Heterogeneous Cross-Coupling over Gold Nanoclusters
Abstract
1. Introduction
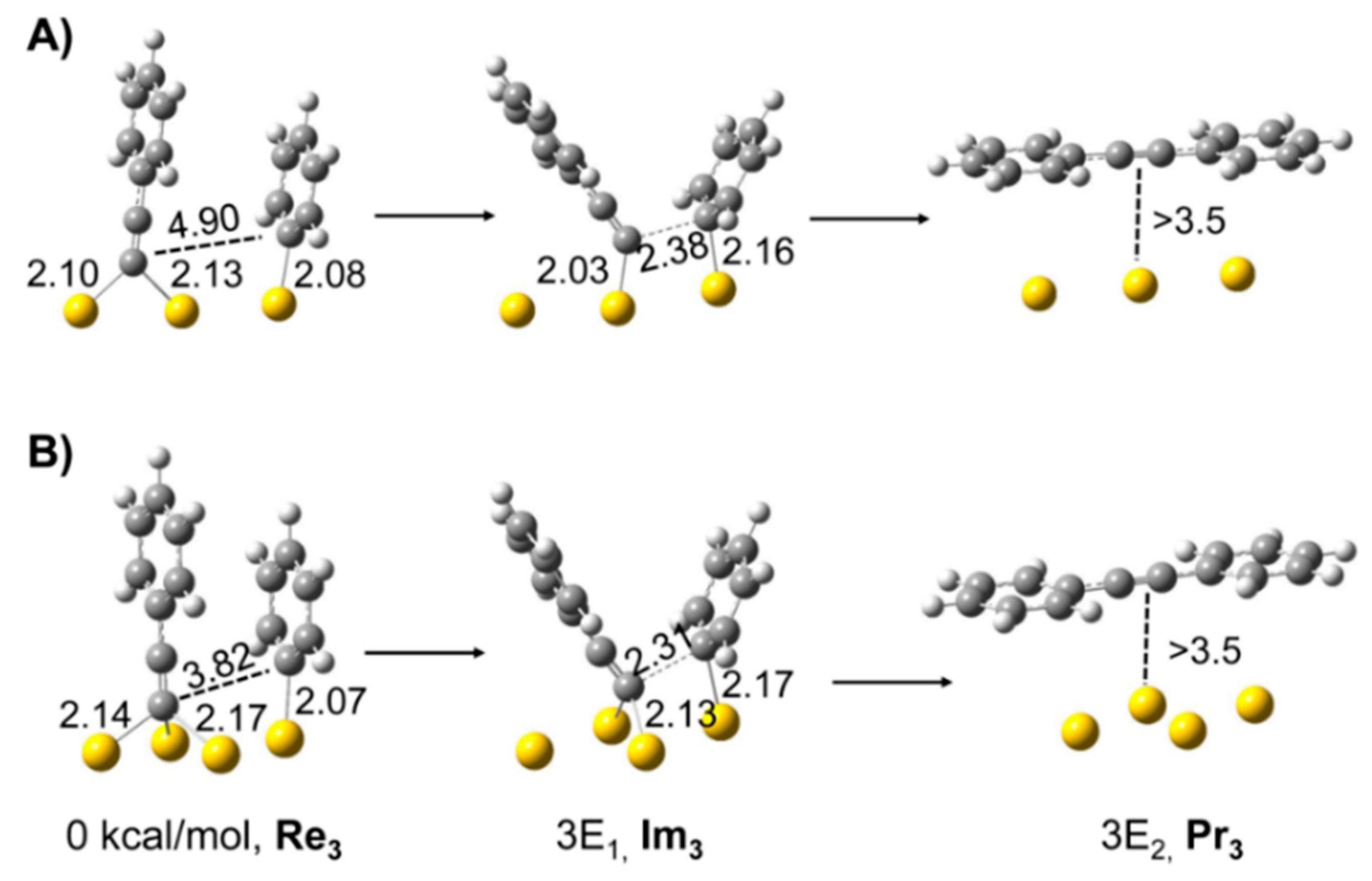
2. Activation of Ph–B(OH)2, Ph–I, and C≡C–H Bonds over Au: Theoretical Simulation
3. Physical Property of Au Nanoclusters
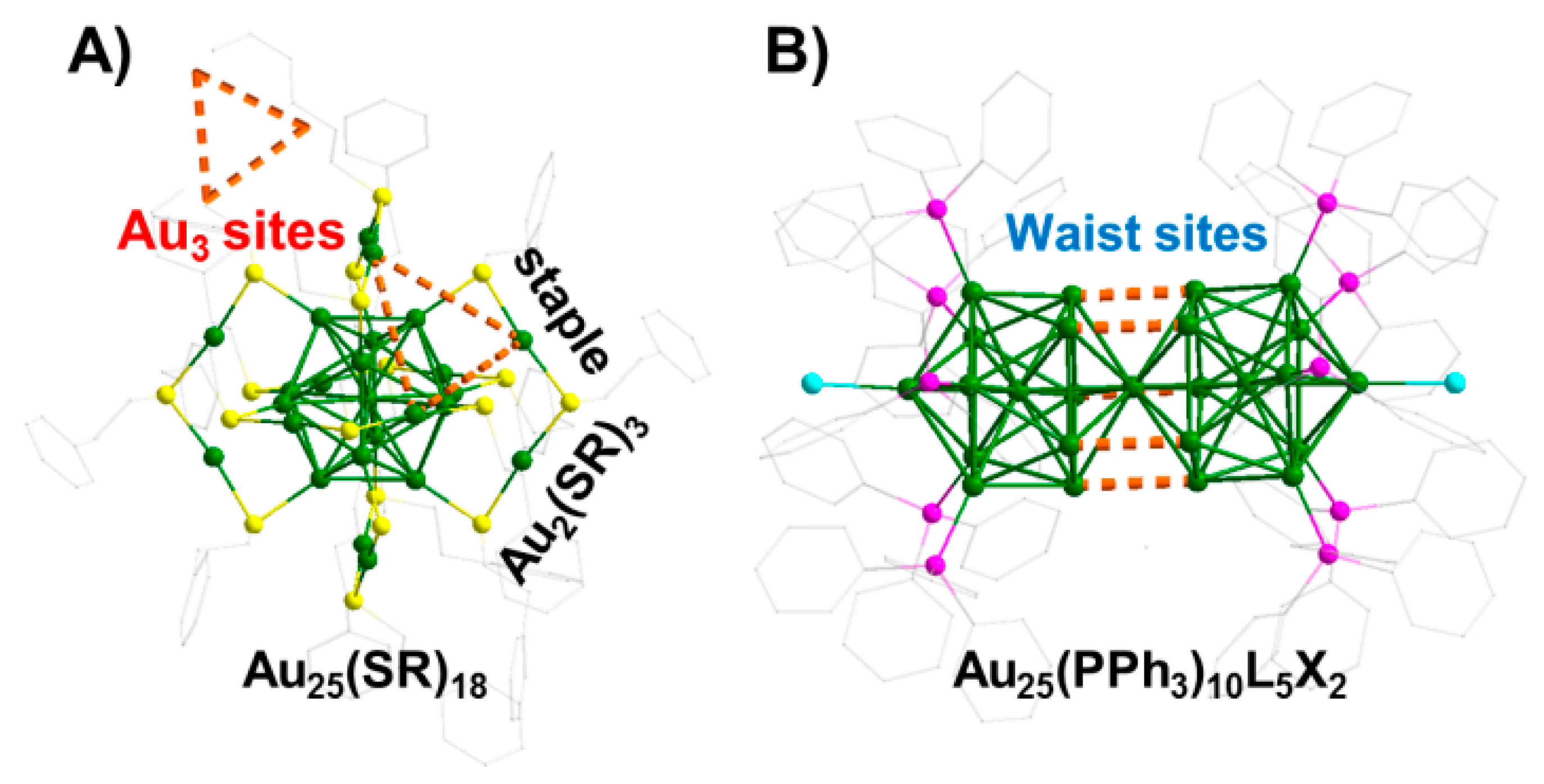
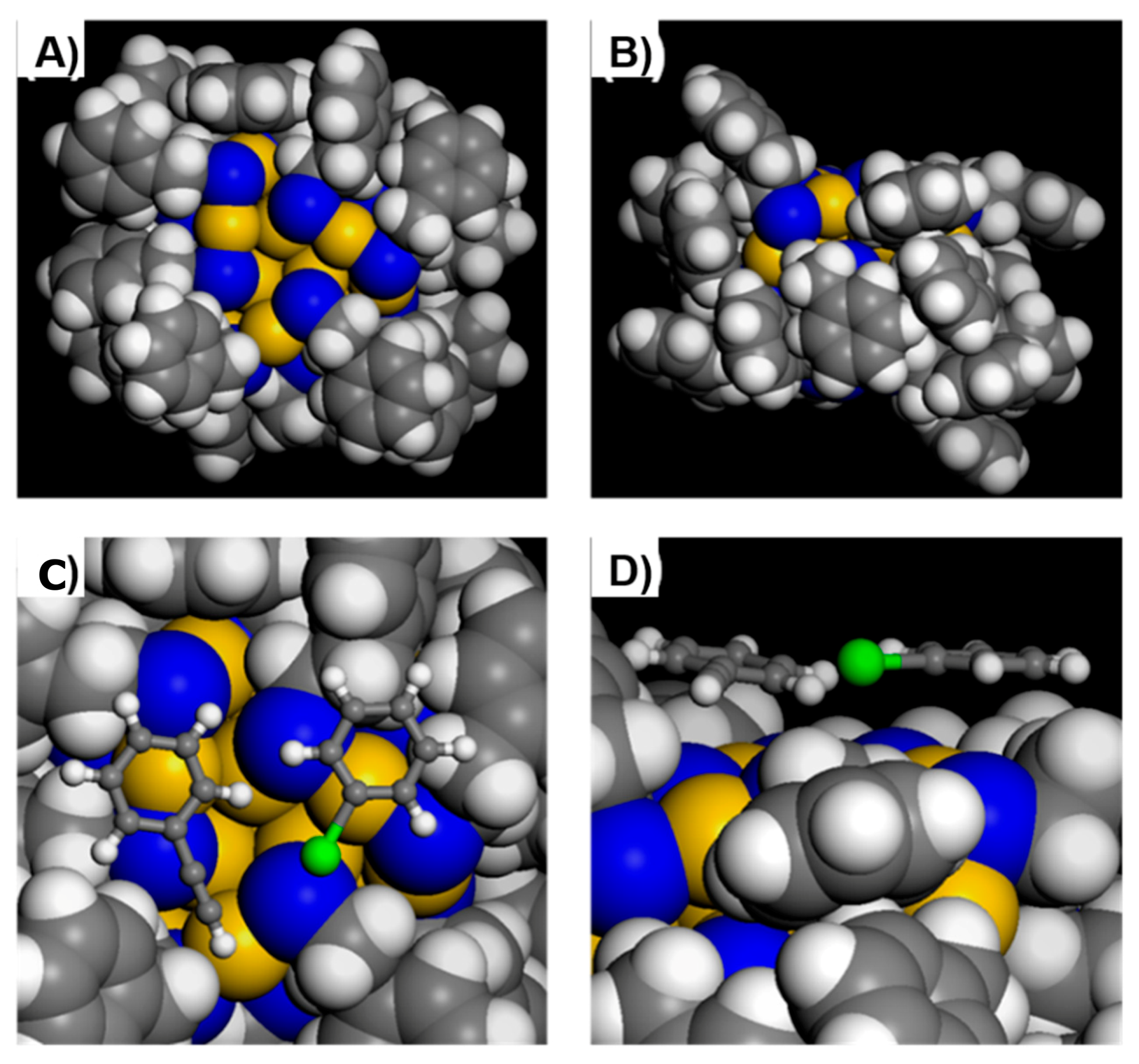
3.1. Framework
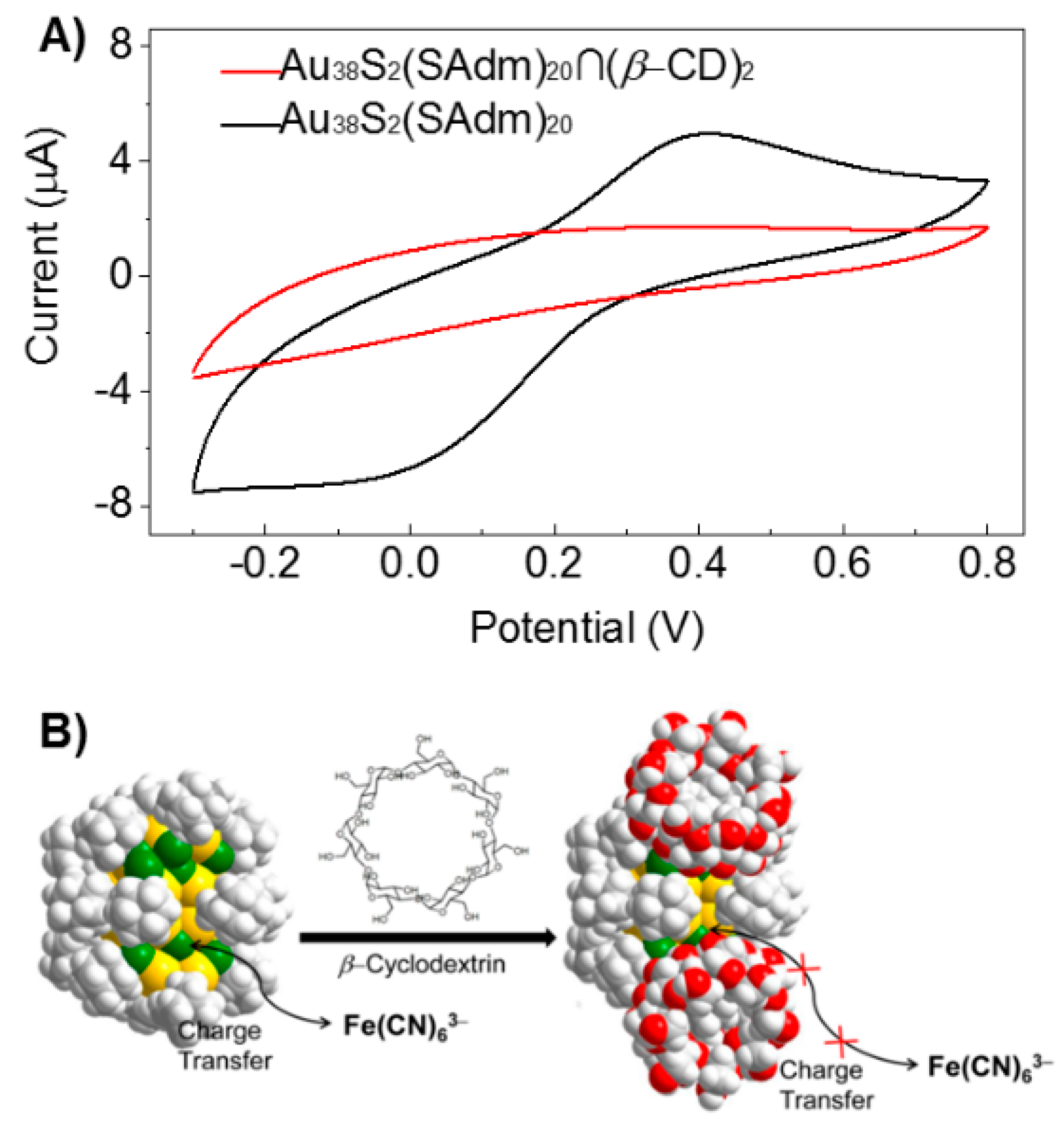
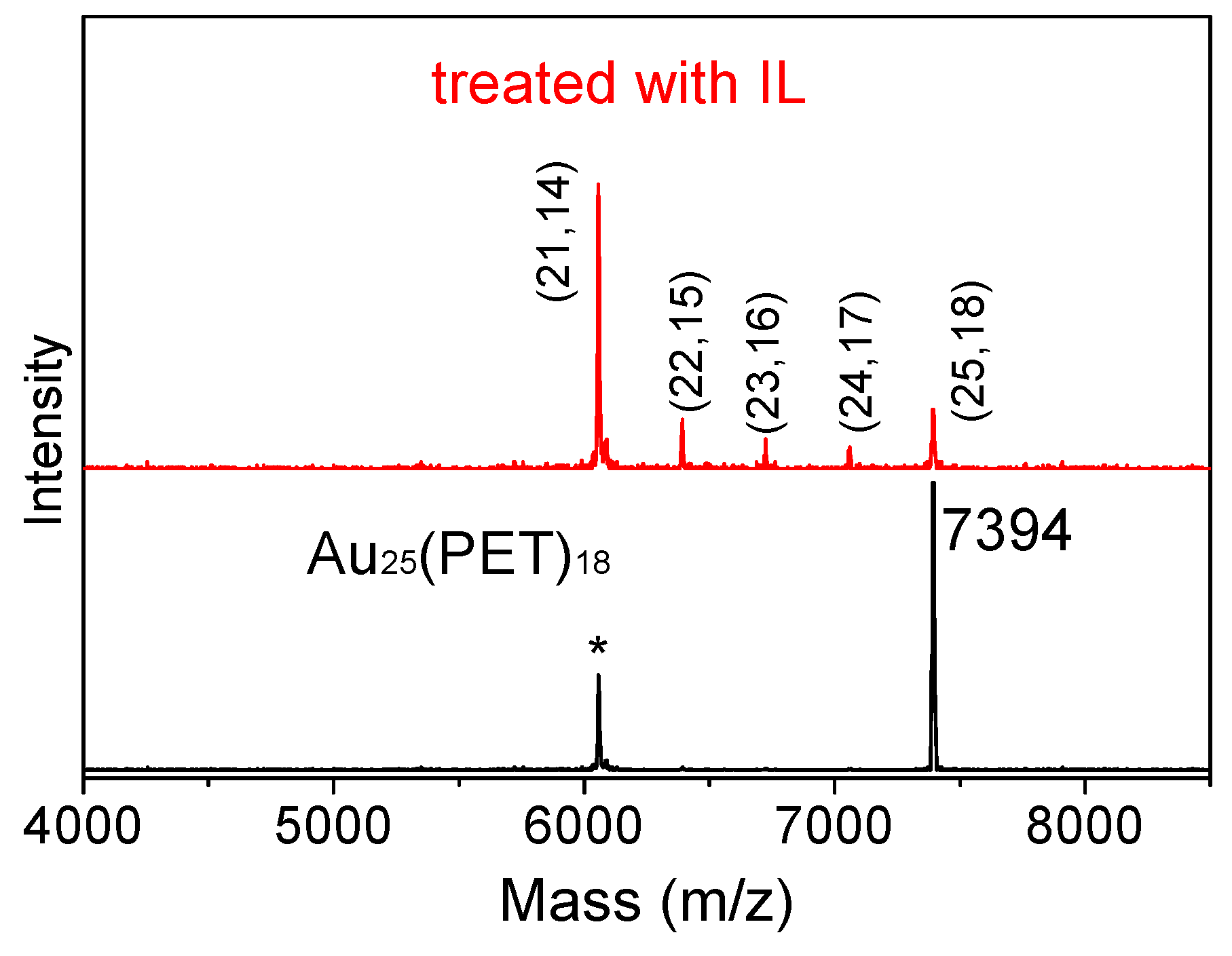
3.2. Redox Property of Au cluster
4. Catalytic Properties
4.1. Ullmann Coupling
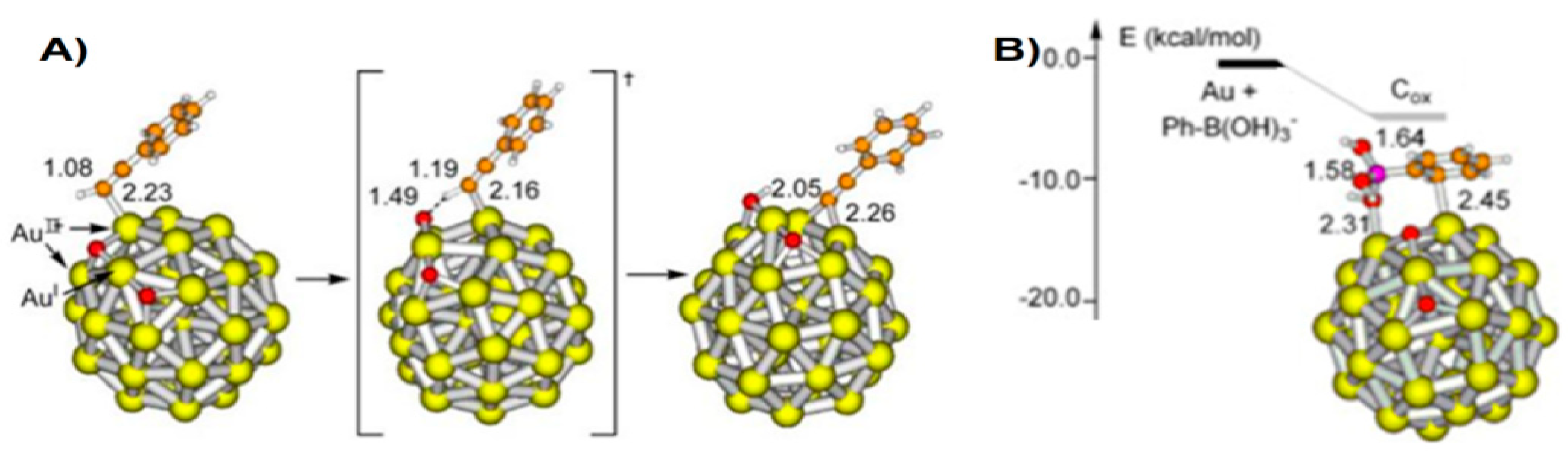
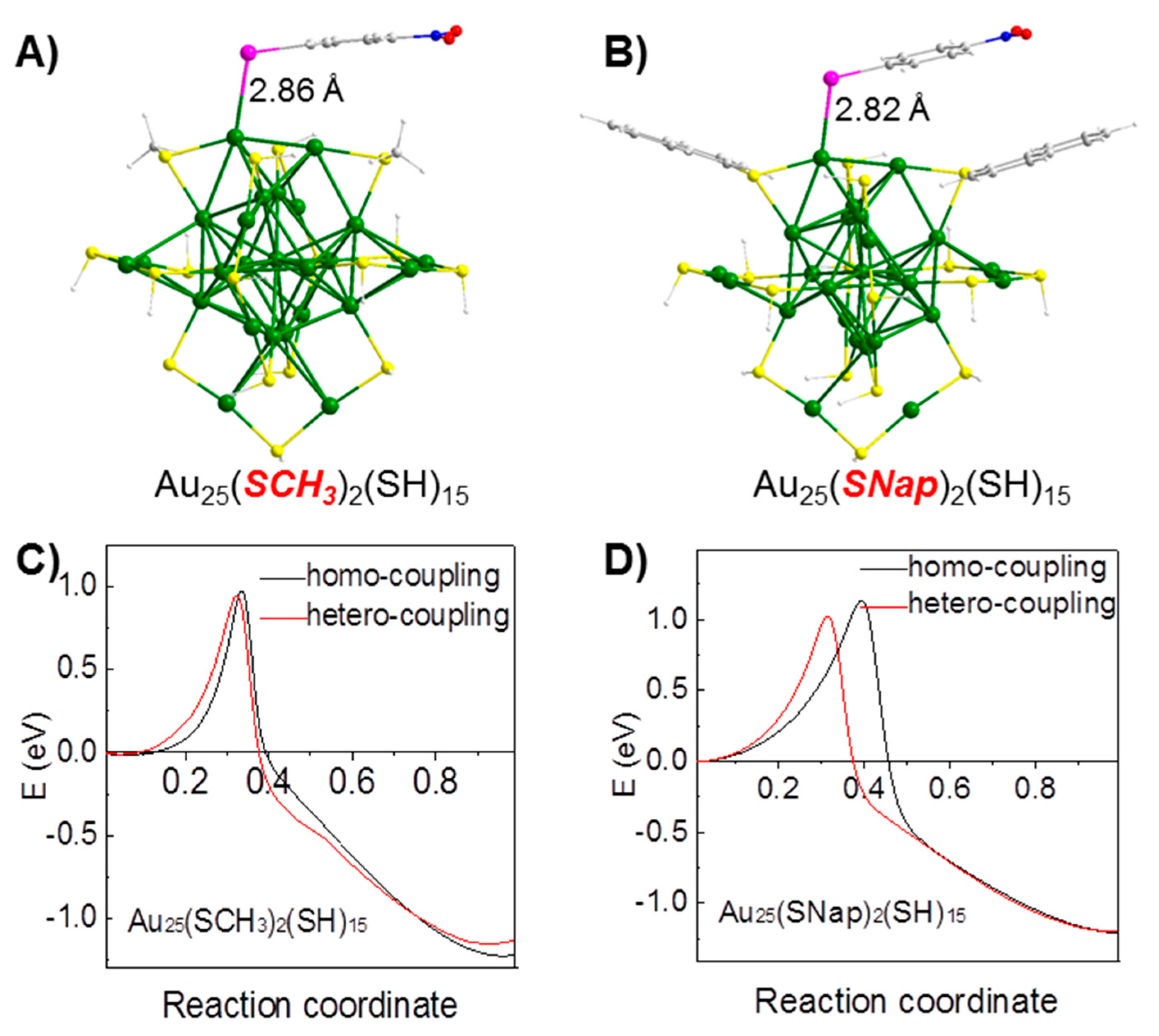
4.2. Suzuki Coupling
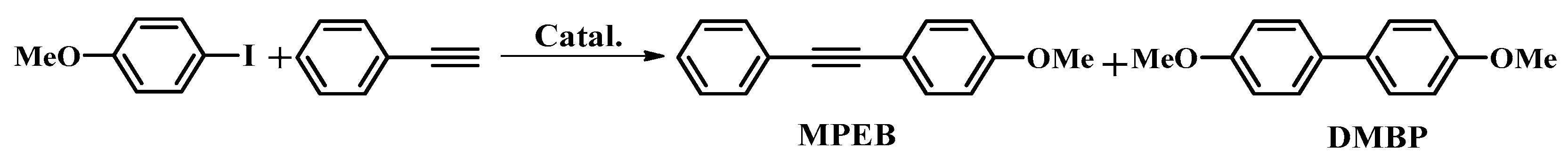
4.3. Sonogashira Coupling
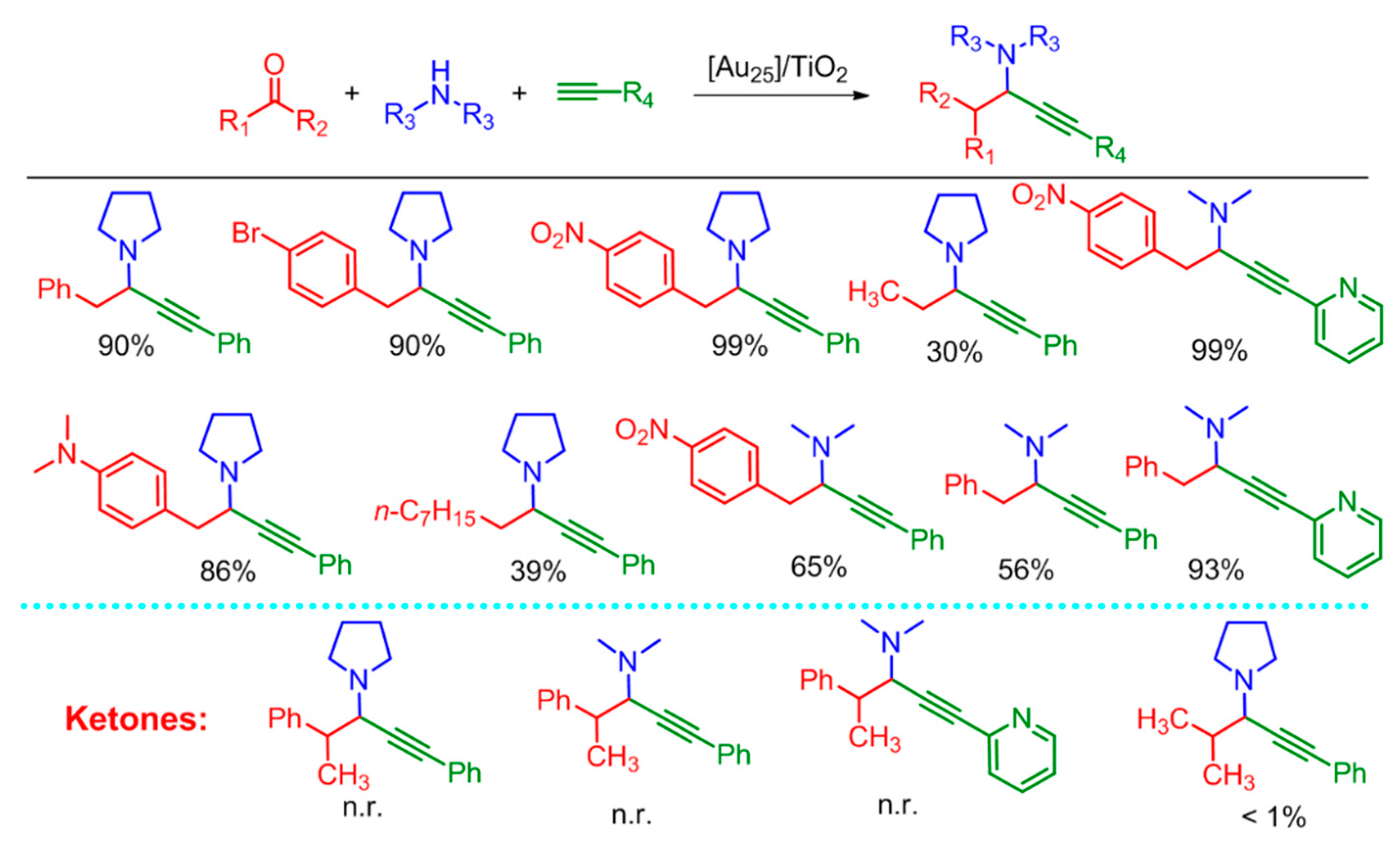
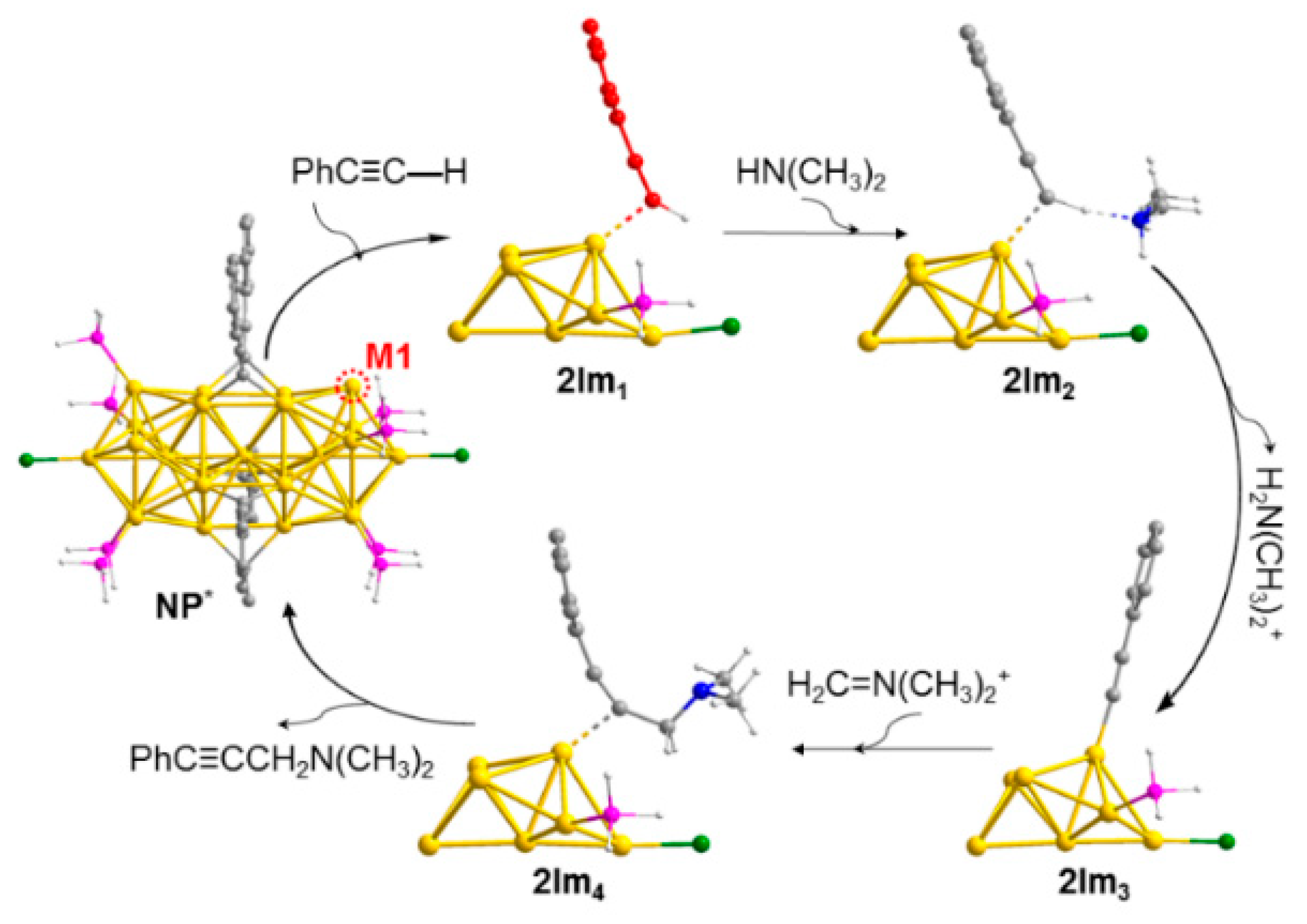
4.4. A3-Coupling
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon-monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Tsukuda, T.; Tsunoyama, H.; Sakurai, H. Aerobic oxidations catalyzed by colloidal nanogold. Chem. Asian J. 2011, 6, 736–748. [Google Scholar] [CrossRef]
- Della, P.C.; Falletta, E.; Rossi, M. Update on selective oxidation using gold. Chem. Soc. Rev. 2012, 41, 350–369. [Google Scholar]
- Li, G.; Qian, H.F.; Jin, R.C. Gold nanocluster-catalyzed selective oxidation of sulfide to sulfoxide. Nanoscale 2012, 4, 6714–6717. [Google Scholar] [CrossRef]
- Liu, C.; Yan, C.Y.; Lin, J.Z.; Yu, C.L.; Huang, J.H.; Li, G. One-pot synthesis of Au144(SCH2Ph)60 nanoclusters and their catalytic application. J. Mater. Chem. A 2015, 3, 20167–20173. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Li, G.; Zeng, C.J.; Jin, R.C. Thermally robust Au99(SPh)42 nanoclusters for chemoselective hydrogenation of nitrobenzaldehyde derivatives in water. J. Am. Chem. Soc. 2014, 136, 3673–3679. [Google Scholar] [CrossRef]
- Li, G.; Jiang, D.E.; Kumar, S.; Chen, Y.X.; Jin, R.C. Size dependence of atomically precise gold nanoclusters in chemoselective hydrogenation and active site structure. ACS Catal. 2014, 4, 2463–2469. [Google Scholar] [CrossRef]
- Naya, S.; Kimura, K.; Tada, H. One-step selective aerobic oxidation of amines to imines by gold nanoparticle-loaded Rutile titanium(IV) oxide plasmon photocatalyst. ACS Catal. 2013, 3, 10–13. [Google Scholar] [CrossRef]
- Chen, H.J.; Liu, C.; Wang, M.; Zhang, C.F.; Luo, N.C.; Wang, Y.H.; Abroshan, H.; Li, G.; Wang, F. Visible light gold nanocluster photocatalyst: Selective aerobic oxidation of amines to imines. ACS Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Taketoshi, A.; Haruta, M. Size- and structure-specificity in catalysis by gold clusters. Chem. Lett. 2014, 43, 380–387. [Google Scholar] [CrossRef]
- Lohse, S.E.; Murphy, C.J. The quest for shape control: A history of gold nanorod synthesis. Chem. Mater. 2013, 25, 1250–1261. [Google Scholar] [CrossRef]
- Lu, C.L.; Prasad, K.S.; Wu, H.L.; Ho, J.A.A.; Huang, M.H. Au nanocube-directed fabrication of Au-Pd core-shell nanocrystals with tetrahexahedral, concave octahedral, and octahedral structures and their electrocatalytic activity. J. Am. Chem. Soc. 2010, 132, 14546–14553. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zheng, K.; Li, G. Synthesis and characterization of size-controlled atomically-precise gold clusters. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Jin, R.C. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Li, G.; Jin, R.C. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef]
- Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 2014, 47, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Abroshan, H.; Liu, C.; Li, G. A Critical review on the catalytic applications of non-metallic gold nanoclusters: Selective oxidation, hydrogenation, and coupling reactions. Curr. Org. Chem. 2017, 21, 476–488. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Jin, R.C. Catalysis by gold NPs: Carbon-carbon coupling reactions. Nanotechnol. Rev. 2013, 5, 529–545. [Google Scholar]
- Zhou, Y.; Li, G. A Critical review on carbon-carbon coupling over ultra-small gold nanoclusters. Acta Phys.-Chim. Sin. 2017, 33, 1297–1309. [Google Scholar]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar] [CrossRef]
- Seechurn, C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Kanuru, V.K.; Kyriakou, G.; Beaumont, S.K.; Papageorgiou, A.C.; Watson, D.J.; Lambert, R.M. Sonogashira coupling on an extended gold surface in vacuo: Reaction of phenylacetylene with iodobenzene on Au(111). J. Am. Chem. Soc. 2010, 132, 8081–8086. [Google Scholar] [CrossRef]
- González-Arellano, C.; Abad, A.; Corma, A.; García, H.; Iglesias, M.; Sánchez, F. Catalysis by gold(I) and gold(III): A parallelism between homo- and heterogeneous catalysts for copper-free Sonogashira cross-coupling reactions. Angew. Chem. Int. Ed. 2007, 46, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Abroshan, H.; Liu, C.; Zhu, M.Z.; Li, G.; Haruta, M. Sonogashira cross-coupling on the Au(111) and Au(100) facets of gold nanorod catalysts: Experimental and computational investigation. J. Catal. 2015, 330, 354–361. [Google Scholar] [CrossRef]
- Li, G.; Zeng, C.J.; Jin, R.C. Chemoselective hydrogenation of nitrobenzaldehyde to nitrobenzyl alcohol with unsupported au nanorod catalysts in water. J. Phys. Chem. C 2015, 119, 11143–11147. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Chemical sensing and imaging with metallic nanorods. Chem. Commun. 2008, 5, 544–547. [Google Scholar] [CrossRef]
- Bai, X.T.; Gao, Y.N.; Liu, H.G.; Zheng, L.Q. Synthesis of amphiphilic ionic liquids terminated gold nanorods and their superior catalytic activity for the reduction of nitro compounds. J. Phys. Chem. C 2009, 113, 17730–17736. [Google Scholar] [CrossRef]
- Boronat, M.; Combita, D.; Concepción, P.; Corma, A.; García, H.; Juárez, R.; Laursen, S.; López-Castro, J.D. Making C-C bonds with gold: Identification of selective gold sites for homo- and cross-coupling reactions between iodobenzene and alkynes. J. Phys. Chem. C 2012, 116, 24855–24867. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Molecular approaches to catalysis naked gold NPs as quasi-molecular catalysts for green processes. J. Catal. 2011, 284, 138–147. [Google Scholar] [CrossRef]
- Corma, A.; Juárez, R.; Boronat, M.; Sánchez, F.; Iglesiasc, M.; García, H. Gold catalyzes the Sonogashira coupling reaction without the requirement of palladium impurities. Chem. Commun. 2011, 47, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Zheng, K.; Abroshan, H.; Kauffman, D.R.; Sun, J.; Li, G. Diphosphine-induced chiral propeller arrangement of gold nanoclusters for singlet oxygen photogeneration. Nano Res. 2018, 11, 5787–5798. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R.C. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, J.W.; Zhao, D.; Yang, Y.; Li, Z.M.; Li, G. Motif Mediated Au25(SPh)5(PPh3)10X2 Nanorod of Conjugated Electron Delocalization. Nano Res. 2019, 12, 501–507. [Google Scholar] [CrossRef]
- Li, G.; Jin, R.C. Gold nanocluster-catalyzed semihydrogenation: A unique activation pathway for terminal alkynes. J. Am. Chem. Soc. 2014, 136, 11347–11354. [Google Scholar] [CrossRef]
- Li, Z.M.; Liu, C.; Abroshan, H.; Kauffman, D.R.; Li, G. Au38S2(SAdm)20 Photocatalyst for One-Step Selective Aerobic Oxidations. ACS Catal. 2017, 7, 3368–3374. [Google Scholar] [CrossRef]
- Yan, C.Y.; Liu, C.; Abroshan, H.; Li, Z.M.; Qiu, R.; Li, G. Surface modification of adamantane-terminated gold nanoclusters using cyclodextrins. Phys. Chem. Chem. Phys. 2016, 18, 23358–23364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Wang, H.; Li, Z.; Zheng, K.; Li, S.; Li, G. Transition-metal-mediated catalytic properties of CeO2-supported gold clusters in aerobic alcohol oxidation. Nano Res. 2018, 11, 2139–2148. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kwak, K.; Lee, D. Amperometric sensing based on glutathione protected Au25 nanoparticles and their pH dependent electrocatalytic activity. Electroanalysis 2011, 23, 2116–2124. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Alfonso, D.; Matranga, C.; Qian, H.; Jin, R. Experimental and computational investigation of Au25 clusters and CO2: A unique interaction and enhanced electrocatalytic activity. J. Am. Chem. Soc. 2012, 134, 10237–10243. [Google Scholar] [CrossRef] [PubMed]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Li, G.; Kumar, K.S.; Kawasaki, H.; Jin, R.C. Stable Au25(SR)18/TiO2 Composite nanostructure with enhanced visible light photocatalytic activity. J. Phys. Chem. Lett. 2013, 4, 2847–2852. [Google Scholar] [CrossRef]
- Chen, H.J.; Liu, C.; Wang, M.; Zhang, C.F.; Li, G.; Wang, F. Thermally robust silica-enclosed Au25 nanocluster and its catalysis. Chin. J. Catal. 2016, 37, 1787–1793. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Lei, Y.; Jin, R.C. Au25 nanocluster-catalyzed Ullmann-type homocoupling reaction of aryl iodides. Chem. Commun. 2012, 48, 12005–12007. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Abroshan, H.; Liu, C.; Zhuo, S.; Li, Z.M.; Xie, Y.; Kim, H.J.; Rosi, N.L.; Jin, R.C. Tailoring the electronic and catalytic properties of Au25 nanoclusters via ligand engineering. ACS Nano 2016, 10, 7998–8005. [Google Scholar] [CrossRef] [PubMed]
- Abroshan, H.; Li, G.; Lin, J.Z.; Kim, H.J.; Jin, R.C. Molecular mechanism for the activation of Au25(SCH2CH2Ph)18 nanoclusters by imidazolium-based ionic liquids for catalysis. J. Catal. 2016, 337, 72–79. [Google Scholar] [CrossRef]
- Li, G.; Abroshan, H.; Chen, Y.X.; Jin, R.C.; Kim, H.J. Experimental and mechanistic understanding of aldehyde hydrogenation using Au25 nanoclusters with Lewis acids: Unique sites for catalytic reactions. J. Am. Chem. Soc. 2015, 137, 14295–14304. [Google Scholar] [CrossRef]
- Li, G.; Jiang, D.E.; Liu, C.; Yu, C.L.; Jin, R.C. Oxide-supported atomically precise gold nanocluster for catalyzing Sonogashira cross-coupling. J. Catal. 2013, 306, 177–183. [Google Scholar] [CrossRef]
- Kumara, C.; Aikens, C.M.; Dass, A. X-ray crystal structure and theoretical analysis of Au25−xAgx(SCH2CH2Ph)18- alloy. J. Phys. Chem. Lett. 2014, 5, 461–466. [Google Scholar] [CrossRef]
- Li, W.L.; Liu, C.; Abroshan, H.; Ge, Q.J.; Yang, X.J.; Xu, H.Y.; Li, G. Catalytic CO oxidation using bimetallic MxAu25-x clusters: A combined experimental and computational study on doping effects. J. Phys. Chem. C 2016, 120, 10261–10267. [Google Scholar] [CrossRef]
- Jiang, D.E.; Whetten, R.L. Magnetic doping of a thiolated-gold superatom: First-principles density functional theory calculations. Phys. Rev. B 2009, 80, 115402. [Google Scholar] [CrossRef]
- Qian, H.F.; Jiang, D.E.; Li, G.; Gayathri, C.; Das, A.; Gil, R.R.; Jin, R.C. Monoplatinum Doping of Gold Nanoclusters and Catalytic Application. J. Am. Chem. Soc. 2012, 134, 16159–16162. [Google Scholar] [CrossRef]
- Xie, S.H.; Tsunoyama, H.; Kurashige, W.; Negishi, Y.; Tsukuda, T. Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal. 2012, 2, 1519–1523. [Google Scholar] [CrossRef]
- Li, G.; Jin, R.C. Atomic level tuning of the catalytic properties: Doping effects of 25-atom bimetallic nanoclusters on styrene oxidation. Catal. Today 2016, 278, 187–191. [Google Scholar] [CrossRef]
- Li, Z.M.; Yang, X.J.; Liu, C.; Wang, J.; Li, G. Effects of doping in 25-atom bimetallic nanocluster catalysts for carbon-carbon coupling reaction of iodoanisole and phenylacetylene. Proc. Nat. Sci. Mater. Int. 2016, 26, 477–482. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, D.; Zhao, L.; Xiao, Q.; Wu, T.; Zhang, J.; Wan, C.-Q.; Li, G. Tailoring the Stability, Photocatalysis and Photoluminescence Properties of Au11 Nanocluster via Doping Engineering. Nanoscale Adv. 2019, 2. [Google Scholar] [CrossRef]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012, 2, 16–58. [Google Scholar] [CrossRef]
- Wei, C.M.; Li, C.J. A highly efficient three-component coupling of aldehyde, alkyne, and amines via C-H activation catalyzed by gold in water. J. Am. Chem. Soc. 2003, 125, 9584–9585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Liu, C.; Abroshan, H.; Li, Z.M.; Wang, J.; Li, G.; Haruta, M. Phosphine/phenylacetylide-ligated Au clusters for multicomponent coupling reactions. J. Catal. 2016, 337, 287–294. [Google Scholar] [CrossRef]
- Liu, C.; Abroshan, H.; Yan, C.Y.; Li, G.; Haruta, M. One-pot synthesis of Au11(PPh2Py)7Br3 for the highly chemoselective hydrogenation of nitrobenzaldehyde. ACS Catal. 2016, 6, 92–99. [Google Scholar] [CrossRef]
- Li, Q.; Das, A.; Wang, S.X.; Chen, Y.X.; Jin, R.C. Highly efficient three-component coupling reaction catalysed by atomically precise ligand-protected Au38(SC2H4Ph)24 nanoclusters. Chem. Commun. 2016, 52, 14298–14301. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.; Wu, Z. Improving the catalytic activity of Au25 nanocluster by peeling and doping. Chin. J. Chem. 2017, 35, 567–571. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Li, Z.M.; Ge, Q.J.; Zhou, Y.; Sun, J.; Li, G. Robust nickel cluster@Mes-HZSM-5 composite nanostructure with enhanced catalytic activity in the DTG reaction. J. Catal. 2018, 363, 26–33. [Google Scholar] [CrossRef]
- Li, Z.M.; Li, W.L.; Abroshan, H.; Ge, Q.J.; Zhou, Y.; Zhang, C.L.; Li, G.; Jin, R.C. Dual Effects of Water Vapor over Ceria-Supported Gold Clusters. Nanoscale 2018, 10, 6558–6565. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Z.; Fang, Q.; Ge, Q.; Sun, J.; Li, G. Manganese cluster induce the control synthesis of RHO- and CHA-type silicoaluminaphosphates for dimethylether to light olefin conversion. Fuel 2019, 244, 104–109. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.H.; Fang, Q.H.; Abroshan, H.; Kim, H.; Haruta, M.; Li, G. Gold-palladium nanoalloys supported by graphene oxide and lamellar TiO2 for direct synthesis of hydrogen peroxide. ACS Appl. Mater. Interfaces 2018, 10, 40599–40607. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.Y.; Huang, J.H.; Zhang, C.L.; Hong, F.; Zhou, Y.; Li, G.; Haruta, M. Efficient aerobic oxidation of glucose to gluconic acid over activated carbon-supported gold clusters. ChemSuSChem 2017, 10, 1976–1980. [Google Scholar] [CrossRef]
- Vilhelmsen, L.B.; Walton, K.S.; Sholl, D.S. Structure and mobility of metal clusters in MOFs: Au, Pd, and AuPd clusters in MOF-74. J. Am. Chem. Soc. 2012, 134, 12807–12816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Qin, Z.; Kim, H.J.; Abroshan, H.; Li, G. Silica-encapsulated gold nanoclusters for efficient acetylene hydrogenation to ethylene. ACS Appl. Nano Mater. 2019. [Google Scholar] [CrossRef]
- Ciobanu, M.; Cojocaru, B.; Teodorescu, C.; Vasiliu, F.; Coman, S.M.; Leitner, W.; Parvulescu, V.I. Heterogeneous amination of bromobenzene over titania-supported gold catalysts. J. Catal. 2012, 296, 43–54. [Google Scholar] [CrossRef]
- Fang, Q.; Qin, Z.; Shi, Y.; Liu, F.; Barkaoui, S.; Abroshan, H.; Li, G. Au/NiO composite: A catalyst for one-pot cascade conversion of furfural. ACS Appl. Energy Mater. 2019, 2, 2654–2661. [Google Scholar] [CrossRef]

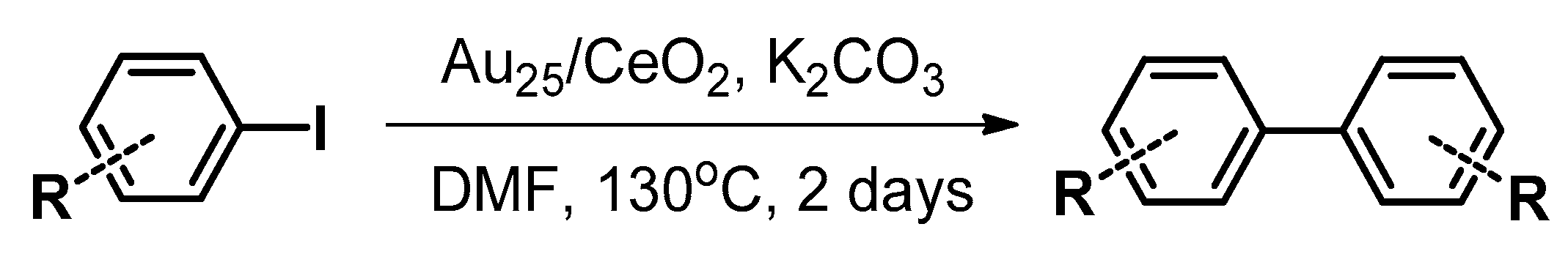
| Entry | Substrate | Product | Conversion (%) |
|---|---|---|---|
| 1 |  |  | 99.8 |
| 2 |  | 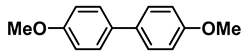 | 99.5 |
| 3 | 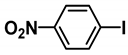 |  | 67.5 |
| 4 | 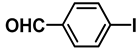 | 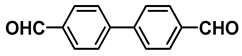 | 78.2 |
| 5 |  |  | 99.7 |

| Catalyst | Conversion (%) | Selectivity (%) |
|---|---|---|
| Au25(SC6H13)18 | 69 | 16 |
| Au25(PET)18 | 72 | 19 |
| Au25(SPh)18 | 80 | 50 |
| Au25(SNap)18 | 91 | 82 |
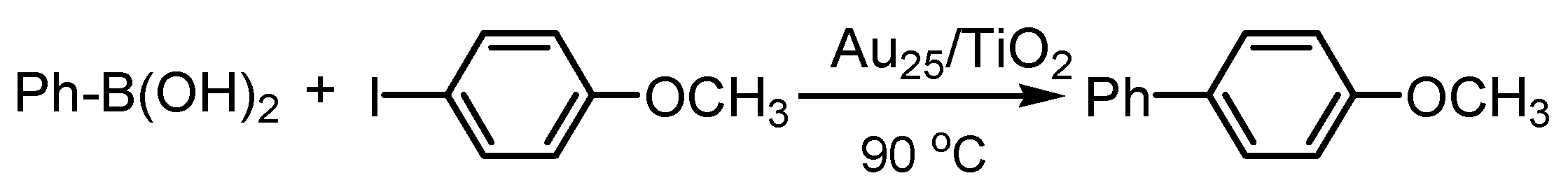
| Solvent | Conversion (%) |
|---|---|
| o-xylene + EtOH:H2O | <0.5 |
| DMF + EtOH:H2O | 4 |
| BMIM·Br + EtOH | 89 |
| BMIM·Cl + EtOH | 90 |
| BMIM·BF4 + EtOH | 94 |
| BMIM·BF4 + EtOH:H2O EtOH:H2O | >99 |
| BDiMIM·BF4 + EtOH | <0.5 |
| Entry | Catalyst | Conv. (%) | Selectivity (%) | |
|---|---|---|---|---|
| MPEB | DMBP | |||
| 1 | Au25(SR)18/CeO2 | 96.1 | 88.1 | 11.9 |
| 2 | Au25(SR)18/TiO2 | 92.8 | 82.9 | 17.1 |
| 3 | Au25(SR)18/SiO2 | 90.8 | 79.3 | 20.7 |
| 4 | Au25(SR)18/MgO | 93.3 | 80.6 | 19.4 |
| 5 | AuNC 2–3 nm (SC6H13)/CeO2 | 65.5 | 57.2 | 42.8 |
| 6 | CeO2 | n.r. | ||
| 7 | TiO2 | n.r. | ||
| Entry | Catalysts | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|
| MPEB | DMBP | |||
| 1 | Au25(SR)18 | 79.5 | 65.7 | 34.3 |
| 2 | AgxAu25−x(SR)18 | 83.0 | 55.4 | 44.6 |
| 3 | CuxAu25−x(SR)18 | 52.4 | 28.3 | 71.7 |
| 4 | Pt1Au24(SR)18 | 48.5 | 67.2 | 32.9 |
| Adsorption Energy (eV) | MxAu25−x(SR)18 Cluster | |||||
|---|---|---|---|---|---|---|
| Au25 | Pt1Au24 | Ag1Au24 | Ag2Au23 | Cu1Au24 | Cu2Au23 | |
| One PA | −0.51 | −0.50 | −0.61 | −0.60 | −0.52 | −0.52 |
| One IB | −0.60 | −0.61 | −0.59 | −0.59 | −0.54 | −0.58 |
| “PA + IB” pair | −1.11 | −1.11 | −1.20 | −1.19 | −1.06 | −1.10 |
| “IB + IB” pair | −1.20 | −1.22 | −1.18 | −1.18 | −1.08 | −1.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials 2019, 9, 838. https://doi.org/10.3390/nano9060838
Shi Q, Qin Z, Xu H, Li G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials. 2019; 9(6):838. https://doi.org/10.3390/nano9060838
Chicago/Turabian StyleShi, Quanquan, Zhaoxian Qin, Hui Xu, and Gao Li. 2019. "Heterogeneous Cross-Coupling over Gold Nanoclusters" Nanomaterials 9, no. 6: 838. https://doi.org/10.3390/nano9060838
APA StyleShi, Q., Qin, Z., Xu, H., & Li, G. (2019). Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials, 9(6), 838. https://doi.org/10.3390/nano9060838





