Understanding of Nanophase Separation and Hydrophilic Morphology in Nafion and SPEEK Membranes: A Combined Experimental and Theoretical Studies
Abstract
1. Introduction
2. Experiment and Simulation Detail
2.1. Materials
2.2. Preparation of Nafion and SPEEK Membranes
2.3. Characterization Methods
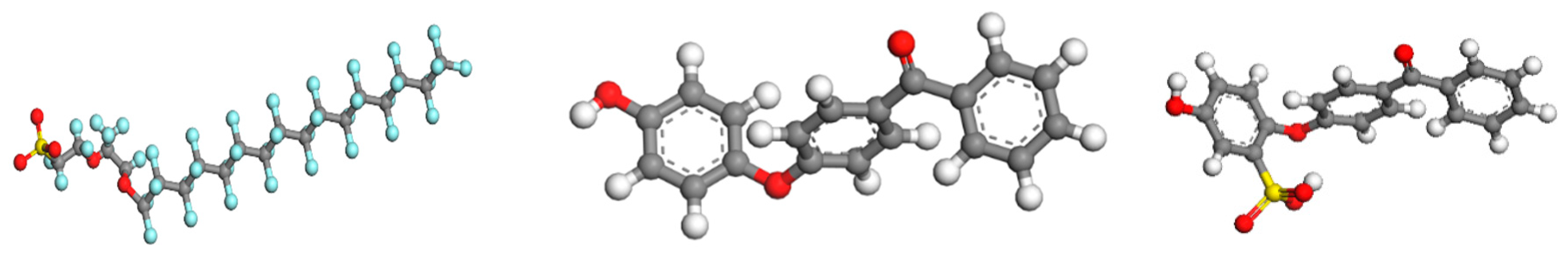
2.4. Model Constructions and Simulation Details
3. Results and Discussion
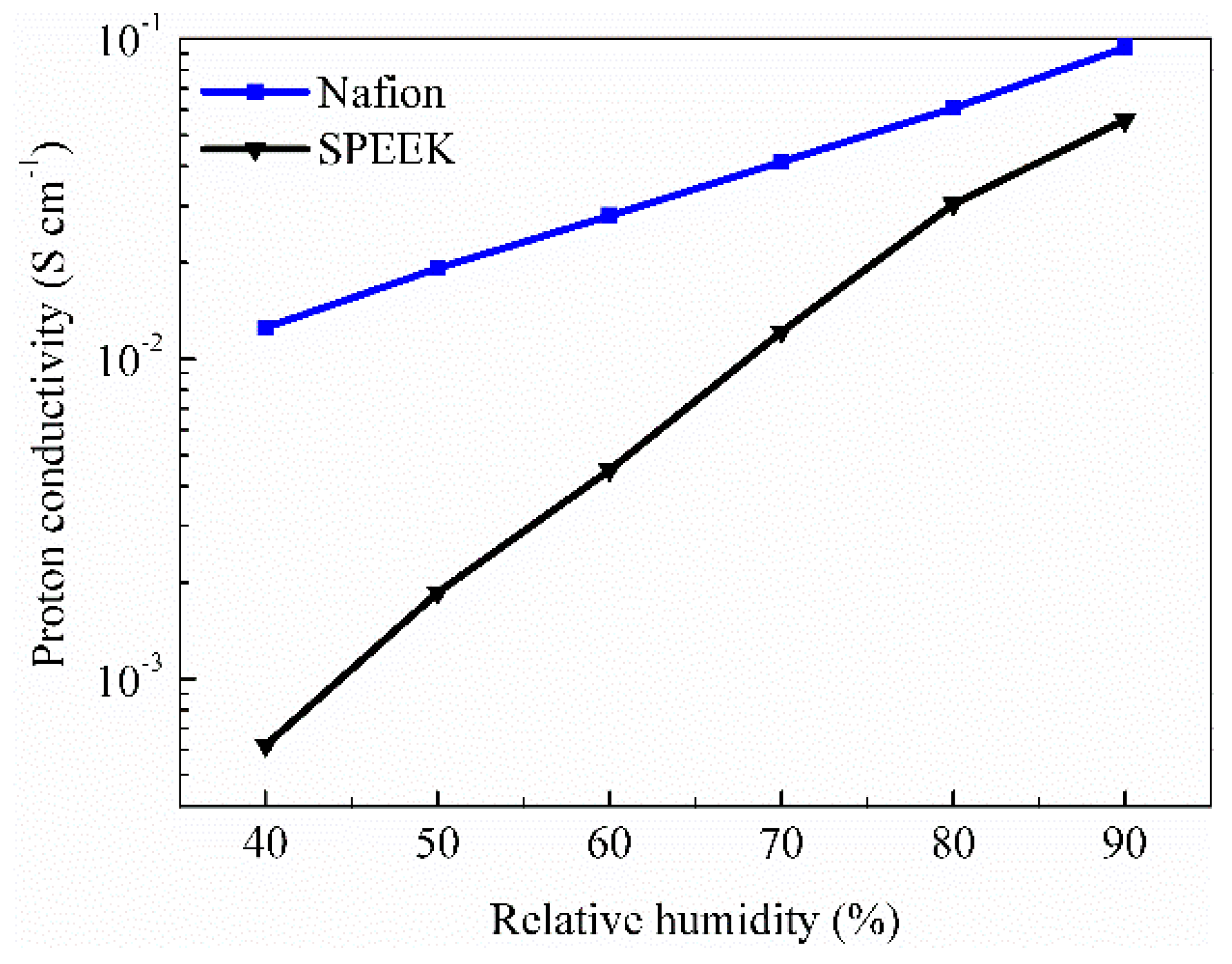
3.1. Water Uptake, Proton Conductivity, Fuel Crossover and Mechanical Performance
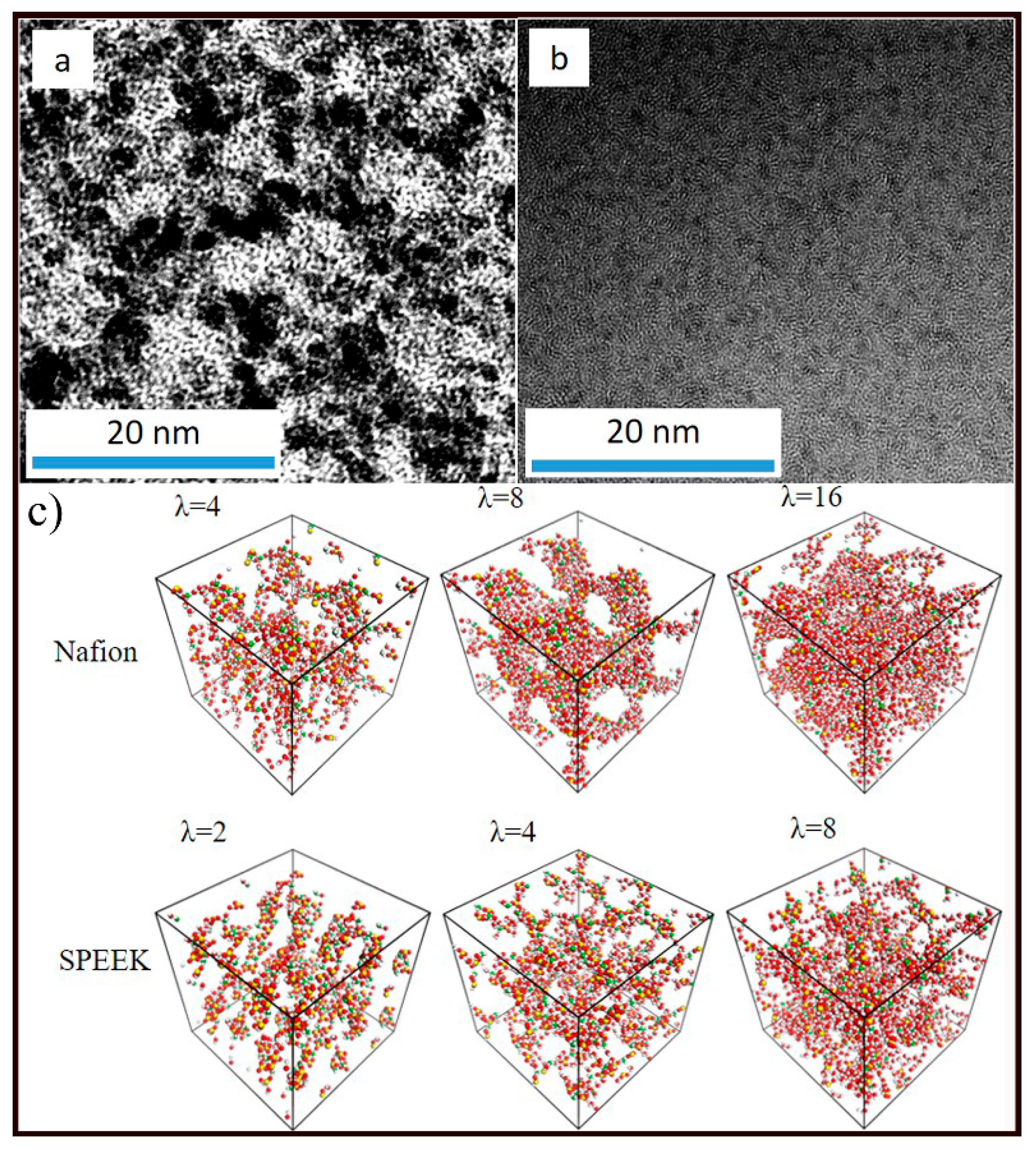
3.2. Nanophase Separation Morphology
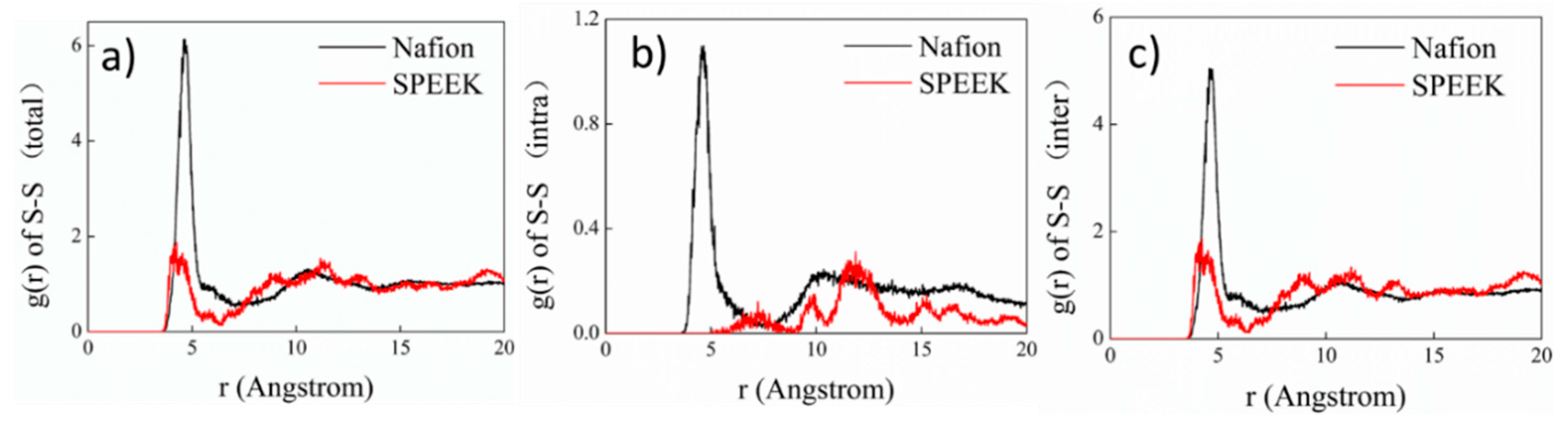
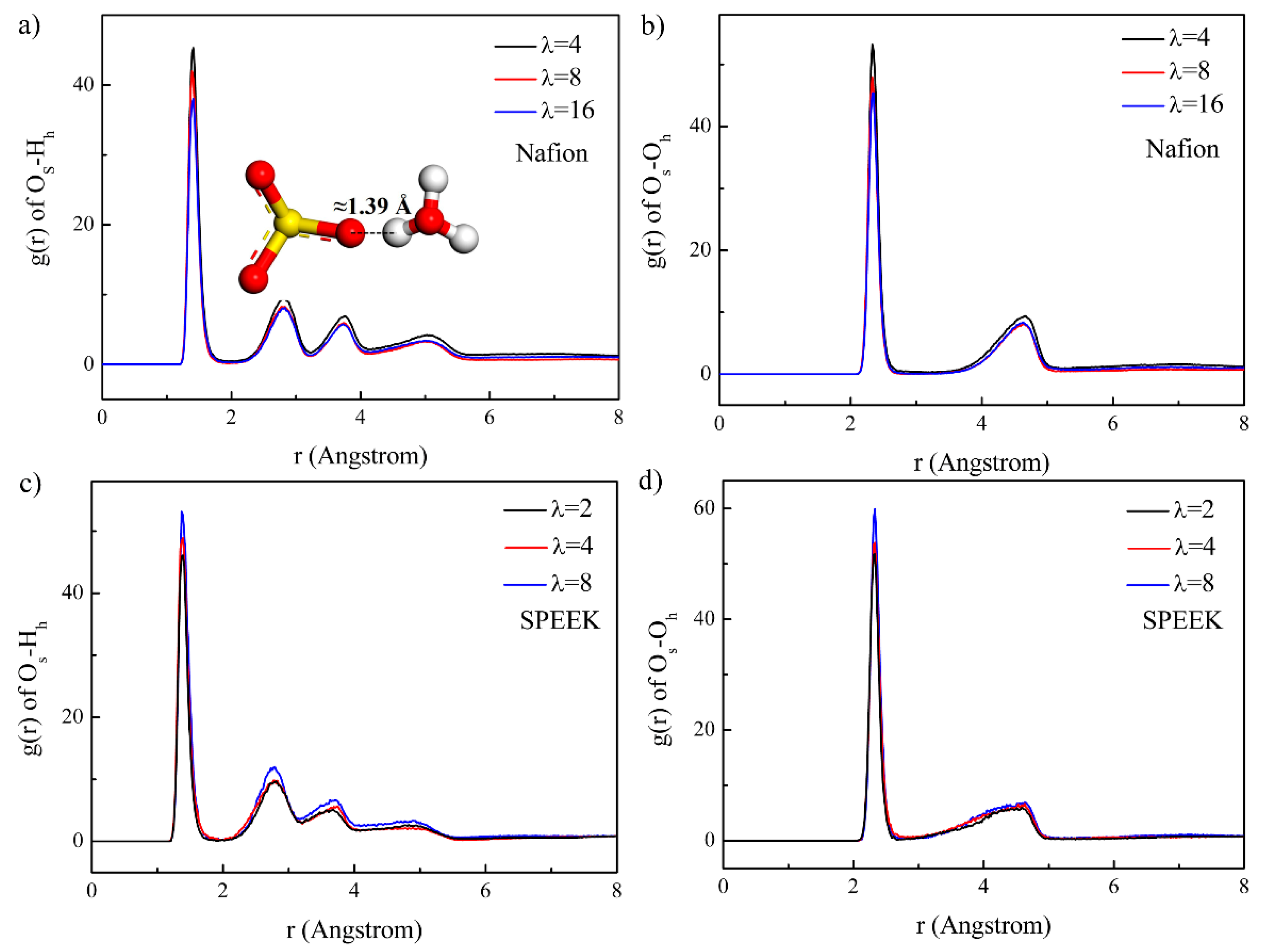
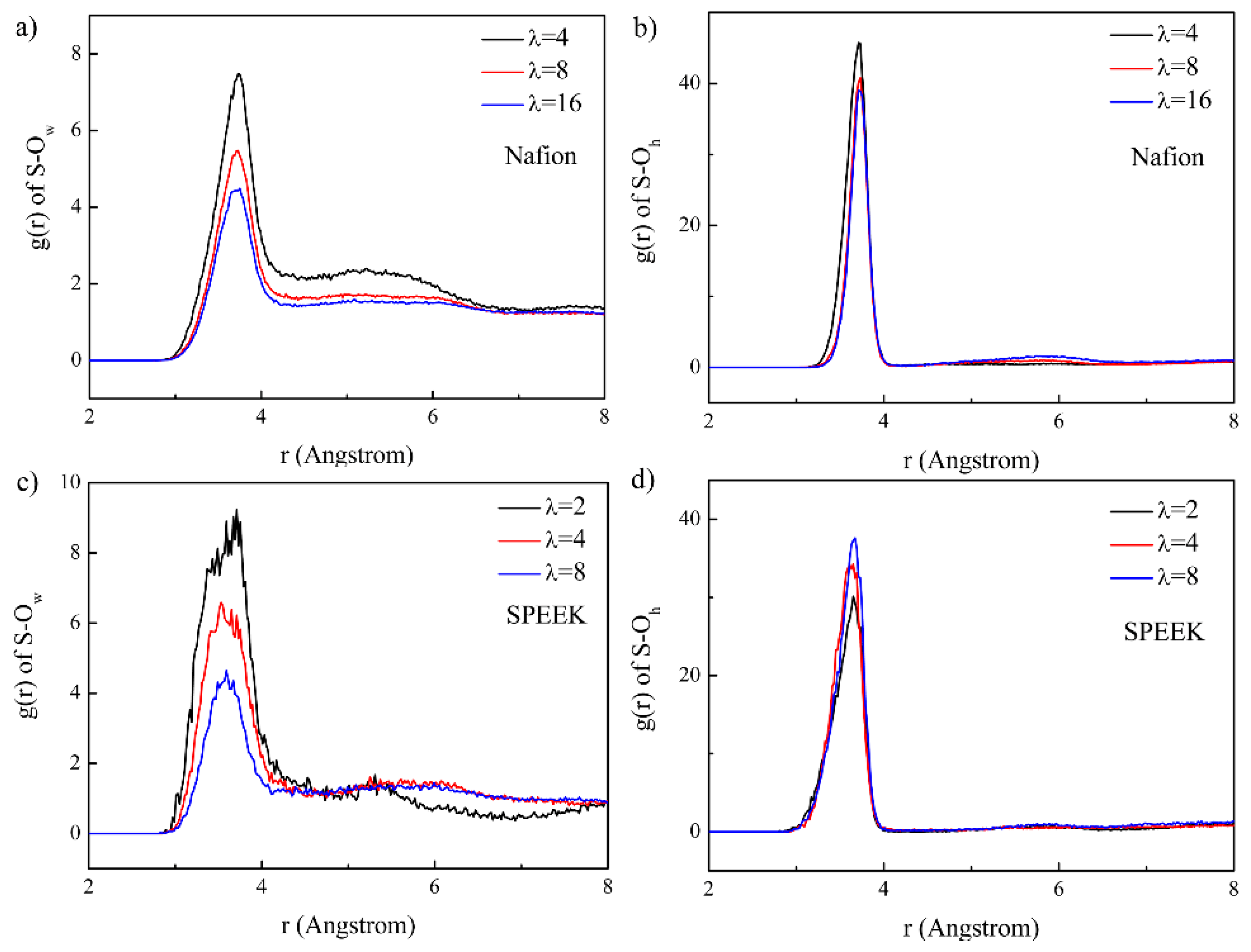
3.3. Hydrophilic Cluster in Nafion and SPEEK Systems
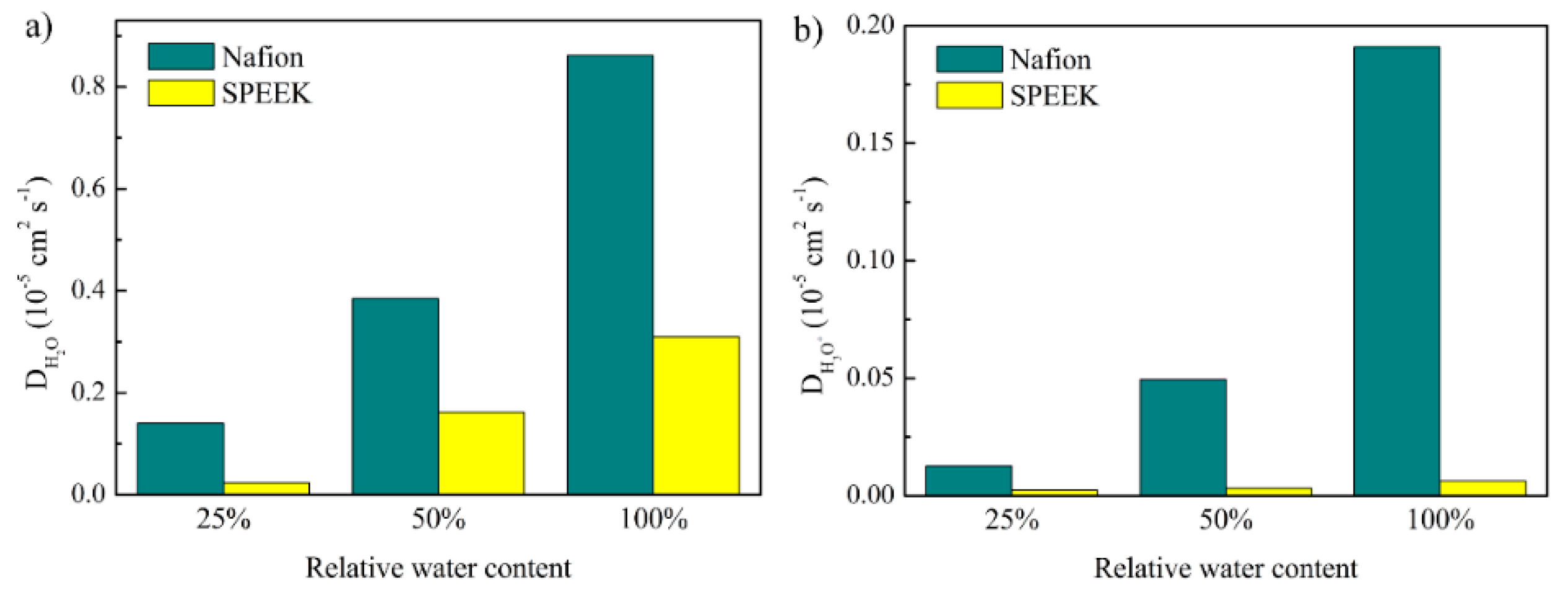
3.4. Diffusion Coefficients of Water and Hydronium
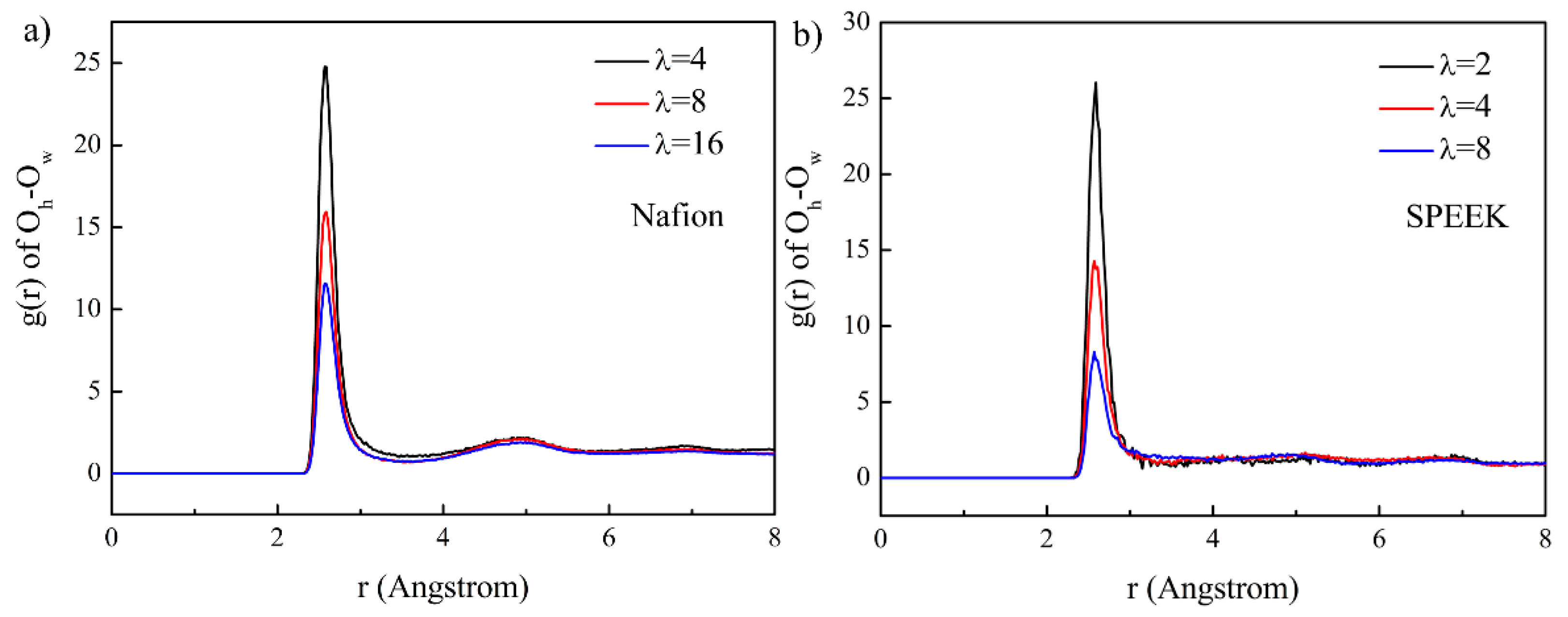
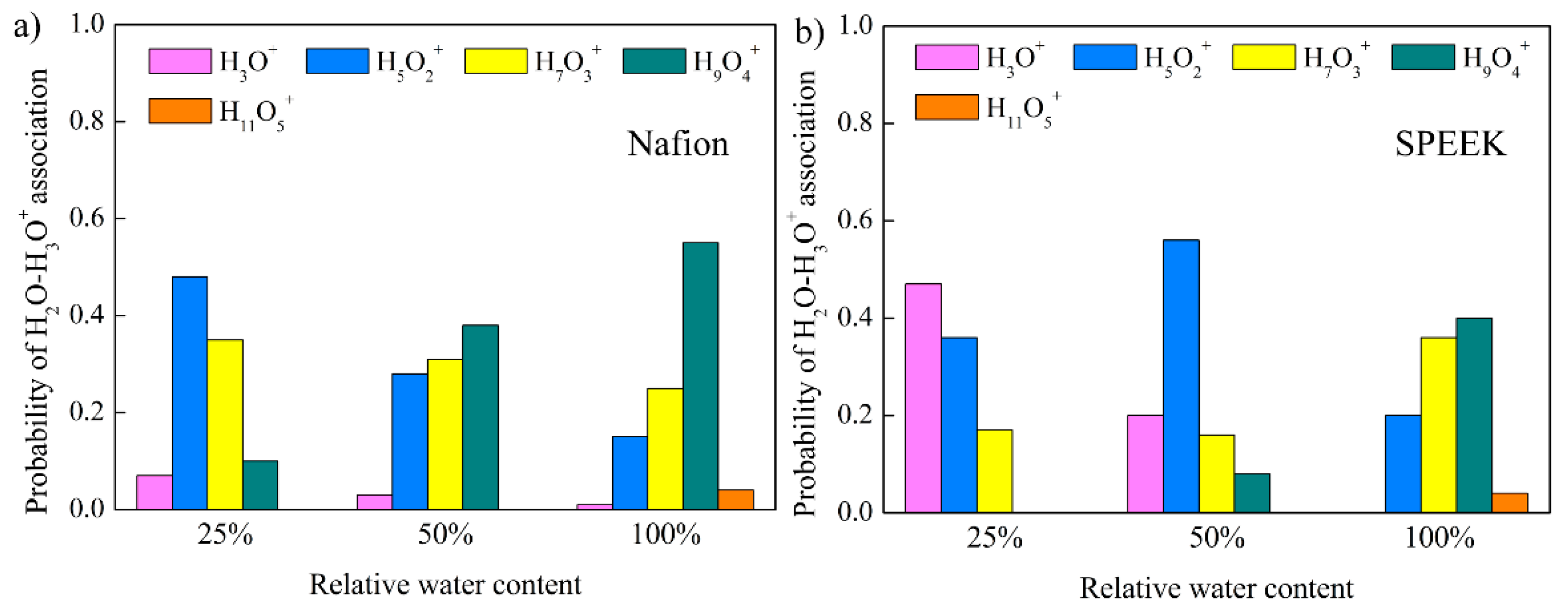
3.5. Hydrated Hydronium Complexes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, Z.; Zhang, Z.; Hu, M.; Hou, J.; Wu, L.; Xu, T. Perylene-based sulfonated aliphatic polyimides for fuel cell applications: Performance enhancement by stacking of polymer chains. J. Membr. Sci. 2018, 547, 43–50. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Lv, P.; Nagao, M.; Teranishi, S.; Mori, T. An Intermediate-Temperature Biomass Fuel Cell UsingWood Sawdust and Pulp Directly as Fuel. J. Electrochem. Soc. 2017, 164, F557–F563. [Google Scholar] [CrossRef]
- Sorte, E.G.; Abbott, L.J.; Frischknecht, A.L.; Wilson, M.A.; Alam, T.M. Hydrophilic domain structure in polymer exchange membranes: Simulations of NMR spin diffusion experiments to address ability for model discrimination. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 62–78. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of nano-size of functionalized silica on overall performance of swelling-filling modified Nafion membrane for direct methanol fuel cell application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Hu, H.; Sui, Y.; Ueda, M.; Qian, J.; Wang, L.; Zhang, X. Multi-block sulfonated poly (arylene ether nitrile) polymers bearing oligomeric benzotriazole pendants with exceptionally high H2/O2 fuel cell performance. J. Membr. Sci. 2018, 564, 342–351. [Google Scholar] [CrossRef]
- Kenneth, A.; Robert, B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar]
- Li, Y.; Shi, Y.; Mehio, N.; Tan, M.; Wang, Z.; Hu, X.; Chen, G.Z.; Dai, S.; Jin, X. More sustainable electricity generation in hot and dry fuel cells with a novel hybrid membrane of Nafion/nano-silica/hydroxyl ionic liquid. Appl. Energy 2016, 175, 451–458. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4611. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, W.; He, G.; Gao, P. Controlling fuel crossover and hydration in ultra-thin proton exchange membrane-based fuel cells using Pt-nanosheet catalysts. J. Mater. Chem. A 2014, 2, 16416–16423. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, C.; Dong, Z.; Jiang, B.; Dai, Y.; Wu, X.; He, G. Amphiprotic Side-Chain Functionalization Constructing Highly Proton/Vanadium-Selective Transport Channels for High-Performance Membranes in Vanadium Redox Flow Batteries. ACS Appl. Mater. Interfaces 2018, 10, 32247–32255. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qiu, Z.; Zhang, S.; Yang, X.; Yang, F.; Li, Z. The direct synthesis of wholly aromatic poly (p-phenylene) s bearing sulfobenzoyl side groups as proton exchange membranes. Polymer 2006, 47, 6993–7000. [Google Scholar] [CrossRef]
- Largier, T.D.; Wang, D.; Mueller, J.; Cornelius, C.J. Improving electrodialysis based water desalination using a sulfonated Diels-Alder poly(phenylene). J. Membr. Sci. 2017, 531, 103–110. [Google Scholar] [CrossRef]
- Lee, K.-S.; Spendelow, J.S.; Choe, Y.-K.; Fujimoto, C.; Kim, Y.S. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 2016, 1, 16120. [Google Scholar] [CrossRef]
- Ono, Y.; Goto, R.; Hara, M.; Nagano, S.; Abe, T.; Nagao, Y. High Proton Conduction of Organized Sulfonated Polyimide Thin Films with Planar and Bent Backbones. Macromolecules 2018, 51, 3351–3359. [Google Scholar] [CrossRef]
- Miyatake, K.; Chikashige, Y.; Higuchi, E.; Watanabe, M. Tuned polymer electrolyte membranes based on aromatic polyethers for fuel cell applications. J. Am. Chem. Soc. 2007, 129, 3879–3887. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Hwang, W.; Baik, J.-H.; Sung, Y.-E.; Lee, J.-C. Cross-Linked Sulfonated Poly (arylene ether sulfone) Containing a Flexible and Hydrophobic Bishydroxy Perfluoropolyether Cross-Linker for High-Performance Proton Exchange Membrane. ACS Appl. Mater. Interfaces 2018, 10, 21788–21793. [Google Scholar] [CrossRef]
- Jiang, R.C.; Kunz, H.R.; Fenton, J.M. Investigation of membrane property and fuel cell behavior with sulfonated poly(ether ether ketone) electrolyte: Temperature and relative humidity effects. J. Power Sources 2005, 150, 120–128. [Google Scholar] [CrossRef]
- Sambandam, S.; Ramani, V. SPEEK/functionalized silica composite membranes for polymer electrolyte fuel cells. J. Power Sources 2007, 170, 259–267. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Sohn, J.-Y.; Park, H.B.; Guiver, M.D.; Lee, Y.M. Phase separation and water channel formation in sulfonated block copolyimide. J. Phys. Chem. B 2010, 114, 12036–12045. [Google Scholar] [CrossRef]
- Yan, X.; Sun, J.; Gao, L.; Zheng, W.; Dai, Y.; Ruan, X.; He, G. A novel long-side-chain sulfonated poly (2,6-dimethyl-1,4-phenylene oxide) membrane for vanadium redox flow battery. Int. J. Hydrogen Energy 2018, 43, 301–310. [Google Scholar] [CrossRef]
- Elabd, Y.A.; Hickner, M.A. Block copolymers for fuel cells. Macromolecules 2010, 44, 1–11. [Google Scholar] [CrossRef]
- O’Dea, J.R.; Economou, N.J.; Buratto, S.K. Surface morphology of Nafion at hydrated and dehydrated conditions. Macromolecules 2013, 46, 2267–2274. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Ran, J.; Zhou, D.; Li, C.; Xu, T. Advances in proton-exchange membranes for fuel cells: An overview on proton conductive channels (PCCs). Phys. Chem. Chem. Phys. 2013, 15, 4870–4887. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wu, X.; Yan, X.; Gao, L.; Zhu, Y.; Zhang, D.; Li, J.; Wang, Q.; He, G. Polybenzimidazole membranes with nanophase-separated structure induced by non-ionic hydrophilic side chains for vanadium flow batteries. J. Mater. Chem. A 2018, 6, 3895–3905. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, S.Y.; Hwang, D.S.; Shin, D.W.; Cho, D.H.; Lee, K.H.; Kim, T.-W.; Lee, M.; Doherty, C.M.; Thornton, A.W.; et al. Nanocrack-regulated self-humidifying membranes. Nature 2016, 532, 480. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Thampan, T.; Malhotra, S.; Tang, H.; Datta, R. Modeling of conductive transport in proton-exchange membranes for fuel cells. J. Electrochem. Soc. 2000, 147, 3242–3250. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.J.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Tse, Y.-L.S.; Herring, A.M.; Kim, K.; Voth, G.A. Molecular dynamics simulations of proton transport in 3M and Nafion perfluorosulfonic acid membranes. J. Phys. Chem. C 2013, 117, 8079–8091. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Tsuchida, E.; Choe, Y.K.; Ikeshoji, T.; Barique, M.A.; Ohira, A. Ab initio studies on the proton dissociation and infrared spectra of sulfonated poly (ether ether ketone) (SPEEK) membranes. Phys. Chem. Chem. Phys. 2014, 16, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.; Tse, Y.-L.S.; Voth, G.A. Proton transport mechanism of perfluorosulfonic acid membranes. J. Phys. Chem. C 2014, 118, 17436–17445. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.A.; Vilčiauskas, L.; Melchior, J.-P.; Bester, G.; Kreuer, K.-D. Mechanism of Efficient Proton Conduction in Diphosphoric Acid Elucidated via First-Principles Simulation and NMR. J. Phys. Chem. B 2015, 119, 15866–15875. [Google Scholar] [CrossRef] [PubMed]
- Telfah, A.; Majer, G.; Kreuer, K.D.; Schuster, M.; Maier, J. Formation and mobility of protonic charge carriers in methyl sulfonic acid–water mixtures: A model for sulfonic acid based ionomers at low degree of hydration. Solid State Ion. 2010, 181, 461–465. [Google Scholar] [CrossRef]
- Paddison, S.J.; Kreuer, K.-D.; Maier, J. About the choice of the protogenic group in polymer electrolyte membranes: Ab initio modelling of sulfonic acid, phosphonic acid, and imidazole functionalized alkanes. Phys. Chem. Chem. Phys. 2006, 8, 4530–4542. [Google Scholar] [CrossRef]
- Brandell, D.; Karo, J.; Liivat, A.; Thomas, J. Molecular dynamics studies of the Nafion®, Dow® and Aciplex® fuel-cell polymer membrane systems. J. Mol. Model. 2007, 13, 1039–1046. [Google Scholar] [CrossRef]
- Urata, S.; Irisawa, J.; Takada, A.; Shinoda, W.; Tsuzuki, S.; Mikami, M. Molecular dynamics simulation of swollen membrane of perfluorinated ionomer. J. Phys. Chem. B 2005, 109, 4269–4278. [Google Scholar] [CrossRef]
- Elliott, J.A.; Paddison, S.J. Modelling of morphology and proton transport in PFSA membranes. Phys. Chem. Chem. Phys. 2007, 9, 2602–2618. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Ruan, X.; Yan, X.; Song, Y.; Shen, Z.; Wu, X.; He, G. Molecular dynamics study of confined structure and diffusion of hydrated proton in Hyfion® perfluorosulfonic acid membranes. Chem. Eng. Sci. 2017, 158, 234–244. [Google Scholar] [CrossRef]
- Mabuchi, T.; Tokumasu, T. Relationship between Proton Transport and Morphology of Perfluorosulfonic Acid Membranes: A Reactive Molecular Dynamics Approach. J. Phys. Chem. B 2018, 122, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yan, X.; He, G.; Wu, X.; Hu, Z.; Wang, Y. SPEEK proton exchange membranes modified with silica sulfuric acid nanoparticles. Int. J. Hydrogen Energy 2012, 37, 11853–11861. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Yan, X.; He, G.; Hu, Z. Proton conductivity enhancement of SPEEK membrane through n-BuOH assisted self-organization. J. Membr. Sci. 2015, 479, 46–54. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Wu, X.; He, G.; Du, L.; Hu, Z.; Tan, M. Modification of hydrophilic channels in Nafion membranes by DMBA: Mechanism and effects on proton conductivity. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1107–1117. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Nikazar, M.; Hafezi, M.J.; Dashtimoghadam, E.; Hasani-Sadrabadi, M.M. Molecular dynamics simulation study of proton diffusion in polymer electrolyte membranes based on sulfonated poly (ether ether ketone). Int. J. Hydrogen Energy 2012, 37, 10256–10264. [Google Scholar] [CrossRef]
- Cui, S.; Liu, J.; Selvan, M.E.; Keffer, D.J.; Edwards, B.J.; Steele, W.V. A molecular dynamics study of a Nafion polyelectrolyte membrane and the aqueous phase structure for proton transport. J. Phys. Chem. B 2007, 111, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.Z.; Newman, J. Transport in Polymer-Electrolyte Membranes: II. Mathematical Model. J. Electrochem. Soc. 2004, 151, A311–A325. [Google Scholar] [CrossRef]
- Morris, D.R.; Sun, X. Water-sorption and transport properties of Nafion 117 H. J. Appl. Polym. Sci. 1993, 50, 1445–1452. [Google Scholar] [CrossRef]
- Devanathan, R.; Venkatnathan, A.; Dupuis, M. Atomistic simulation of nafion membrane: I. Effect of hydration on membrane nanostructure. J. Phys. Chem. B 2007, 111, 8069–8079. [Google Scholar] [CrossRef]
- Komarov, P.V.; Veselov, I.N.; Chu, P.P.; Khalatur, P.G.; Khokhlov, A.R. Atomistic and mesoscale simulation of polymer electrolyte membranes based on sulfonated poly (ether ether ketone). Chem. Phys. Lett. 2010, 487, 291–296. [Google Scholar] [CrossRef]
- Mahajan, C.V.; Ganesan, V. Atomistic Simulations of Structure of Solvated Sulfonated Poly (ether ether ketone) Membranes and Their Comparisons to Nafion: I. Nanophase Segregation and Hydrophilic Domains. J. Phys. Chem. B 2010, 114, 8357–8366. [Google Scholar] [CrossRef]
- Mahajan, C.V.; Ganesan, V. Influence of Hydrogen Bonding Effects on Methanol and Water Diffusivities in Acid–Base Polymer Blend Membranes of Sulfonated Poly (ether ether ketone) and Base Tethered Polysulfone. J. Phys. Chem. B 2013, 117, 5315–5329. [Google Scholar] [CrossRef] [PubMed]
- Awatani, T.; Midorikawa, H.; Kojima, N.; Ye, J.; Marcott, C. Morphology of water transport channels and hydrophobic clusters in Nafion from high spatial resolution AFM-IR spectroscopy and imaging. Electrochem. Commun. 2013, 30, 5–8. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Gierke, T.; Hsu, W. The cluster-network nodel of ion clustering in perfluorosulfonated membranes. ACS Symp. Ser. 1982, 180, 283–307. [Google Scholar]
- Nimmanpipug, P.; Kodchakorn, K.; Lee, V.S.; Yana, J.; Jarumaneeroj, C.; Phongtamrug, S.; Chirachanchai, S. Structural and transport phenomena of urocanate-based proton carrier in sulfonated poly (ether ether ketone) membrane composite. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1625–1635. [Google Scholar] [CrossRef]
- Perrin, J.-C.; Lyonnard, S.; Volino, F. Quasielastic Neutron Scattering Study of Water Dynamics in Hydrated Nafion Membranes. J. Phys. Chem. C 2007, 111, 3393–3404. [Google Scholar] [CrossRef]
- Wang, J.; Hou, T. Application of molecular dynamics simulations in molecular property prediction II: Diffusion coefficient. J. Comput. Chem. 2011, 32, 3505–3519. [Google Scholar] [CrossRef]
- Selvan, M.E.; Liu, J.; Keffer, D.J.; Cui, S.; Edwards, B.J.; Steele, W.V. Molecular dynamics study of structure and transport of water and hydronium ions at the membrane/vapor interface of Nafion. J. Phys. Chem. C 2008, 112, 1975–1984. [Google Scholar] [CrossRef]

| PEM | Water Uptake at 20 °C | Proton Conductivity in DI Water at 80 °C (S cm−1) | Methanol Permeability (cm2 s−1) | Tensile Strength (MPa) | Modulus (MPa) |
|---|---|---|---|---|---|
| Nafion | 26.5% | 0.167 | 2.41 × 10−6 | 13.9 | 215 |
| SPEEK | 22.5% | 0.120 | 1.97 × 10−7 | 23.9 | 632 |
| PEM | Atomic Types | Water Content (25%) | Water Content (50%) | Water Content (100%) |
|---|---|---|---|---|
| Nafion | λ | 4 | 8 | 16 |
| Os–Oh | 0.658 | 0.526 | 0.384 | |
| S–Ow | 2.53 | 3.63 | 5.70 | |
| S–Oh | 2.02 | 1.62 | 1.16 | |
| Oh–Ow | 1.41 | 2.26 | 2.90 | |
| SPEEK | Λ | 2 | 4 | 8 |
| Os–Oh | 0.759 | 0.712 | 0.621 | |
| S–Ow | 1.24 | 2.52 | 3.62 | |
| S–Oh | 2.20 | 2.12 | 1.97 | |
| Oh–Ow | 0.70 | 1.14 | 1.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Liu, S.; Wang, L.; Li, M.; Gao, C. Understanding of Nanophase Separation and Hydrophilic Morphology in Nafion and SPEEK Membranes: A Combined Experimental and Theoretical Studies. Nanomaterials 2019, 9, 869. https://doi.org/10.3390/nano9060869
Wang R, Liu S, Wang L, Li M, Gao C. Understanding of Nanophase Separation and Hydrophilic Morphology in Nafion and SPEEK Membranes: A Combined Experimental and Theoretical Studies. Nanomaterials. 2019; 9(6):869. https://doi.org/10.3390/nano9060869
Chicago/Turabian StyleWang, Rujie, Shanshan Liu, Lidong Wang, Ming Li, and Chong Gao. 2019. "Understanding of Nanophase Separation and Hydrophilic Morphology in Nafion and SPEEK Membranes: A Combined Experimental and Theoretical Studies" Nanomaterials 9, no. 6: 869. https://doi.org/10.3390/nano9060869
APA StyleWang, R., Liu, S., Wang, L., Li, M., & Gao, C. (2019). Understanding of Nanophase Separation and Hydrophilic Morphology in Nafion and SPEEK Membranes: A Combined Experimental and Theoretical Studies. Nanomaterials, 9(6), 869. https://doi.org/10.3390/nano9060869






