Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7)
Abstract
1. Introduction
2. Computational Methods
3. Results
3.1. Structural Modelling of Bulk Tc and C12A7
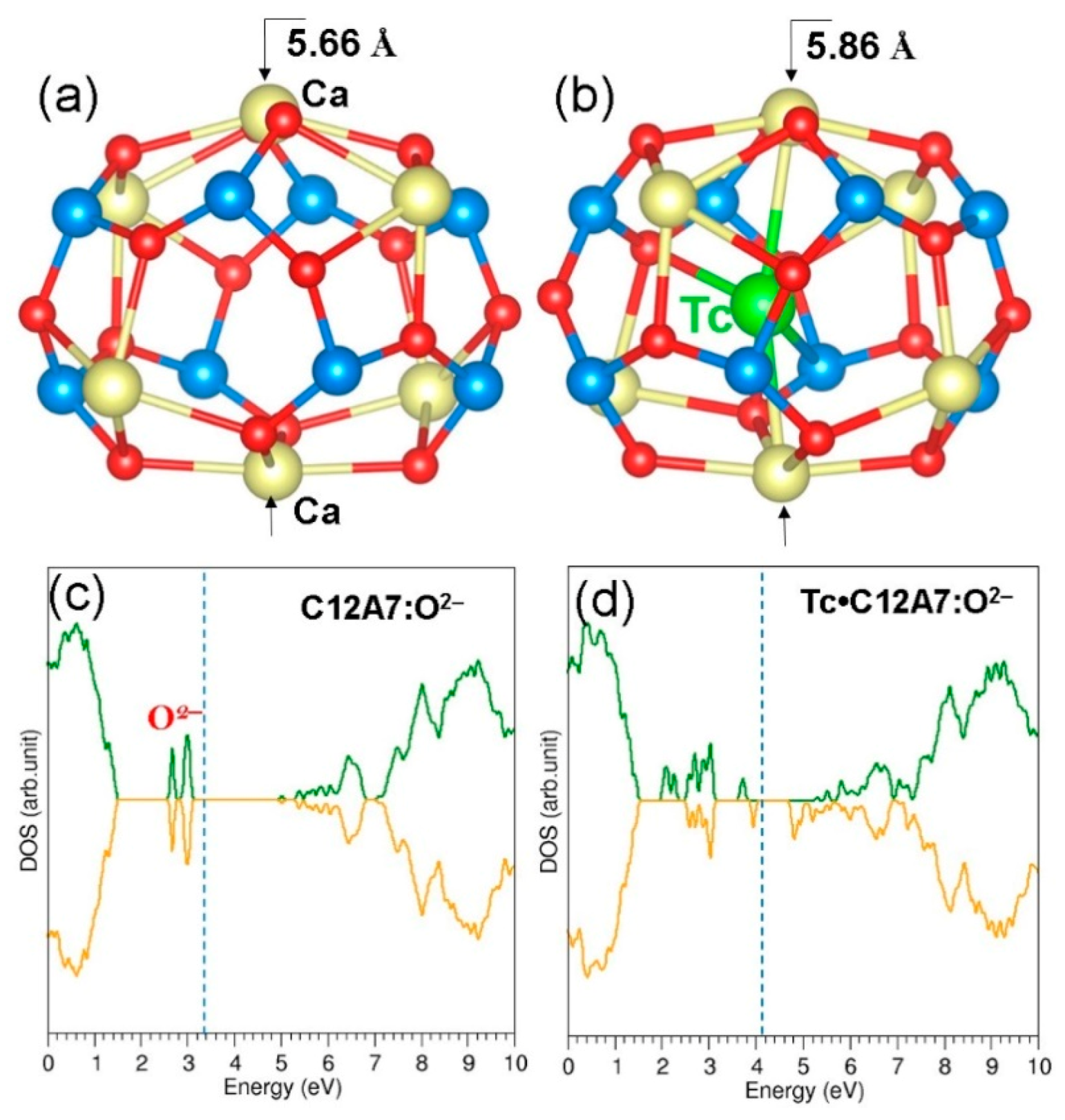
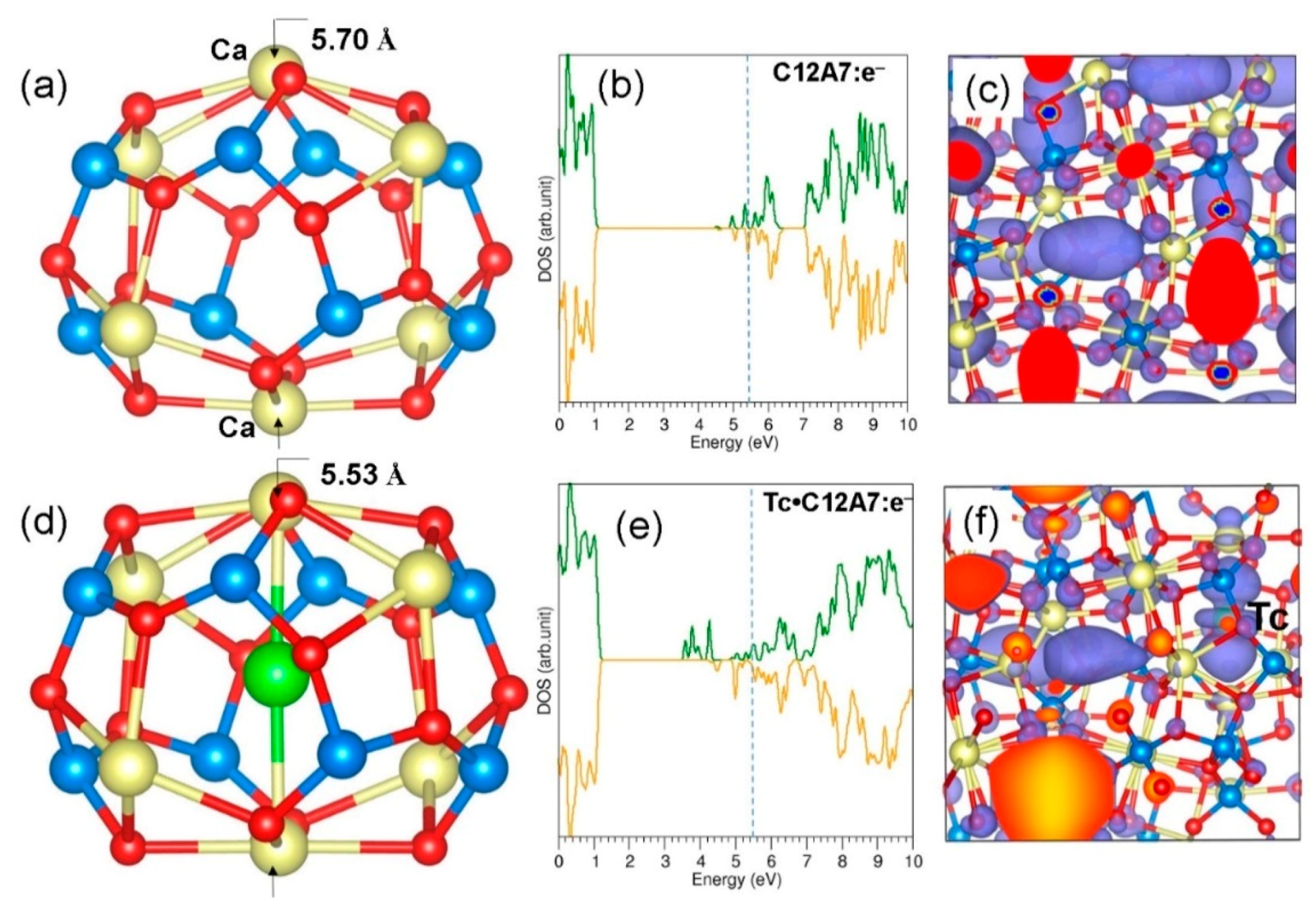
3.2. Encapsulation of Single Tc Atoms in A Cage of C12A7:O2−
3.3. Encapsulation of Single Tc Atoms in C12A7:e−
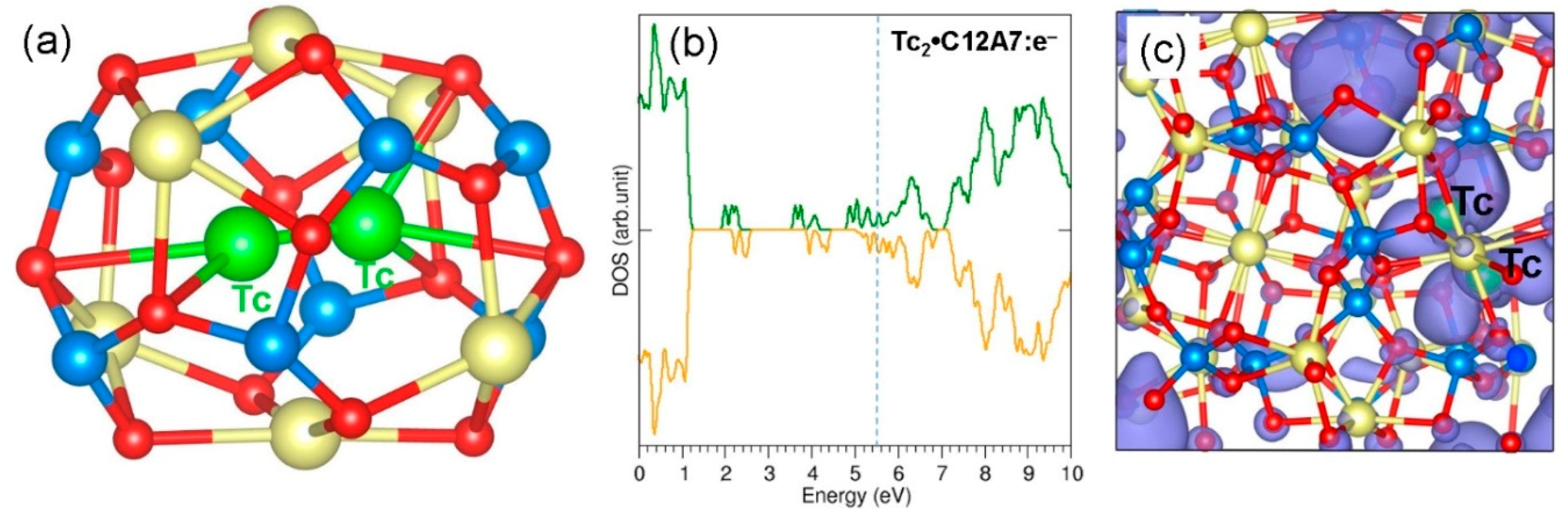
3.4. Stability of a Tc Dimer in C12A7:O2−
3.5. Stability of a Tc Dimer in C12A7:e−
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beals, D.M. Determination of technetium-99 in aqueous samples by isotope dilution inductively coupled plasma-mass spectrometry. J. Radioanal. Nucl. Chem. 1996, 204, 253–263. [Google Scholar] [CrossRef]
- Garcia-Leon, M. 99Tc in the environment: Sources, distribution and methods. J. Nucl. Radiochem. Sci. 2005, 6, 253–259. [Google Scholar]
- Schulte, E.H.; Scoppa, P. Sources and behavior of technetium in the environment. Sci. Total Environ. 1987, 64, 163–179. [Google Scholar] [CrossRef]
- Shi, K.; Hou, X.; Roos, P.; Wu, W. Determination of technetium-99 in environmental samples: A review. Anal. Chim. Acta 2012, 709, 1–20. [Google Scholar] [CrossRef]
- Berlyn, G.P.; Dhillon, S.S.; Koslow, E.E. Technetium: A toxic waste product of the nuclear fuel cycle: Effects on soybean growth and development. Environ. Manag. 1980, 4, 149–156. [Google Scholar] [CrossRef]
- McMaster, S.A.; Ram, R.; Faris, N.; Pownceby, M.I. Radionuclide disposal using the pyrochlore super group of minerals as a host matrix—A review. J. Hazard. Mater. 2018, 360, 257–269. [Google Scholar] [CrossRef]
- Higgo, J.J.W. Clay as a barrier to radionuclide migration. Prog. Nucl. Energy 1987, 19, 173–207. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, L.; Aguila, B.; Thallapally, P.K.; Xu, C.; Chen, J.; Wang, S.; Rogers, D.; Ma, S. Optimizing radionuclide sequestration in anion nanotraps with record pertechnetate sorption. Nat. Commun. 2019, 10, 1646. [Google Scholar] [CrossRef]
- Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review. Materials 2019, 12, 1399. [Google Scholar] [CrossRef]
- Shilina, A.S.; Bakhtin, V.D.; Burukhin, S.B.; Askhadullin, S.R. Sorption of cations of heavy metals and radionuclides from the aqueous media by new synthetic zeolite-like sorbent. Nucl. Energy Technol. 2017, 3, 249–254. [Google Scholar] [CrossRef]
- Yang, H.; Luo, M.; Luo, L.; Wang, H.; Hu, D.; Lin, J.; Wang, X.; Wang, Y.; Wang, S.; Bu, X.; et al. Highly Selective and Rapid Uptake of Radionuclide Cesium Based on Robust Zeolitic Chalcogenide via Stepwise Ion-Exchange Strategy. Chem. Mater. 2016, 28, 8774–8780. [Google Scholar] [CrossRef]
- Gadd, G.E.; Evans, P.J.; Hurwood, D.J.; Moricca, S.; McOrist, G.; Wall, T.; Elcombe, M.; Prasad, P. Endohedral Formation from Neutron Activation of Interstitial Rare Cas C60 Fullerides. Fuller. Sci. Technol. 1997, 5, 871–902. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Ohno, K. Radiochemical approaches for formation of endohedral fullerenes and MD simulation. Sci. Technol. Adv. Mater. 2004, 5, 621–624. [Google Scholar] [CrossRef]
- Belloni, F.; Kütahyali, C.; Rondinella, V.V.; Carbol, P.; Wiss, T.; Mangione, A. Can carbon nanotubes play a role in the field of nuclear waste management? Environ. Sci. Technol. 2009, 43, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Whetten, R.L. Beyond C60: The higher fullerenes. Acc. Chem. Res. 1992, 25, 119–126. [Google Scholar] [CrossRef]
- Kuganathan, N.; Arya, A.K.; Rushton, M.J.D.; Grimes, R.W. Trapping of volatile fission products by C60. Carbon 2018, 132, 477–485. [Google Scholar] [CrossRef]
- Kuganathan, N.; Selvanantharajah, N.; Iyngaran, P.; Abiman, P.; Chroneos, A. Cadmium trapping by C60 and B-, Si-, and N-doped C60. J. Appl. Phys. 2019, 125, 054302. [Google Scholar] [CrossRef]
- Kuganathan, N.; Green, J.C.; Himmel, H.-J. Dinitrogen fixation and activation by Ti and Zr atoms, clusters and complexes. New J. Chem. 2006, 30, 1253–1262. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pandey, S.K.; Misra, N. Encapsulation of lawrencium into C60 fullerene: Lr@C60versus Li@C60. Mater. Chem. Phys. 2016, 177, 437–441. [Google Scholar] [CrossRef]
- Kikuchi, K.; Kobayashi, K.; Sueki, K.; Suzuki, S.; Nakahara, H.; Achiba, Y.; Tomura, K.; Katada, M. Encapsulation of Radioactive 159Gd and 161Tb Atoms in Fullerene Cages. J. Am. Chem. Soc. 1994, 116, 9775–9776. [Google Scholar] [CrossRef]
- Saha, S.K.; Chowdhury, D.P.; Das, S.K.; Guin, R. Encapsulation of radioactive isotopes into C60 fullerene cage by recoil implantation technique. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 243, 277–281. [Google Scholar] [CrossRef]
- Weck, P.F.; Kim, E.; Czerwinski, K.R.; Tománek, D. Structural and magnetic properties of Tcn@C60 endohedral metalofullerenes: First-principles predictions. Phys. Rev. B 2010, 81, 125448. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Choi, D.Y.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Liu, D.; Zhong, C. Radioactive Barium Ion Trap Based on Metal–Organic Framework for Efficient and Irreversible Removal of Barium from Nuclear Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 8527–8535. [Google Scholar] [CrossRef]
- Watauchi, S.; Tanaka, I.; Hayashi, K.; Hirano, M.; Hosono, H. Crystal growth of Ca12Al14O33 by the floating zone method. J. Cryst. Growth 2002, 237–239, 801–805. [Google Scholar] [CrossRef]
- Imlach, J.A.; Dent Glasser, L.S.; Glasser, F.P. Excess oxygen and the stability of “12CaO.7A12O3”. Cem. Concr. Res. 1971, 1, 57–61. [Google Scholar] [CrossRef]
- Hayashi, K.; Hirano, M.; Hosono, H. Thermodynamics and Kinetics of Hydroxide Ion Formation in 12CaO·7Al2O3. J. Phys. Chem. B 2005, 109, 11900–11906. [Google Scholar] [CrossRef]
- Miyakawa, M.; Kamioka, H.; Hirano, M.; Kamiya, T.; Sushko, P.V.; Shluger, A.L.; Matsunami, N.; Hosono, H. Photoluminescence from Au ion-implanted nanoporous single-crystal. 12CaO∙7Al2O3. Phys. Rev. B 2006, 73, 205108. [Google Scholar] [CrossRef]
- Kuganathan, N.; Hosono, H.; Shluger, A.L.; Sushko, P.V. Enhanced N2 Dissociation on Ru-Loaded Inorganic Electride. J. Am. Chem. Soc. 2014, 136, 2216–2219. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef]
- Toda, Y.; Hirayama, H.; Kuganathan, N.; Torrisi, A.; Sushko, P.V.; Hosono, H. Activation and splitting of carbon dioxide on the surface of an inorganic electride material. Nat. Commun. 2013, 4, 2378. [Google Scholar] [CrossRef]
- Kuganathan, N.; Grimes, R.W.; Chroneos, A. Encapsulation of heavy metals by a nanoporous complex oxide 12CaO·7Al2O3. J. Appl. Phys. 2019, 125, 165103. [Google Scholar] [CrossRef]
- Song, C.; Sun, J.; Qiu, S.; Yuan, L.; Tu, J.; Torimoto, Y.; Sadakata, M.; Li, Q. Atomic Fluorine Anion Storage Emission Material C12A7-F−and Etching of Si and SiO2 by Atomic Fluorine Anions. Chem. Mater. 2008, 20, 3473–3479. [Google Scholar] [CrossRef]
- Hayashi, F.; Tomota, Y.; Kitano, M.; Toda, Y.; Yokoyama, T.; Hosono, H. NH2− Dianion Entrapped in a Nanoporous 12CaO·7Al2O3 Crystal by Ammonothermal Treatment: Reaction Pathways, Dynamics, and Chemical Stability. J. Am. Chem. Soc. 2014, 136, 11698–11706. [Google Scholar] [CrossRef]
- Matsuishi, S.; Toda, Y.; Miyakawa, M.; Hayashi, K.; Kamiya, T.; Hirano, M.; Tanaka, I.; Hosono, H. High-Density Electron Anions in a Nanoporous Single Crystal: [Ca24Al28O64]4+(4e-). Science 2003, 301, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Matsuishi, S.; Nomura, T.; Kubota, Y.; Takata, M.; Hayashi, K.; Kamiya, T.; Hirano, M.; Hosono, H. Metallic State in a Lime−Alumina Compound with Nanoporous Structure. Nano Lett. 2007, 7, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kuganathan, N.; Green, J.C. Mercury telluride crystals encapsulated within single walled carbon nanotubes: A density functional study. Int. J. Quantum Chem. 2008, 108, 797–807. [Google Scholar] [CrossRef]
- Kuganathan, N.; Green, J.C. 1D lead iodide crystals encapsulated within single walled carbon nanotubes. Int. J. Quantum Chem. 2009, 109, 171–175. [Google Scholar] [CrossRef]
- Kuganathan, N.; Green, J.C. Crystal structure of low-dimensional Cu(i) iodide: DFT prediction of cuprophilic interactions. Chem. Commun. 2008, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N. Antimony Selenide Crystals Encapsulated within Single Walled Carbon Nanotubes-A DFT Study. J. Chem. 2009, 6, S147–S152. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Ge, H.; Kuganathan, N.; Mirabello, V.; Jacobs, R.M.J.; Rees, N.H.; Stoppiello, C.T.; Khlobystov, A.N.; Tyrrell, R.M.; Como, E.D.; Pascu, S.I. Encapsulation of Cadmium Selenide Nanocrystals in Biocompatible Nanotubes: DFT Calculations, X-ray Diffraction Investigations, and Confocal Fluorescence Imaging. ChemistryOpen 2018, 7, 144–158. [Google Scholar] [CrossRef]
- Bichoutskaia, E.; Liu, Z.; Kuganathan, N.; Faulques, E.; Suenaga, K.; Shannon, I.J.; Sloan, J. High-precision imaging of an encapsulated Lindqvist ion and correlation of its structure and symmetry with quantum chemical calculations. Nanoscale 2012, 4, 1190–1199. [Google Scholar] [CrossRef]
- Hu, Z.; Pantoş, G.D.; Kuganathan, N.; Arrowsmith, R.L.; Jacobs, R.M.J.; Kociok-Köhn, G.; O’Byrne, J.; Jurkschat, K.; Burgos, P.; Tyrrell, R.M.; Botchway, S.W.; Sanders, J.K.M.; Pascu, S.I. Interactions Between Amino Acid-Tagged Naphthalenediimide and Single Walled Carbon Nanotubes for the Design and Construction of New Bioimaging Probes. Adv. Funct. Mater. 2012, 22, 503–518. [Google Scholar] [CrossRef]
- Mao, B.; Calatayud, D.G.; Mirabello, V.; Kuganathan, N.; Ge, H.; Jacobs, R.M.J.; Shepherd, A.M.; Ribeiro Martins, J.A.; Bernardino De La Serna, J.; Hodges, B.J.; Botchway, S.W.; Pascu, S.I. Fluorescence-Lifetime Imaging and Super-Resolution Microscopies Shed Light on the Directed- and Self-Assembly of Functional Porphyrins onto Carbon Nanotubes and Flat Surfaces. Chem. A Eur. J. 2017, 23, 9772–9789. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.; Bichoutskaia, E.; Liu, Z.; Kuganathan, N.; Faulques, E.; Suenaga, K.; Shannon, I.J. Aberration corrected imaging of a carbon nanotube encapsulated Lindqvist Ion and correlation with Density Functional Theory. J. Phys. Conf. Ser. 2012, 371, 012018. [Google Scholar] [CrossRef]
- Zoberbier, T.; Chamberlain, T.W.; Biskupek, J.; Kuganathan, N.; Eyhusen, S.; Bichoutskaia, E.; Kaiser, U.; Khlobystov, A.N. Interactions and Reactions of Transition Metal Clusters with the Interior of Single-Walled Carbon Nanotubes Imaged at the Atomic Scale. J. Am. Chem. Soc. 2012, 134, 3073–3079. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Encapsulation of cadmium telluride nanocrystals within single walled carbon nanotubes. Inorg. Chim. Acta 2019, 488, 246–254. [Google Scholar] [CrossRef]
- Pascu, S.I.; Kuganathan, N.; Tong, L.H.; Jacobs, R.M.J.; Barnard, P.J.; Chu, B.T.; Huh, Y.; Tobias, G.; Salzmann, C.G.; Sanders, J.K.M.; Green, M.L.H.; Green, J.C. Interactions between tripodal porphyrin hosts and single walled carbon nanotubes: An experimental and theoretical (DFT) account. J. Mater. Chem. 2008, 18, 2781–2788. [Google Scholar] [CrossRef]
- Chuvilin, A.; Bichoutskaia, E.; Gimenez-Lopez, M.C.; Chamberlain, T.W.; Rance, G.A.; Kuganathan, N.; Biskupek, J.; Kaiser, U.; Khlobystov, A.N. Self-assembly of a sulphur-terminated graphene nanoribbon within a single-walled carbon nanotube. Nat. Mater. 2011, 10, 687. [Google Scholar] [CrossRef]
- Kuganathan, N. DFT Modelling of Tripeptides (Lysine-Tryptophan-Lysine) Interacting with Single Walled Carbon Nanotubes. J. Chem. 2010, 7, 870–874. [Google Scholar] [CrossRef][Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C: The Art of Scientific Computing, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; p. 994. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Lam, D.J.; Darby, J.B.; Downey, J.W.; Norton, L.J. α-Manganese Phases containing Technetium-99. Nature 1961, 192, 744. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]




| Parameter | Calcd | Exptl [56] | |∆|(%) |
|---|---|---|---|
| a = b (Å) | 2.723 | 2.743 | 0.73 |
| c (Å) | 4.378 | 4.400 | 0.50 |
| α = β | 90.00 | 90.00 | 0.00 |
| γ (°) | 120.0 | 120.0 | 0.00 |
| V (Å3) | 28.26 | 28.67 | 1.43 |
| Parameter | Calcd | Exptl [26] | |∆|(%) |
|---|---|---|---|
| a (Å) | 12.04 | 11.99 | 0.42 |
| b (Å) | 12.01 | 11.99 | 0.17 |
| c (Å) | 12.01 | 11.99 | 0.17 |
| α (°) | 90.02 | 90.0 | 0.02 |
| β (°) | 89.95 | 90.0 | 0.06 |
| γ (°) | 89.93 | 90.0 | 0.08 |
| V (Å3) | 1738.66 | 1727.38 | 0.65 |
| Reaction | Encapsulation Energy (eV/atom) with Respect to Tc Atom | Bader Charge |e| on Tc |
|---|---|---|
| Tc + Tc + C12A7:O2− → Tc2: C12A7:O2− | −3.78 | +0.33, −0.44 |
| Tc + Tc: C12A7:O2− → Tc2: C12A7:O2− | −2.65 | |
| with respect to Tc dimer (eV/atom) | ||
| Tc2 + C12A7:O2− → Tc2: C12A7:O2− | −1.16 |
| Reaction | Encapsulation Energy (eV/atom) with Respect to Tc Atom | Bader Charge |e| on Tc |
|---|---|---|
| Tc + Tc + C12A7:e− → Tc2: C12A7:e− | −3.93 | −0.38, −0.51 |
| Tc + Tc: C12A7:e− → Tc2: C12A7:e− | −2.15 | |
| with respect to Tc dimer (eV/atom) | ||
| Tc2 + C12A7:e− → Tc2: C12A7:e− | −1.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuganathan, N.; Chroneos, A. Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7). Nanomaterials 2019, 9, 816. https://doi.org/10.3390/nano9060816
Kuganathan N, Chroneos A. Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7). Nanomaterials. 2019; 9(6):816. https://doi.org/10.3390/nano9060816
Chicago/Turabian StyleKuganathan, Navaratnarajah, and Alexander Chroneos. 2019. "Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7)" Nanomaterials 9, no. 6: 816. https://doi.org/10.3390/nano9060816
APA StyleKuganathan, N., & Chroneos, A. (2019). Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7). Nanomaterials, 9(6), 816. https://doi.org/10.3390/nano9060816







