Pulse Duration and Wavelength Effects of Laser Ablation on the Oxidation, Hydrolysis, and Aging of Aluminum Nanoparticles in Water
Abstract
:1. Introduction
2. Experimental
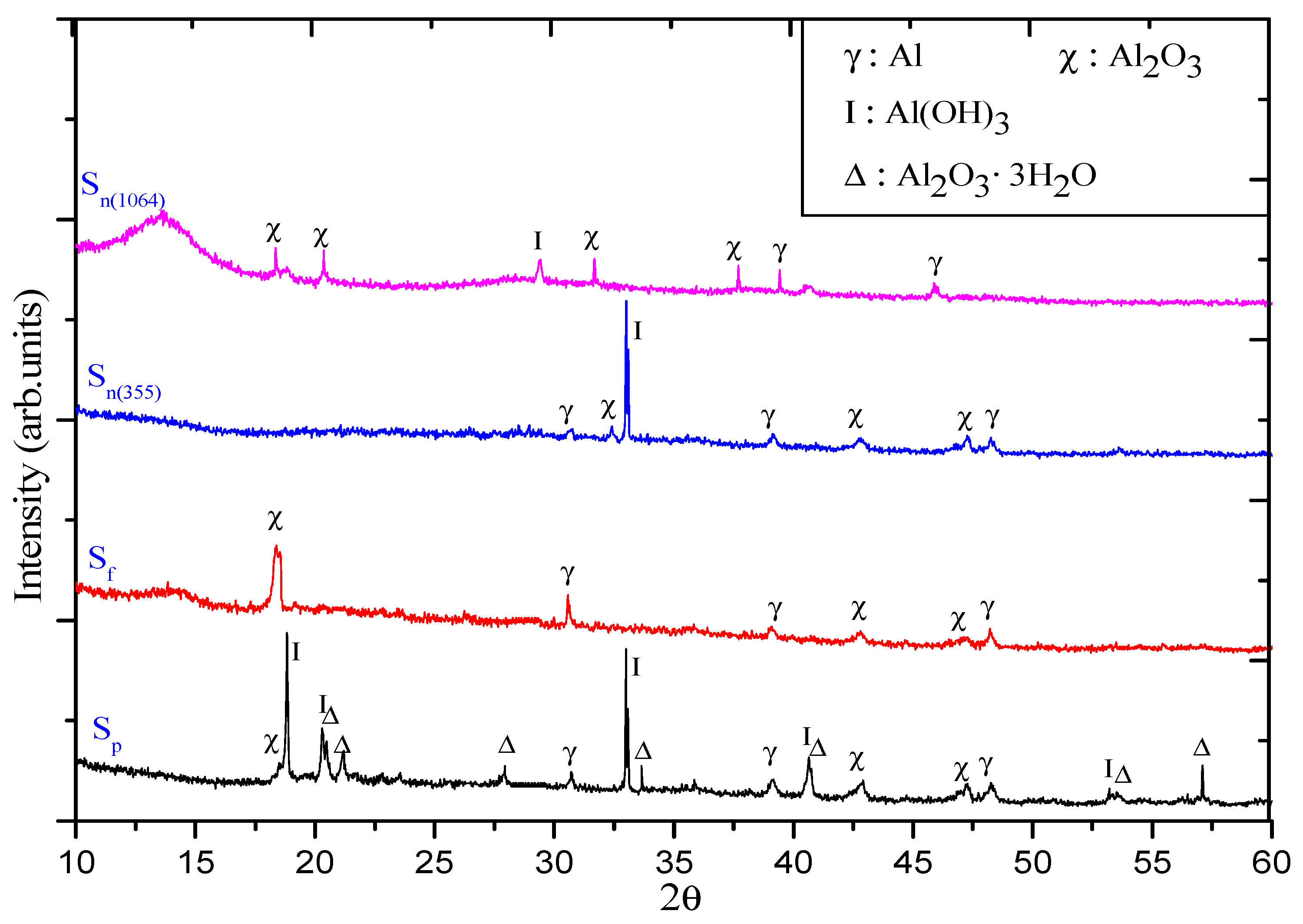
3. Results and Discussion
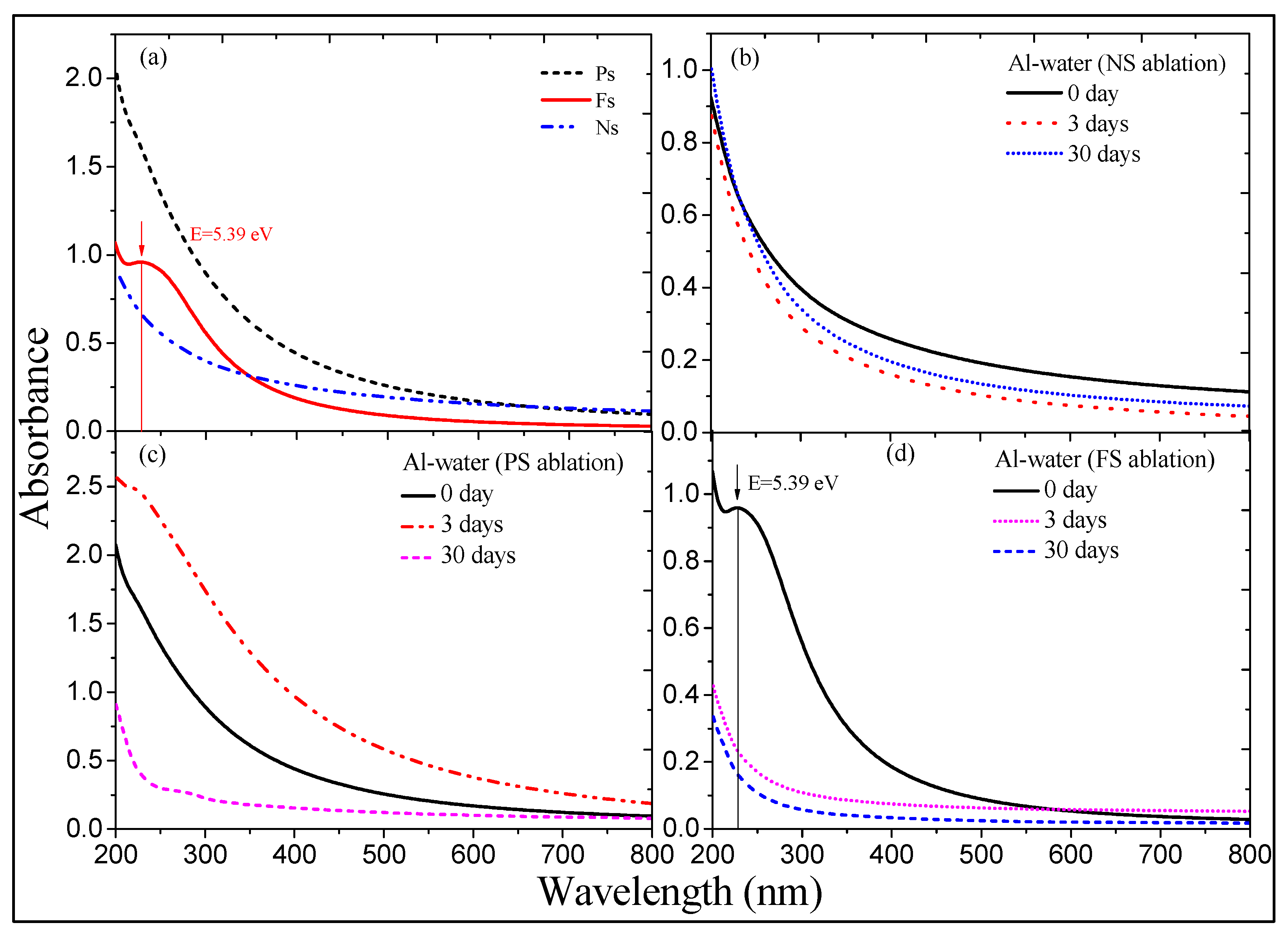
3.1. Color and Spectral Variations of ns-, ps-, and fs-Induced Al Suspensions
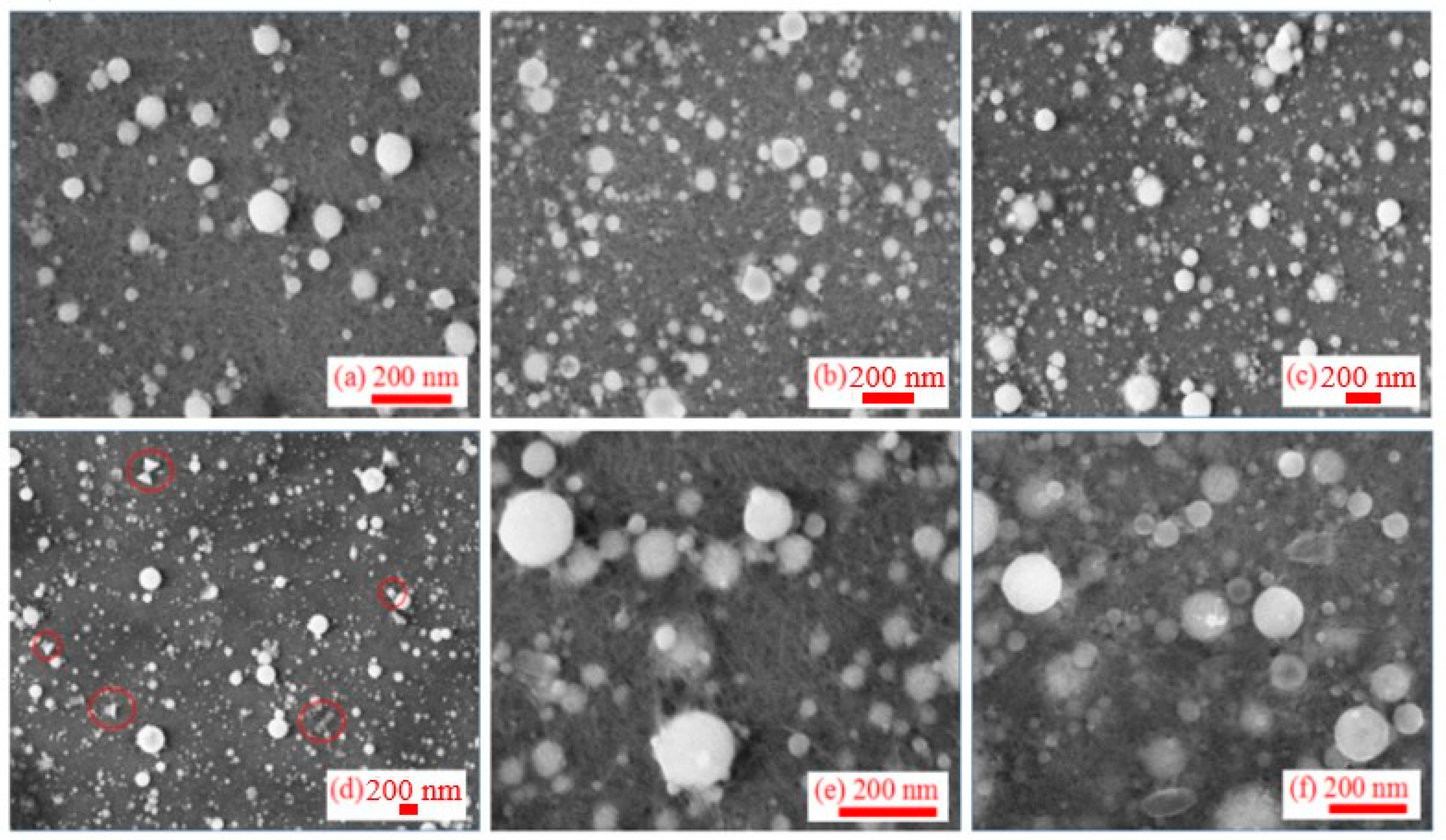
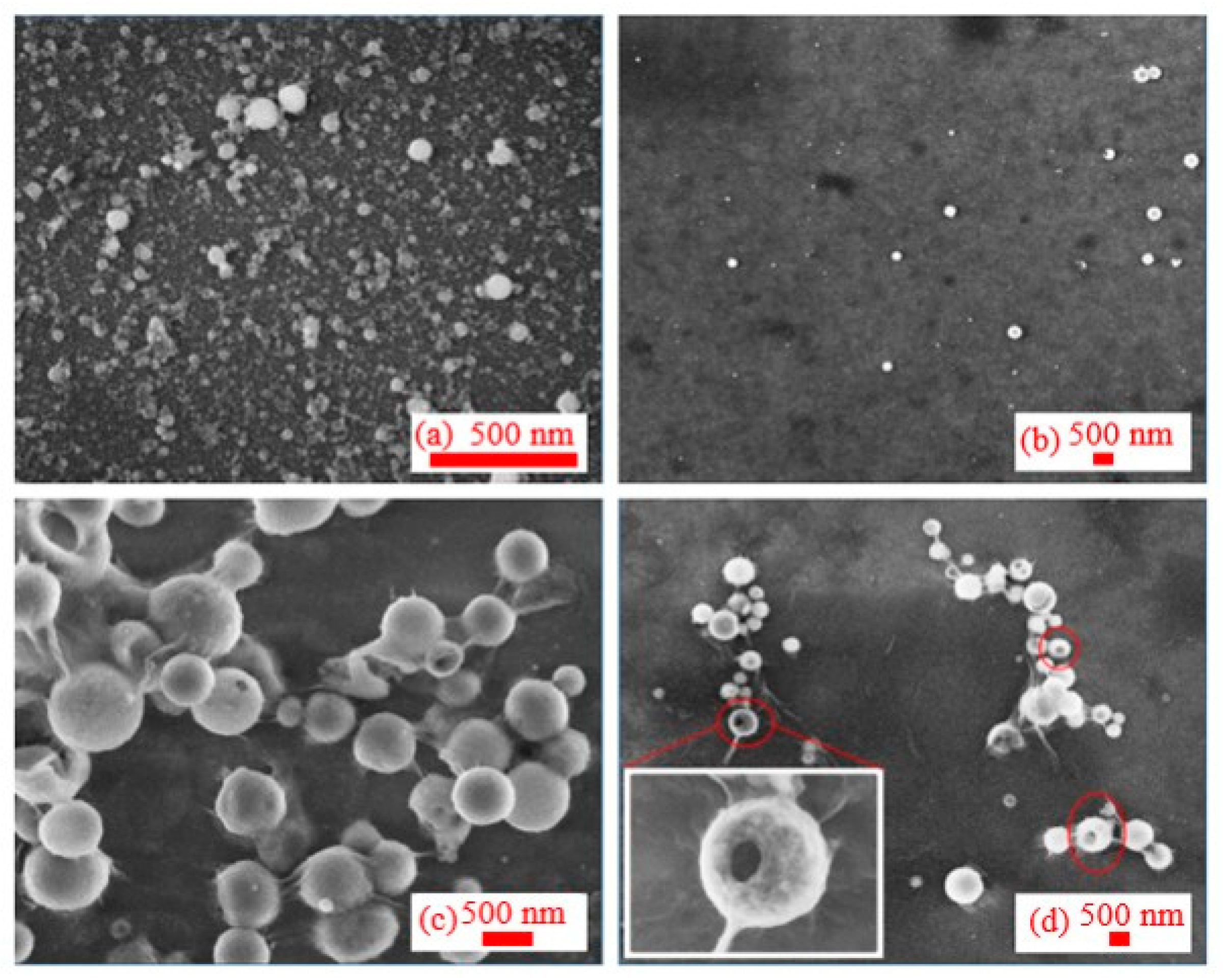
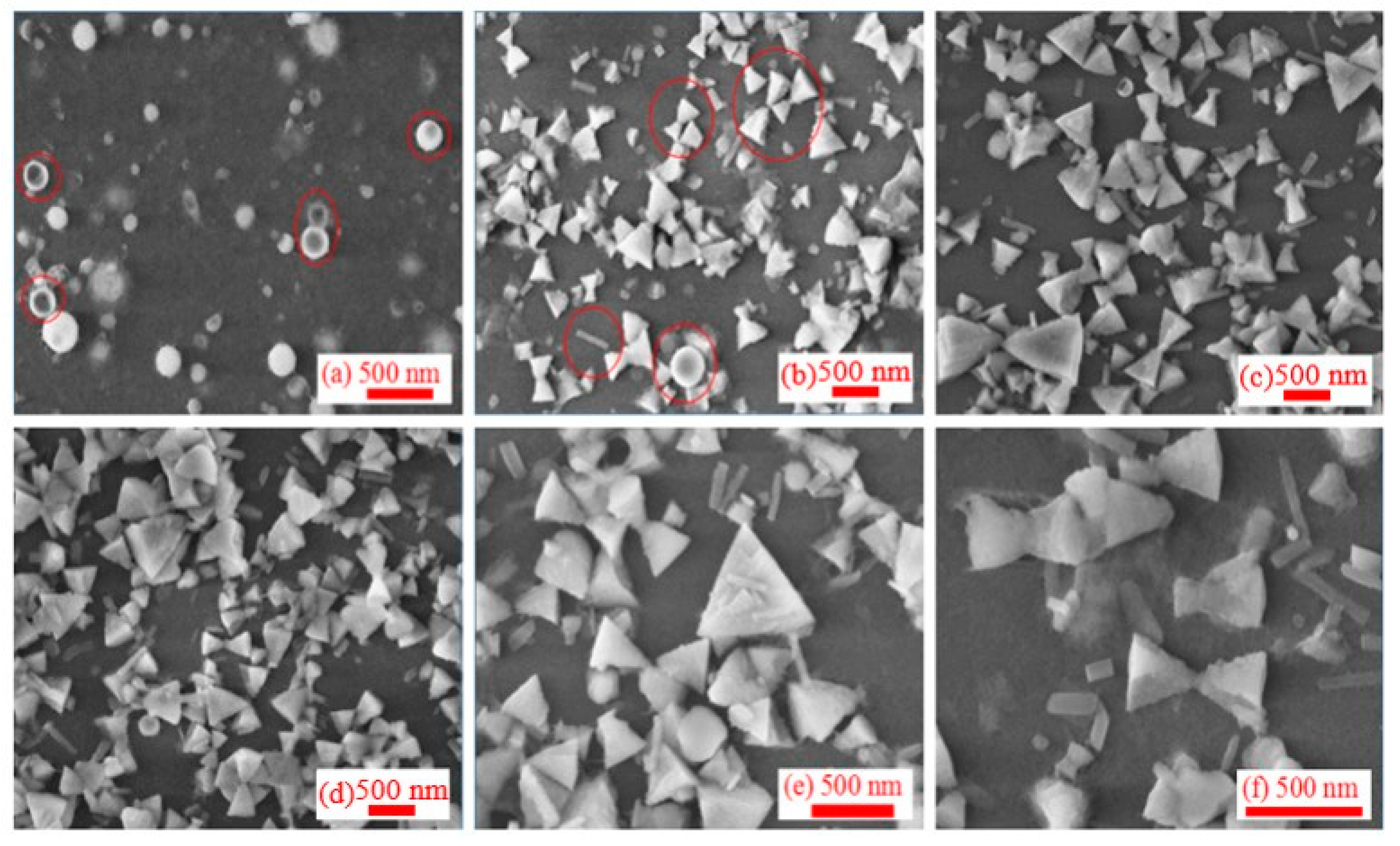
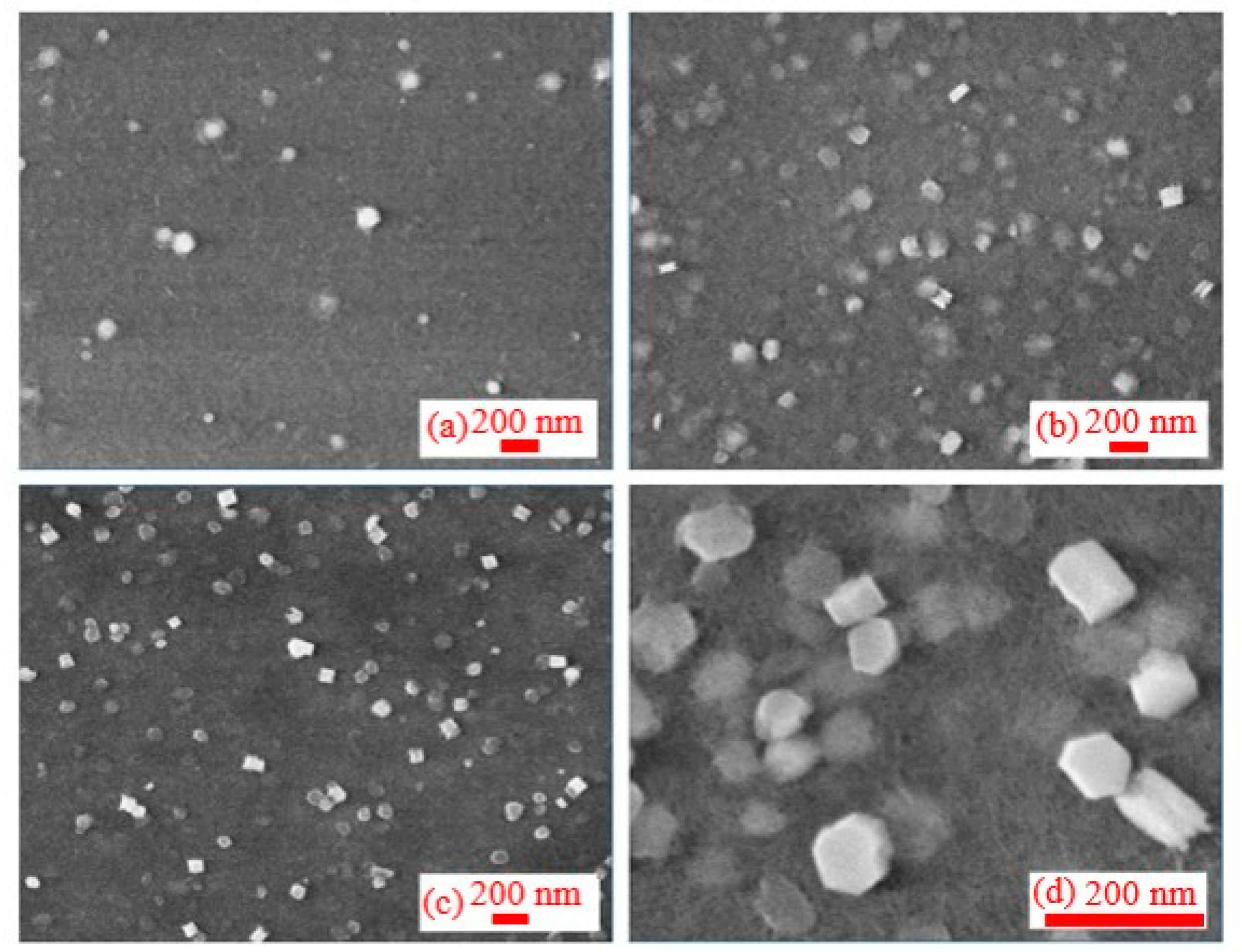
3.2. Morphology of Synthesized Al NPs
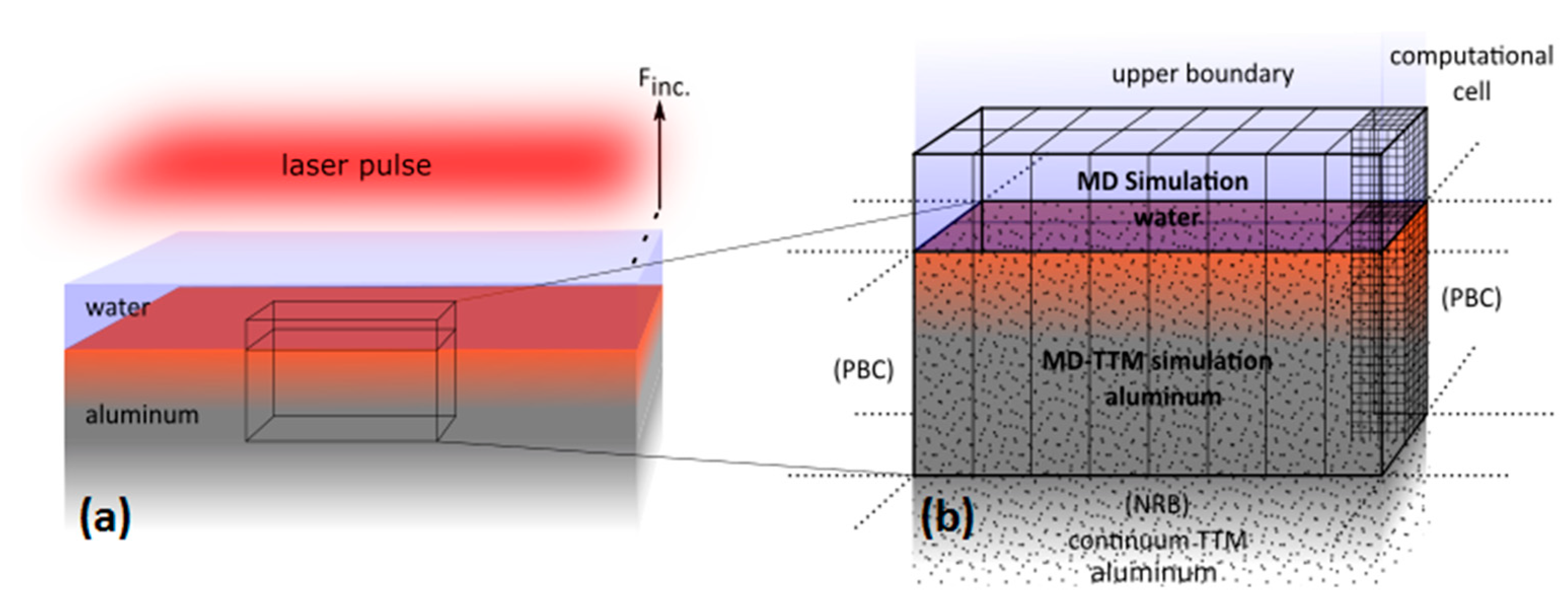
4. Theoretical Analysis of Nanoparticles Formation during Ablation of Bulk Aluminum in Water
4.1. Model
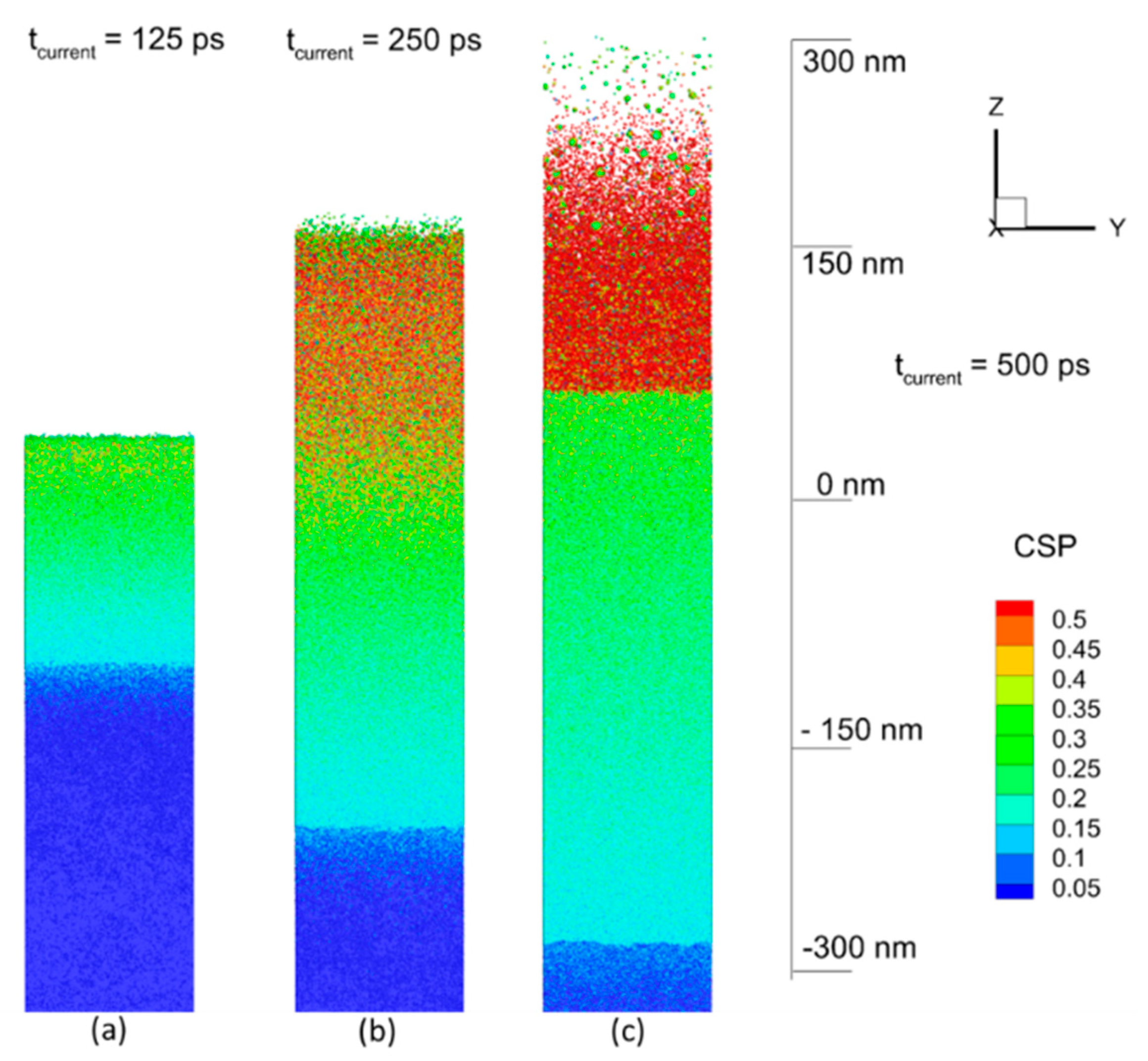
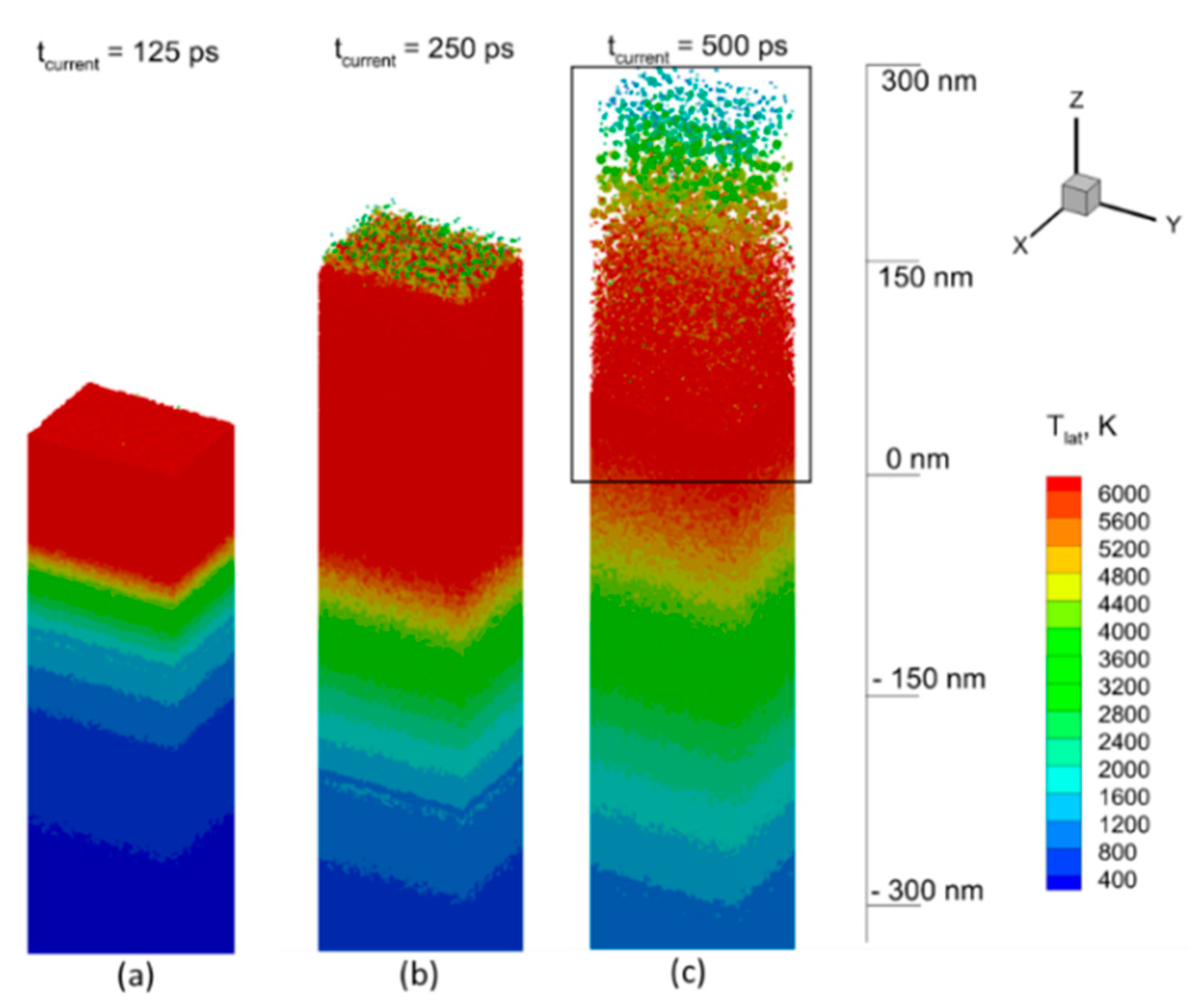
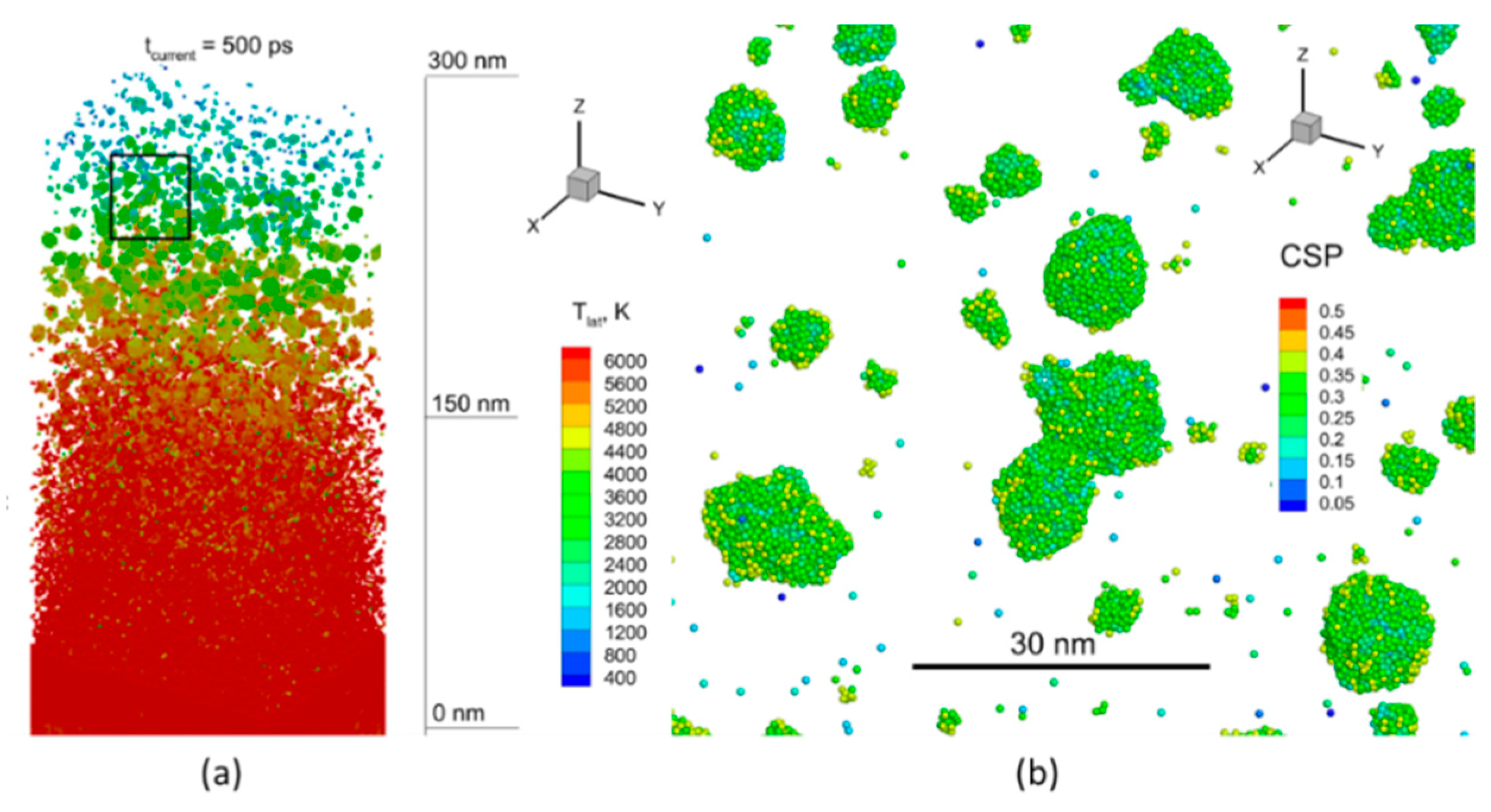
4.2. Results of Modeling and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pincella, F.; Isozaki, K.; Miki, K. A visible light-driven plasmonic photocatalyst. Light Sci. Appl. 2014, 3, e133. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Func. Mater. 2012, 22, 1333. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Mosayyebi, A.; Kavokin, A.V. Tuning the chemiluminescence of a luminol flow using plasmonic nanoparticles. Light Sci. Appl. 2016, 5, e16164. [Google Scholar] [CrossRef] [PubMed]
- Kudryashov, S.I.; Samokhvalov, A.A.; Nastulyavichus, A.A.; Saraeva, I.N.; Mikhailovskii, V.Y.; Ionin, A.A.; Veiko, V.P. Nanosecond-laser generation of nanoparticles in liquids: From ablation through bubble dynamics to nanoparticle yield. Materials 2019, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Bao, H.; Gan, X.; Stokes, N.; Wu, J. Tweezing and manipulating micro- and nanoparticles by optical nonlinear endoscopy. Light: Sci. Appl. 2014, 3, e126. [Google Scholar] [CrossRef]
- Galfetti, L.; De Luca, L.T.; Severini, F.; Meda, L.U.; Marra, G.; Marchetti, M.; Regi, M.; Bellucci, S. Nanoparticles for solid rocket propulsion. J. Phys. Condens. Matter 2006, 18, 1991–2005. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.E.; Prasher, R.; Peck, R.; Lee, T.; Pacheco, J.R.; Arentzen, P. Increased hot-plate ignition probability for nanoparticle-laden diesel fuel. Nano Lett. 2008, 8, 1410–1416. [Google Scholar] [CrossRef]
- Chen, X.; Jia, B.; Zhang, Y.; Gu, M. Exceeding the limit of plasmonic light trapping in textured screen-printed solar cells using Al nanoparticles and wrinkle-like graphene sheets. Light Sci. Appl. 2013, 2, e92. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, Y.; Zhang, P. A new substrate for surface enhanced Raman scattering of dye Sudan molecules. Spectrosc. Acta Part A 2007, 67, 122–124. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Ray, K.; Gray, S.K.; Pond, J.; Lakowicz, J.R. Al nanoparticles as substrates for metal-enhanced fluorescence in the ultraviolet for the label-free detection of biomolecules. Anal. Chem. 2009, 81, 1397–1403. [Google Scholar] [CrossRef]
- Wang, J.J.; Walters, F.; Liu, X.; Sciortino, P.; Deng, X. High-performance, large area, deep ultraviolet to infrared polarizers based on 40 nm line/78 nm space nanowire grids. Appl. Phys. Lett. 2007, 90, 061104. [Google Scholar] [CrossRef]
- Shimojo, F.; Ohmura, S.; Kalia, R.K.; Nakano, A.; Vashishta, P. Molecular dynamics simulations of rapid hydrogen production from water using Al clusters as catalyzers. Phys. Rev. Lett. 2010, 104, 126102. [Google Scholar] [CrossRef] [PubMed]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed Al in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Roach, P.J.; Woodward, W.H., Jr.; Castleman, A.W.; Reber, A.C.; Khanna, S.N. Complementary active sites cause size-selective reactivity of Al cluster anions with water. Science 2009, 323, 492–495. [Google Scholar] [CrossRef]
- Zheng, S.; Fang, F.; Zhou, G.; Chen, G.; Ouyang, L.; Zhu, M.; Sun, D. Hydrogen storage properties of space-confined NaAlH4 nanoparticles in ordered mesoporous silica. Chem. Mater. 2008, 20, 3954–3958. [Google Scholar] [CrossRef]
- Balde, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; de Jong, K.P. Sodium alanate nanoparticles - linking size to hydrogen storage properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Sun, Z.; Dlott, D.D. Near-infrared laser ablation of poly tetrafluoroethylene (Teflon) sensitized by nanoenergetic materials. Appl. Phys. Lett. 2004, 85, 1493–1495. [Google Scholar] [CrossRef]
- Henz, B.J.; Hawa, T.; Zachariah, M.R. On the role of built-in electric fields on the ignition of oxide coated nano Al: Ion mobility versus Fickian diffusion. J. Appl. Phys. 2010, 107, 024901. [Google Scholar] [CrossRef]
- Yoon, B.; Häkkinen, H.; Landman, U.; Wörz, A.S.; Antonietti, J.M.; Abbet, S.; Judai, K.; Heiz, U. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science 2005, 307, 403–407. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Singhal, H.; Naik, P.A.; Chakera, J.A.; Srivastava, A.K.; Dhami, T.S.; Joshi, M.P.; Gupta, P.D. Influence of C60 morphology on high-order harmonic generation enhancement in fullerene-containing plasma. J. Appl. Phys. 2009, 106, 103103. [Google Scholar] [CrossRef]
- Yamamoto, T. The relation between surface plasmon resonance and morphology of Ag nanodots prepared by pulsed laser deposition. Solid State Ion. 2004, 172, 299–302. [Google Scholar] [CrossRef]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Fan, G.-H.; Qu, S.-L.; Guo, Z.-Y.; Wang, Q.; Li, Z.-G. Size-dependent nonlinear absorption and refraction of Ag nanoparticles excited by fs lasers. Chin. Phys. B 2012, 21, 047804. [Google Scholar] [CrossRef]
- Wang, K.; Long, H.; Fu, M.; Yang, G.; Lu, P. Size-related third-order optical nonlinearities of Au nanoparticle arrays. Opt. Express 2010, 18, 13874–13879. [Google Scholar]
- Lee, S.; Ahn, A.; Choi, M.Y. Direct observation of aluminium ions produced via pulsed laser ablation in liquid: A ‘turn-on’ fluorescence study. Phys. Chem. Chem. Phys. 2012, 14, 15677–15681. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-Y.; Liu, Y.-F.; Tanaka, Y.; Ye, J.; Sakka, Y. Modification of Al particle surfaces by c-Al2O3 and its effect on the corrosion behavior of Al. J. Am. Ceram. Soc. 2005, 88, 977–979. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Liu, Y.-F.; Tanaka, Y.; Zhang, H.-W.; Ye, J.; Kagawa, Y. Temperature effect on hydrogen generation by the reaction of c-Al2O3-modified Al powder with distilled water. J. Am. Ceram. Soc. 2005, 88, 2975–2977. [Google Scholar] [CrossRef]
- Patil, P.P.; Phase, D.M.; Kulkarni, S.A.; Ghaisas, S.V.; Kulkarni, S.K.; Kanetkar, S.M.; Ogale, S.B.; Bhide, V.G. Pulsed-laser-induced reactive quenching at liquid-solid interface: Aqueous oxidation of iron. Phys. Rev. Lett. 1987, 58, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Santillán, J.M.J.; Videla, F.A.; van Raap, M.B.F.; Schinca, D.C.; Scaffardi, L.B. Analysis of the structure, configuration, and sizing of Cu and Cu oxide nanoparticles generated by fs laser ablation of solid target in liquids. J. Appl. Phys. 2013, 113, 134305. [Google Scholar] [CrossRef]
- Kim, K.K.; Kim, D.; Kim, S.K.; Park, S.M.; Song, J.K. Formation of ZnO nanoparticles by laser ablation in neat water. Chem. Phys. Lett. 2011, 511, 116–120. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Logothetidis, S.; Frangis, N.; Tsiaoussis, I.; Perrie, W.; Dearden, G. Laser ablation in water: A route to synthesize nanoparticles of titanium monoxide. Chem. Phys. Lett. 2010, 496, 113–116. [Google Scholar] [CrossRef]
- Acacia, N.; Barreca, F.; Barletta, E.; Spadaro, D.; Currò, G.; Neri, F. Laser ablation synthesis of indium oxide nanoparticles in water. Appl. Surf. Sci. 2010, 256, 6918–6922. [Google Scholar] [CrossRef]
- Musaev, O.R.; Yan, J.; Dusevich, V.; Wrobel, J.M.; Kruger, M.B. Ni nanoparticles fabricated by laser ablation in water. Appl. Phys. A 2014, 116, 735–739. [Google Scholar] [CrossRef]
- Tilaki, R.M.; zad, A.I.; Mahdavi, S.M. Stability, size and optical properties of silver nanoparticles prepared by laser ablation in different carrier media. Appl. Phys. A 2006, 84, 215–219. [Google Scholar] [CrossRef]
- Boardman, A.D.; Tsai, D.P.; Arboleda, D.M.; Santillán, J.M.J.; Herrera, L.J.M.; van Raap, M.B.F. Structure, configuration, and sizing of Ni nanoparticles generated by ultrafast laser ablation in different media. Plasmon. Met. Nanostruct. Their Opt. Prop. XIII 2015, 9547, 95473. [Google Scholar]
- Ganeev, R.A.; Baba, M.; Ryasnyansky, A.I.; Suzuki, M.; Kuroda, H. Characterization of optical and nonlinear optical properties of silver nanoparticles prepared by laser ablation in various liquids. Opt. Commun. 2004, 240, 437–448. [Google Scholar] [CrossRef]
- Jasbi, N.E.; Dorranian, D. Dependence of laser ablation produced TiO2 NPs on the ablation environment temperature. Opt. Quantum Electron. 2017, 49, 123–134. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Preparation of zinc oxide nanorods using pulsed laser ablation in water media at high temperature. J. Colloid Interface Sci. 2006, 300, 612–615. [Google Scholar] [CrossRef]
- Solati, E.; Dorranian, D. Effect of temperature on the characteristics of ZnO nanoparticles produced by laser ablation in water. Bull. Mater. Sci. 2016, 39, 1677–1684. [Google Scholar] [CrossRef]
- Giorgetti, E.; Miranda, M.M.; Caporali, S.; Canton, P.; Marsili, P.; Vergari, C. TiO2 nanoparticles obtained by laser ablation in water: Influence of pulse energy and duration on the crystalline phase. J. Alloys Compd. 2015, 643, S75–S79. [Google Scholar] [CrossRef]
- Dorranian, D.; Tajmir, S.; Khazanehfar, F. Effect of laser fluence on the characteristics of Ag nanoparticles produced by laser ablation. Soft Nanosci. Lett. 2013, 03, 93–100. [Google Scholar] [CrossRef]
- Shazia, B.; Shazaib, K.; Mahreen, A.; Nisar, A.; Umm, K.; Shahbaz, A. Pulsed laser ablation of Ni in vacuum and N2 atmosphere at various fluencies. Quantum Electron. 2015, 45, 640–647. [Google Scholar] [CrossRef]
- Nath, A.; Laha, S.S.; Khare, A. Effect of focusing conditions on synthesis of titanium oxide nanoparticles via laser ablation in titanium–water interface. Appl. Surf. Sci. 2011, 257, 3118–3122. [Google Scholar] [CrossRef]
- Tsuji, T.; Thang, D.H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Sukhov, I.A.; Shafeev, G.A.; Voronov, V.V.; Sygletou, M.; Stratakis, E.; Fotakis, C. Generation of nanoparticles of bronze and brass by laser ablation in liquid. Appl. Surf. Sci. 2014, 302, 79–82. [Google Scholar] [CrossRef]
- Lasemi, N.; Pacher, U.; Zhigilei, L.V.; Bomatí-Miguel, O.; Lahoz, R.; Kautek, W. Pulsed laser ablation and incubation of nickel, iron and tungsten in liquids and air. Appl. Surf. Sci. 2018, 433, 772–779. [Google Scholar] [CrossRef]
- Anisimov, S.I.; Kapeliovich, B.L.; Perel’man, T.L. Electron emission from metal surfaces exposed to ultrashort laser pulses. Zh. Eksp. Teor. Fiz 1974, 66, 776. [Google Scholar]
- Ivanov, D.S.; Zhigilei, L.V. Combined atomistic-continuum modeling of short-pulse laser melting and disintegration of metal films. Phys. Rev. B 2003, 68, 064114. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Lipp, V.P.; Blumenstein, A.; Kleinwort, F.; Veiko, V.P.; Yakovlev, E.; Roddatis, V.; Garcia, M.E.; Rethfeld, B.; Ihlemann, J.; et al. Experimental and theoretical investigation of periodic nanostructuring of Au with UV laser near the ablation threshold. Phys. Rev. Appl. 2015, 4, 064006. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Rethfeld, B.C.; O’Connor, G.M.; Glynn, T.J.; Volkov, A.N.; Zhigilei, L.V. The mechanism of nanobump formation in femtosecond pulse laser nanostructuring of thin metal films. Appl. Phys. A 2008, 92, 791. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Kuznetsov, A.I.; Lipp, V.P.; Rethfeld, B.; Chichkov, B.N.; Garcia, M.E.; Schulz, W. Short laser pulse surface nanostructuring on thin metal films: Direct comparison of molecular dynamics modeling and experiment. Appl. Phys. A 2013, 111, 675. [Google Scholar] [CrossRef]
- Corkum, P.B.; Brunel, F.; Sherman, N.K.; Srinivasan-Rao, T. Thermal response of metals to ultrashort-pulse laser excitation. Phys. Rev. Lett. 1988, 61, 2886. [Google Scholar] [CrossRef]
- Boltaev, G.S.; Ganeev, R.A.; Krishnendu, P.S.; Maurya, S.K.; Redkin, P.V.; Rao, K.S.; Zhang, K.; Guo, C. Strong third-order optical nonlinearities of Ag nanoparticles synthesized by laser ablation of bulk silver in water and air. Appl. Phys. A 2018, 124, 766. [Google Scholar] [CrossRef]
- Rao, K.S.; Ganeev, R.A.; Zhang, K.; Fu, Y.; Boltaev, G.S.; Krishnendu, P.S. Laser ablation–induced synthesis and nonlinear optical characterization of titanium and cobalt NPs. J. Nanopart. Res. 2018, 20, 285. [Google Scholar] [CrossRef]
- Zhang, K.; Maurya, S.K.; Ganeev, R.A.; Rao, K.S.; Guo, C. Ablated nickel NPs: Third harmonic generation and optical nonlinearities. J. Opt. 2018, 20, 125502. [Google Scholar] [CrossRef]
- Zeng, H.; Cai, W.; Li, Y.; Hu, J.; Liu, P. Composition/structural evolution and optical properties of ZnO/Zn nanoparticles by laser ablation in liquid media. J. Phys. Chem. B 2005, 109, 18260–18266. [Google Scholar] [CrossRef]
- Barmina, E.V.; Kuzmin, P.G.; Shafeev, G.A. Self-organization of hydrogen gas bubbles rising above laser-etched metallic aluminum in a weakly basic aqueous solution. Phys. Rev. E 2011, 84, 045302. [Google Scholar] [CrossRef]
- Lee, S.; Jung, H.J.; Shin, J.H.; Choi, M.Y. Production of size controlled Al and alumina nanoparticles via pulsed laser ablation in water. J. Nanosci. Nanotechnol. 2012, 12, 8900–8903. [Google Scholar] [CrossRef]
- Lee, S.; Shin, J.H.; Choi, M.Y. Watching the growth of Al hydroxide nanoparticles from Al nanoparticles synthesized by pulsed laser ablation in aqueous surfactant solution. J. Nanopart. Res. 2013, 15, 106. [Google Scholar] [CrossRef]
- Stratakis, E.; Barberoglou, M.; Fotakis, C.; Viau, G.; Garcia, C.; Shafeev, G.A. Generation of Al nanoparticles via ablation of bulk Al in liquids with short laser pulses. Opt. Express 2009, 17, 12650–12662. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.S.; Atanasov, P.A.; Milev, D.R.; Stoyanchov, T.R.; Deleva, A.D.; Peshev, Z.Y. Synthesis and characterization of TiOx nanoparticles prepared by pulsed-laser ablation of Ti target in water. Appl. Surf. Sci. 2009, 255, 5351–5354. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Nastulyavichus, A.A.; Ivanova, A.K.; Smirnov, N.A.; Khmelnitskiy, R.A.; Rudenko, A.A.; Saraeva, I.N.; Tolordava, E.R.; Kharin, A.Y.; Zavestovskaya, I.N.; et al. High-throughput laser generation of Si-nanoparticle based surface coatings for antibacterial applications. Appl. Surf. Sci. 2019, 470, 825. [Google Scholar] [CrossRef]
- Schäfer, C.; Urbassek, H.M.; Zhigilei, L.V.; Garrison, B.J. Pressure-transmitting boundary conditions for molecular dynamics simulations. Comp. Mater. Sci. 2002, 24, 421. [Google Scholar] [CrossRef]
- Zhakhovskii, V.V.; Inogamov, N.A.; Petrov, Y.V.; Ashitkov, S.I.; Nishihara, K. Molecular dynamics simulation of femtosecond ablation and spallation with different interatomic potentials. Appl. Surf. Sci. 2009, 255, 9592. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithell’s Metal. Reference Book, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Nada, H. An intermolecular potential model for the simulation of ice and water near the melting point: A six-site model of H2O. J. Chem. Phys. 2003, 118, 7401. [Google Scholar] [CrossRef]
- Dou, Y.; Zhigilei, L.V.; Postawa, Z.; Winograd, N.; Garrison, B.J. Thickness effects of water overlayer on its explosive evaporation at heated metal surfaces. Nucl. Instrum. Methods B 2001, 180, 105–111. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Wu, C.; Shugaev, M.V.; Zhigilei, L.V. Atomistic modeling of nanoparticle generation in short pulse laser ablation of thin metal films in water. J. Colloid Interface Sci. 2017, 489, 3–17. [Google Scholar] [CrossRef]
- Development of Interatomic EAM Potentials. Available online: https://www.researchgate.net/project/Development-of-interatomic-EAM-potentials (accessed on 1 March 2019).
- The World’s Sales Leader in Thin-Film Thickness Measurement. Available online: https://www.filmetrics.com (accessed on 1 March 2019).
- Kanski, M.; Garrison, B.J.; Postawa, Z. Effect of oxygen chemistry in sputtering of polymers. J. Phys. Chem. Lett. 2016, 7, 1559–1562. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Rethfeld, B.C. The effect of pulse duration on the character of laser heating: Photo-mechanical vs. photo-thermal damage of metal targets. Appl. Surf. Sci. 2009, 255, 9724. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Lipp, V.P.; Veiko, V.P.; Jakovlev, E.; Rethfeld, B.; Garcia, M.E. Molecular dynamics study of the short laser pulse ablation: Quality and efficiency in production. Appl. Phys. A 2014, 117, 2133. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Lin, Z.; Ivanov, D.S. Atomistic modeling of short pulse laser ablation of metals: Connections between melting, spallation, and phase explosion. J. Chem. Phys. 2009, 113, 11892. [Google Scholar] [CrossRef]
- Saraeva, I.N.; Kudryashov, S.I.; Rudenko, A.A.; Zhilnikova, M.I.; Ivanov, D.S.; Zayarnyi, D.A.; Simakin, A.V.; Ionin, A.A.; Garcia, M. Effect of laser pulsewidth on fs/ps laser ablation of metals and silicon in air and liquids on nanoparticle yield and single-shot ablation thresholds. Appl. Surf. Sci. 2018, 470, 1018. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Blumenstein, A.; Ihlemann, J.; Simon, P.; Garcia, M.E.; Rethfeld, B. Molecular dynamics modeling of periodic nanostructuring of metals with a short UV laser pulse under spatial confinement by a water layer. Appl. Phys. A 2017, 123, 744. [Google Scholar] [CrossRef]
| Wt % | At % | Type | Picture | |||
|---|---|---|---|---|---|---|
| Al | O | Al | O | |||
| Sn (1064 nm) | 38.4 | 61.6 | 27 | 73 | Al & Al2O3 & Al(OH)3 |  |
| Sn (355 nm) | 35 | 65 | 24.2 | 75.8 | Al & Al2O3 & Al(OH)3 |  |
| Sp | 32.4 | 67.6 | 22.1 | 77.9 | Al2O3 3H2O |  |
| 18 | 82 | 11.5 | 88.5 | Al(OH)3 |  | |
| Sf | 36.3 | 63.7 | 25.8 | 74.2 | Al & Al2O3 |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ivanov, D.S.; Ganeev, R.A.; Boltaev, G.S.; Krishnendu, P.S.; Singh, S.C.; Garcia, M.E.; Zavestovskaya, I.N.; Guo, C. Pulse Duration and Wavelength Effects of Laser Ablation on the Oxidation, Hydrolysis, and Aging of Aluminum Nanoparticles in Water. Nanomaterials 2019, 9, 767. https://doi.org/10.3390/nano9050767
Zhang K, Ivanov DS, Ganeev RA, Boltaev GS, Krishnendu PS, Singh SC, Garcia ME, Zavestovskaya IN, Guo C. Pulse Duration and Wavelength Effects of Laser Ablation on the Oxidation, Hydrolysis, and Aging of Aluminum Nanoparticles in Water. Nanomaterials. 2019; 9(5):767. https://doi.org/10.3390/nano9050767
Chicago/Turabian StyleZhang, Ke, Dmitry S. Ivanov, Rashid A. Ganeev, Ganjaboy S. Boltaev, Pandiyalackal S. Krishnendu, Subhash C. Singh, Martin E. Garcia, Irina N. Zavestovskaya, and Chunlei Guo. 2019. "Pulse Duration and Wavelength Effects of Laser Ablation on the Oxidation, Hydrolysis, and Aging of Aluminum Nanoparticles in Water" Nanomaterials 9, no. 5: 767. https://doi.org/10.3390/nano9050767
APA StyleZhang, K., Ivanov, D. S., Ganeev, R. A., Boltaev, G. S., Krishnendu, P. S., Singh, S. C., Garcia, M. E., Zavestovskaya, I. N., & Guo, C. (2019). Pulse Duration and Wavelength Effects of Laser Ablation on the Oxidation, Hydrolysis, and Aging of Aluminum Nanoparticles in Water. Nanomaterials, 9(5), 767. https://doi.org/10.3390/nano9050767








