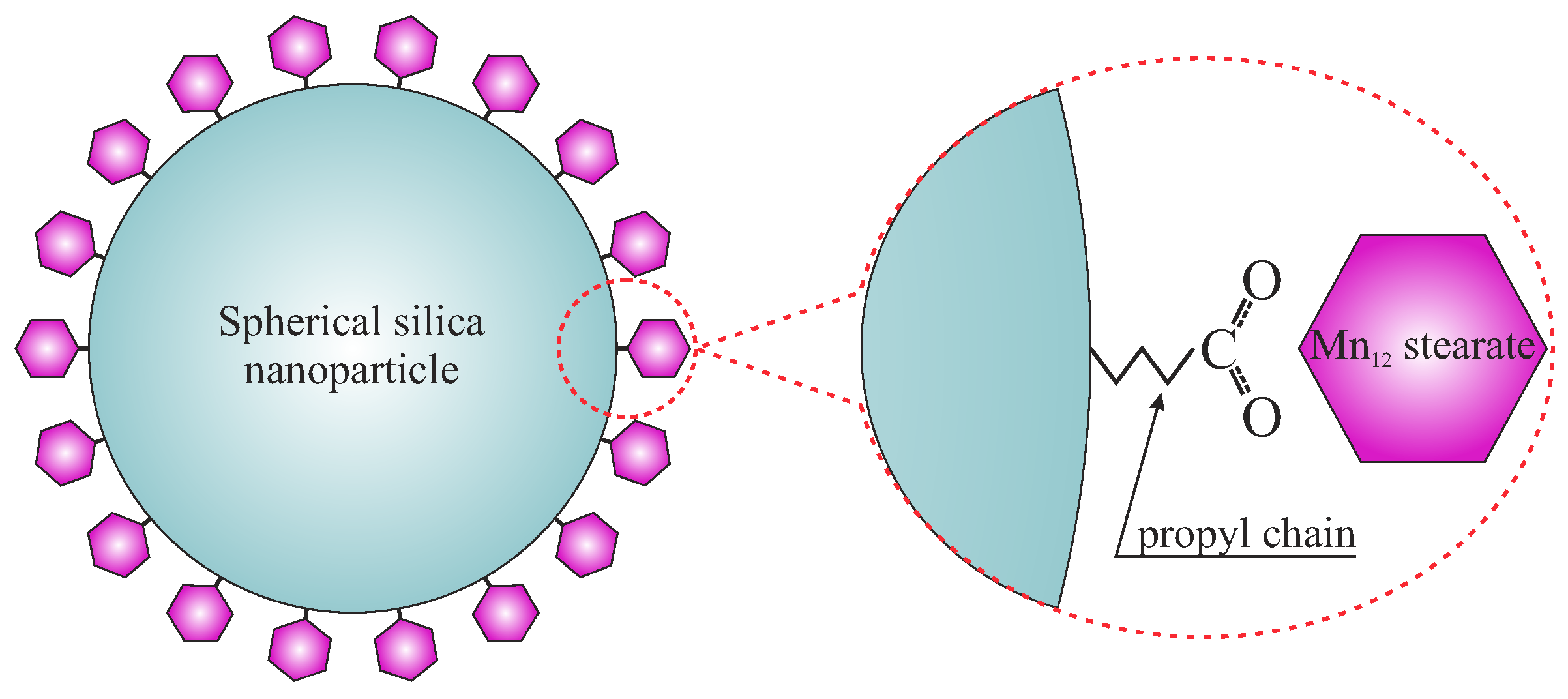
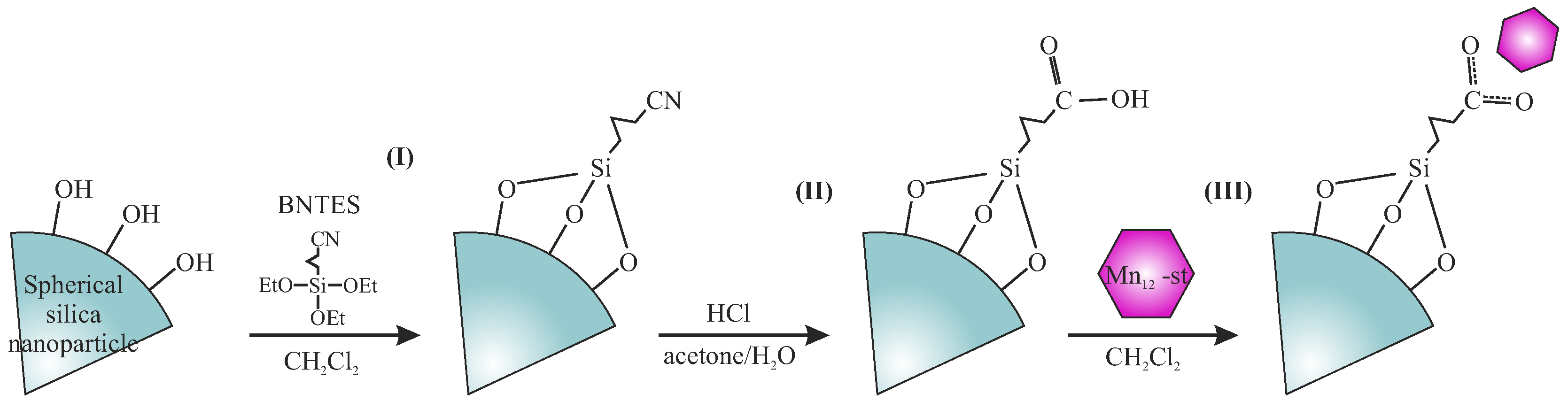
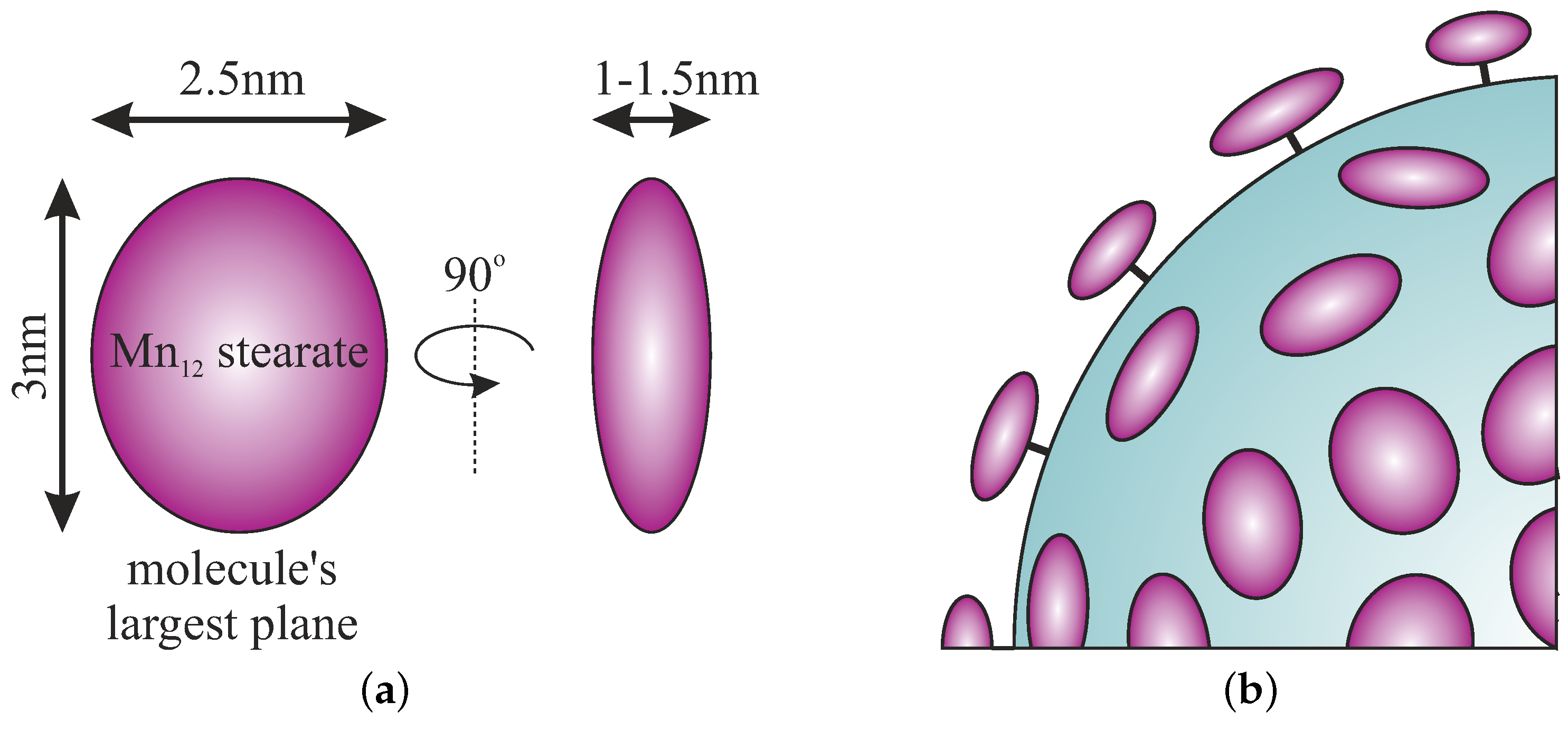
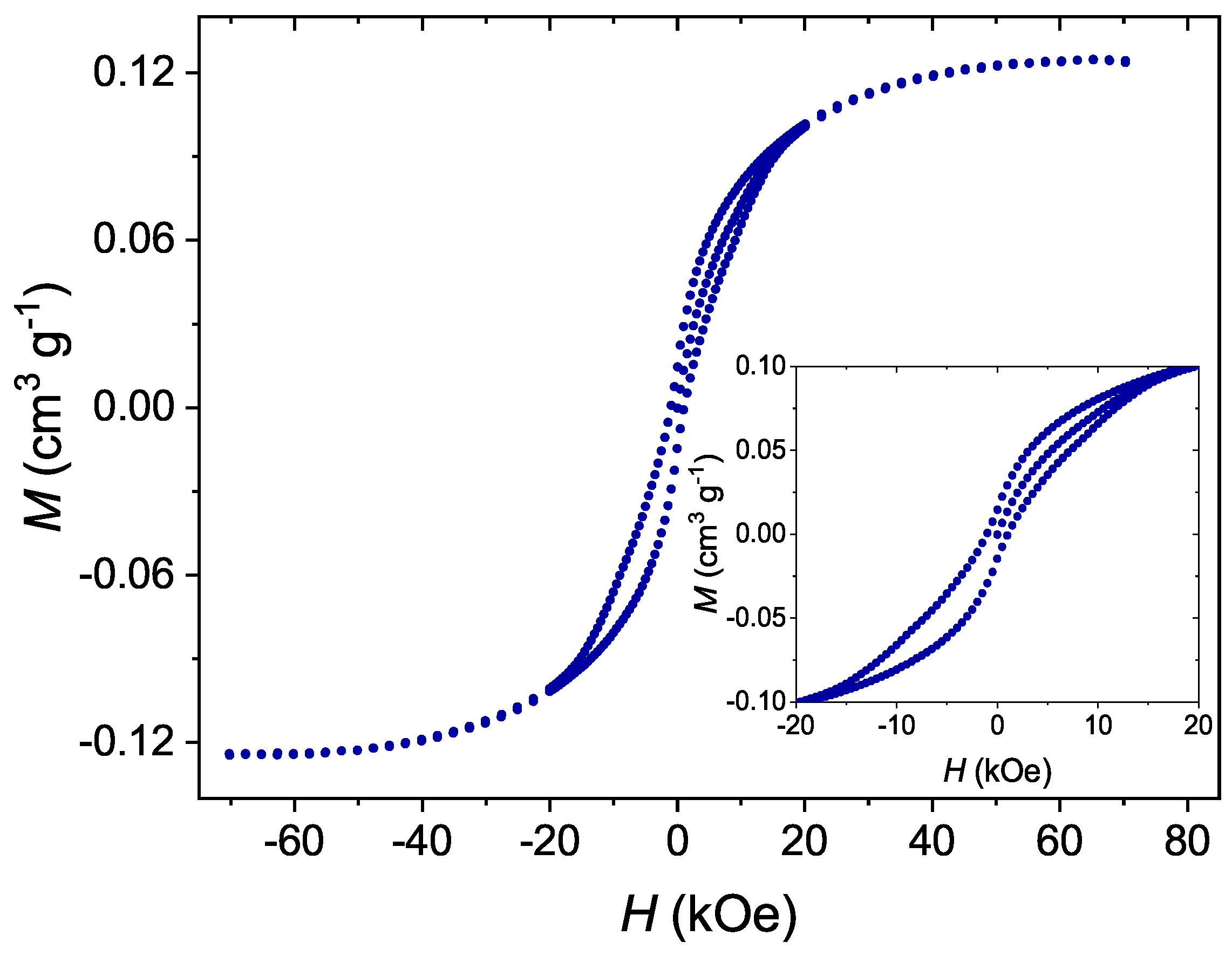
The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles
Abstract
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SMM | single-molecule magnet |
| Mnac | |
| Mn-st | derivative of Mn containing strearic acid ligands |
| BNTES | 4-(triethoxysilyl)butyronitrile |
References
- Lis, T. Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 2042–2046. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-molecule magnets. MRS Bull. 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Park, C.D.; Jeong, D.Y. Soluble Single-Molecule Magnet: Mn12-stearate. Bull. Korean Chem. Soc. 2001, 22, 611–615. [Google Scholar]
- Willemin, S.; Arrachart, G.; Lecren, L.; Larionova, J.; Coradin, T.; Clérac, R.; Mallah, T.; Guérin, C.; Sanchez, C. Immobilisation of single molecule magnets in mesoporous silica hosts. New J. Chem. 2003, 27, 1533–1539. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Forment-Aliaga, A.; Amorós, P.; Ramírez-Castellanos, J.; González-Calbet, J.M. Incorporation of Mn 12 single molecule magnets into mesoporous silica. J. Mater. Chem. 2003, 13, 3089–3095. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Forment-Aliaga, A.; Martínez-Agudo, J.; Amorós, P. Mn12 single-molecule magnets incorporated into mesoporous MCM-41 silica. Polyhedron 2003, 22, 2395–2400. [Google Scholar] [CrossRef]
- del Carmen Giménez-López, M.; Moro, F.; La Torre, A.; Gómez-García, C.J.; Brown, P.D.; Van Slageren, J.; Khlobystov, A.N. Encapsulation of single-molecule magnets in carbon nanotubes. Nat. Commun. 2011, 2, 407. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Bałanda, M.; Fitta, M.; Dulski, M.; Zubko, M.; Pawlik, P.; Laskowski, Ł. Magnetic behaviour of Mn12-stearate single-molecule magnets immobilized inside SBA-15 mesoporous silica matrix. J. Magn. Magn. Mater. 2019. [Google Scholar] [CrossRef]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Verma, S.; Verma, A.; Srivastava, A.K.; Gupta, A.; Singh, S.P.; Singh, P. Structural and magnetic properties of Mn12-Stearate nanomagnets. Mater. Chem. Phys. 2016, 177, 140–146. [Google Scholar] [CrossRef]
- Barra, A.L.; Bianchi, F.; Caneschi, A.; Cornia, A.; Gatteschi, D.; Gorini, L.; Gregoli, L.; Maffini, M.; Parenti, F.; Sessoli, R.; et al. New Single-Molecule Magnets by Site-Specific Substitution: Incorporation of “Alligator Clips” into Fe4 Complexes. Eur. J. Inorg. Chem. 2007, 2007, 4145–4152. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowski, L.; Kityk, I.; Konieczny, P.; Pastukh, O.; Schabikowski, M.; Laskowska, M. The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles. Nanomaterials 2019, 9, 764. https://doi.org/10.3390/nano9050764
Laskowski L, Kityk I, Konieczny P, Pastukh O, Schabikowski M, Laskowska M. The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles. Nanomaterials. 2019; 9(5):764. https://doi.org/10.3390/nano9050764
Chicago/Turabian StyleLaskowski, Lukasz, Iwan Kityk, Piotr Konieczny, Oleksandr Pastukh, Mateusz Schabikowski, and Magdalena Laskowska. 2019. "The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles" Nanomaterials 9, no. 5: 764. https://doi.org/10.3390/nano9050764
APA StyleLaskowski, L., Kityk, I., Konieczny, P., Pastukh, O., Schabikowski, M., & Laskowska, M. (2019). The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles. Nanomaterials, 9(5), 764. https://doi.org/10.3390/nano9050764








