org/10.3390/nano9060884
Sapino S, Chirio D, Peira E, Abellán Rubio E, Brunella V, Jadhav SA, Chindamo G, Gallarate M. Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach. Nanomaterials. 2019; 9(6):884. https://doi.org/10.3390/nano9060884
Chicago/Turabian StyleSapino, Simona, Daniela Chirio, Elena Peira, Elena Abellán Rubio, Valentina Brunella, Sushilkumar A. Jadhav, Giulia Chindamo, and Marina Gallarate. 2019. "Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach" Nanomaterials 9, no. 6: 884. https://doi.org/10.3390/nano9060884
APA StyleSapino, S., Chirio, D., Peira, E., Abellán Rubio, E., Brunella, V., Jadhav, S. A., Chindamo, G., & Gallarate, M. (2019). Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach. Nanomaterials, 9(6), 884. https://doi.org/10.3390/nano9060884








