Asynchronous Evolution of Nanoporous Silver on Dual-Phase Ag–Sn Alloys by Potentiostatic Dealloying in Hydrochloric Acid Solution
Abstract
1. Introduction
2. Experimental Procedure
3. Results
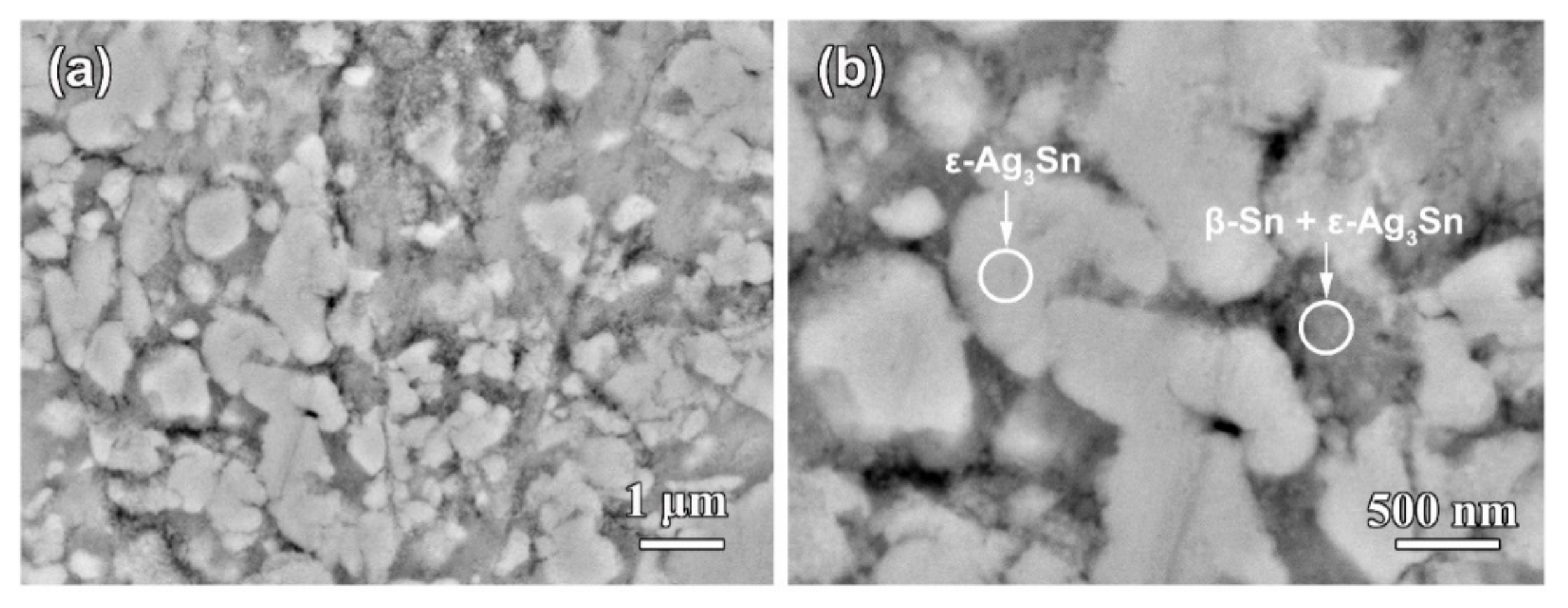
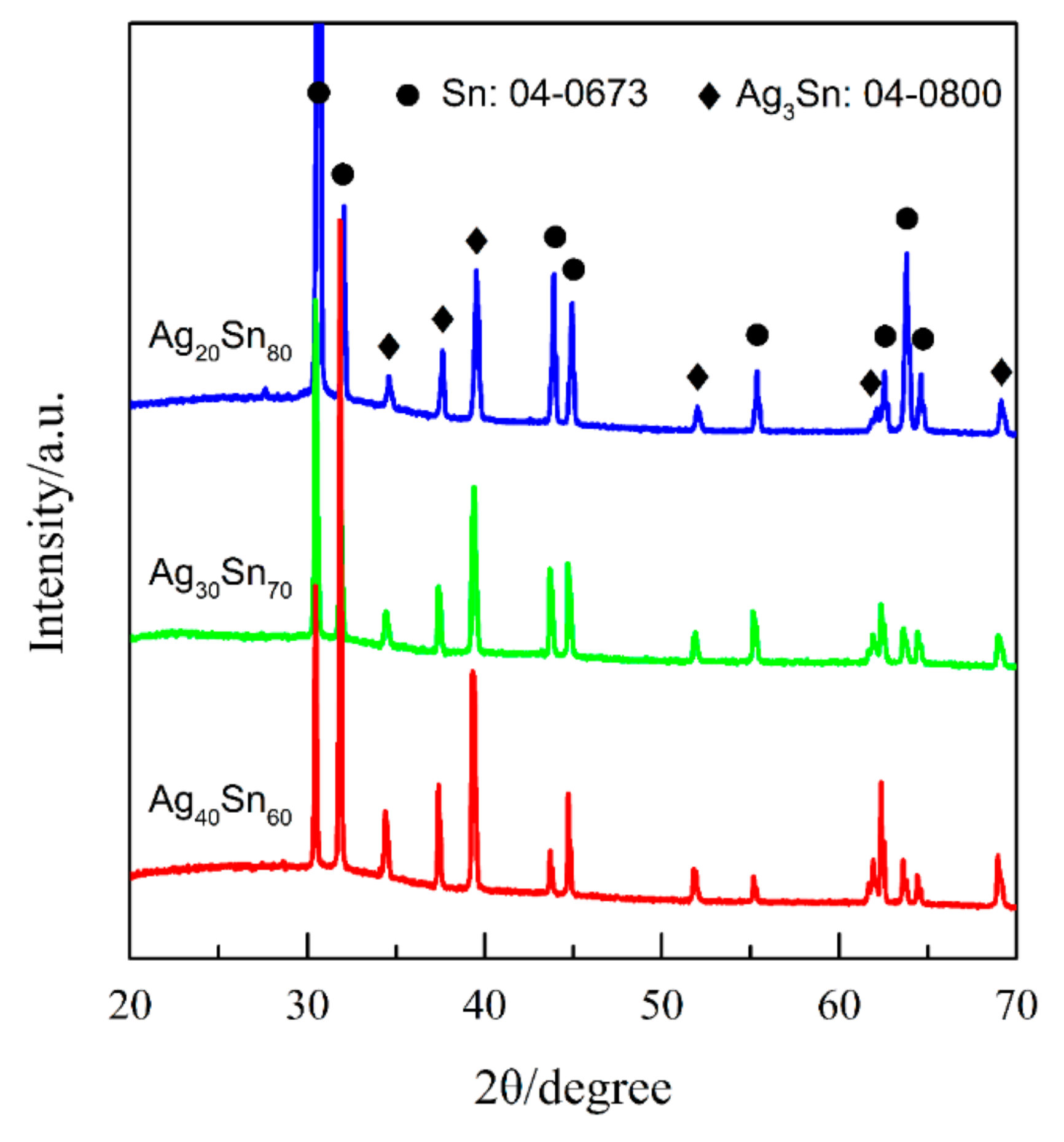
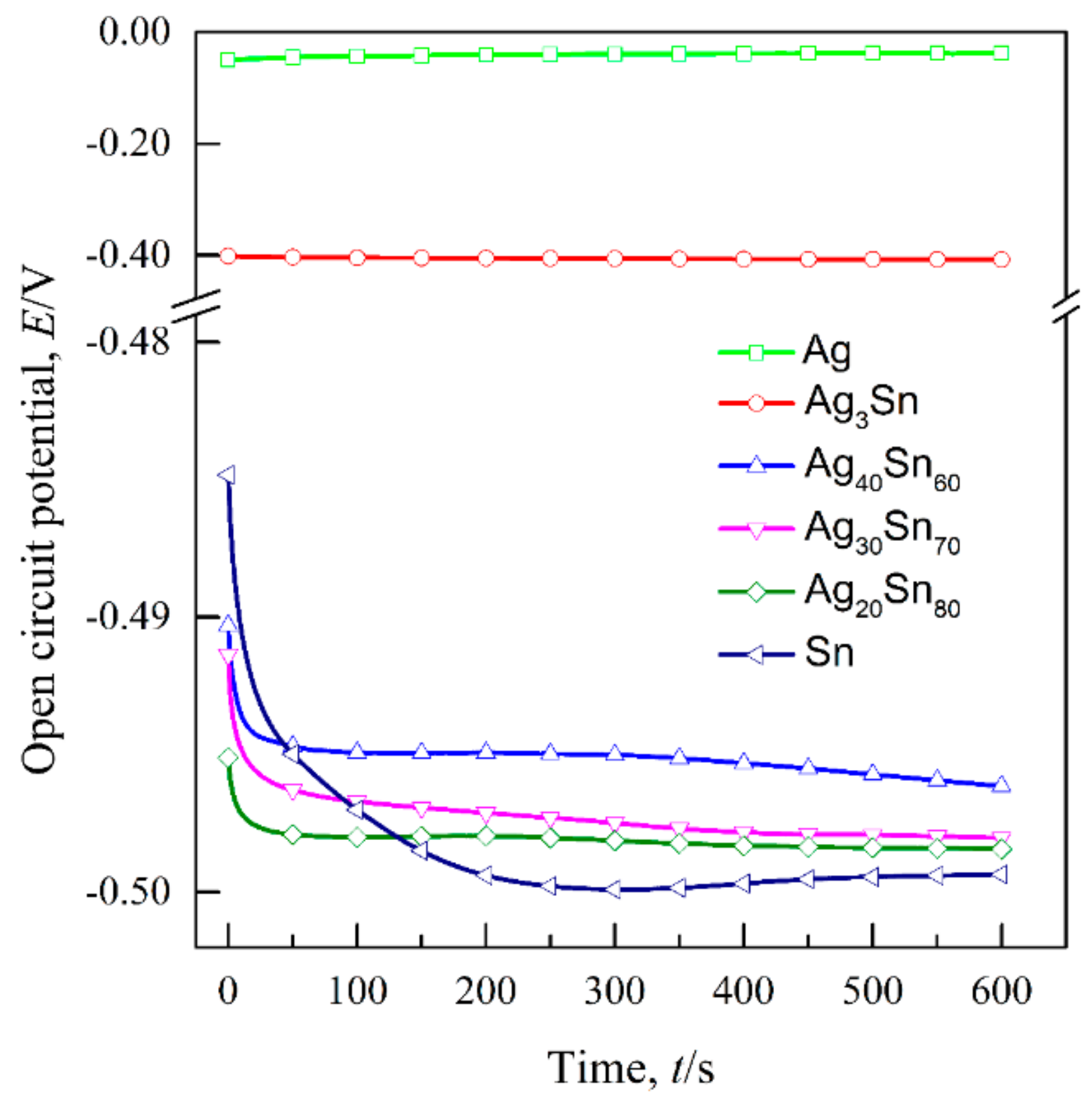
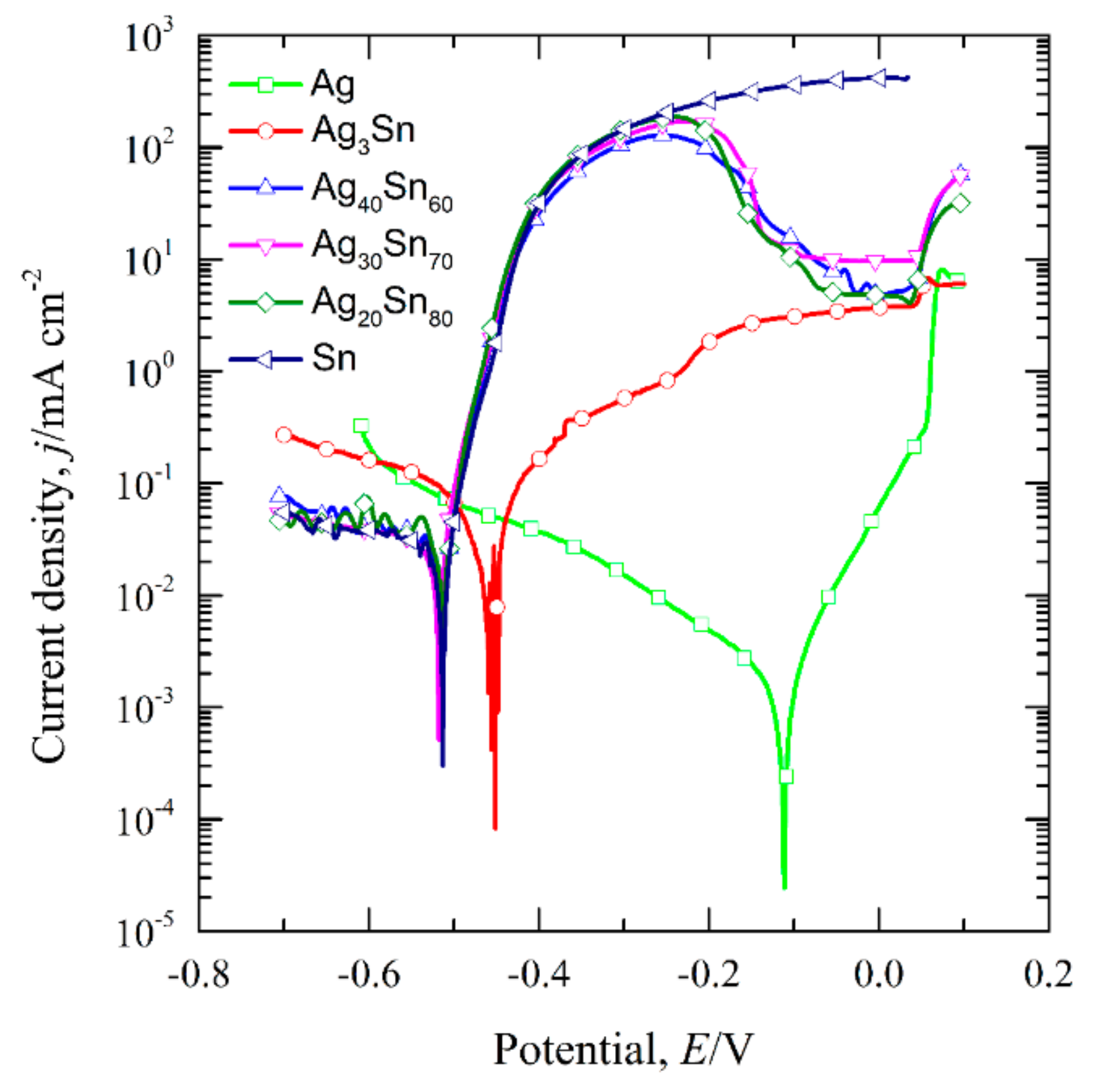
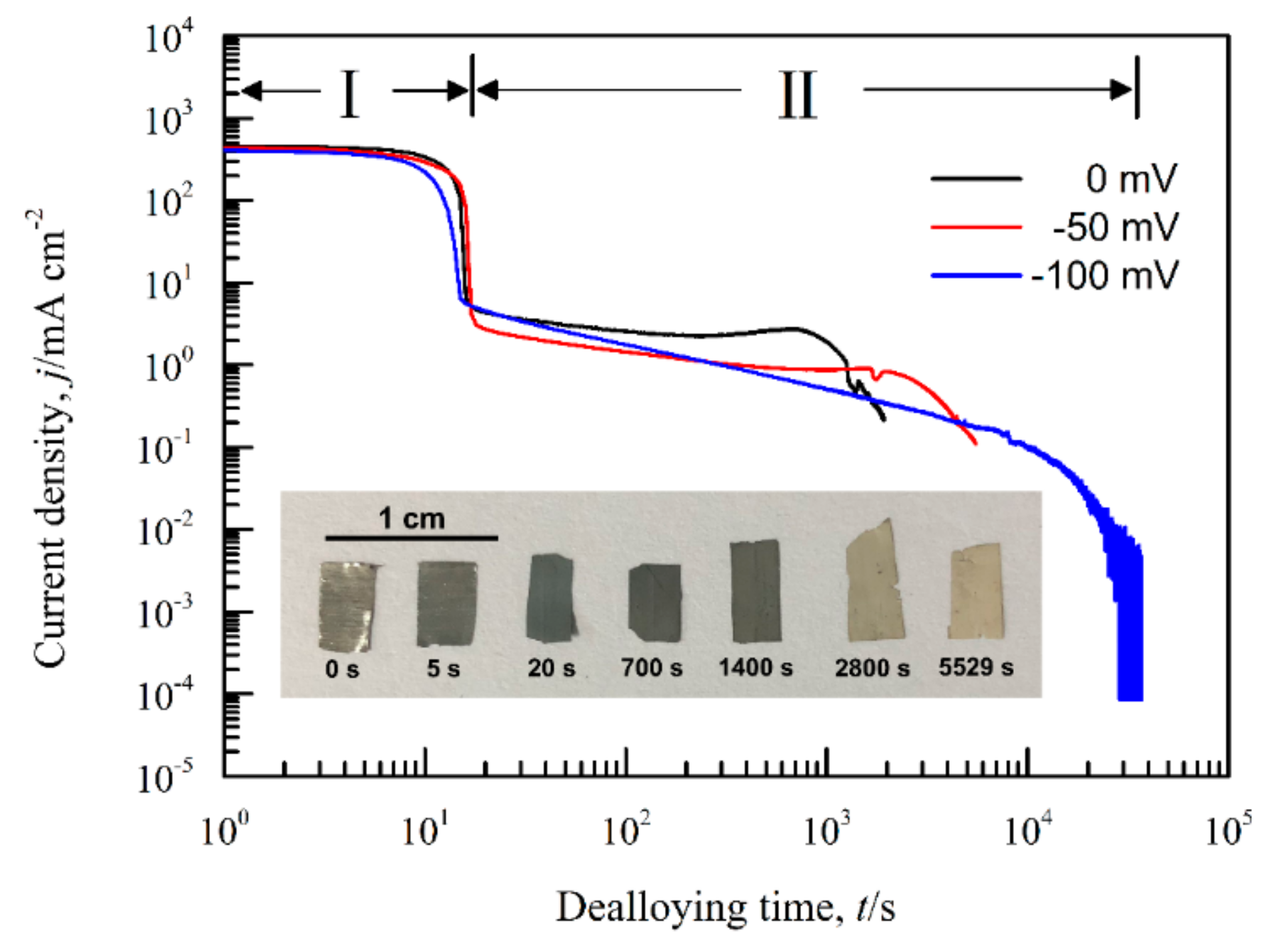
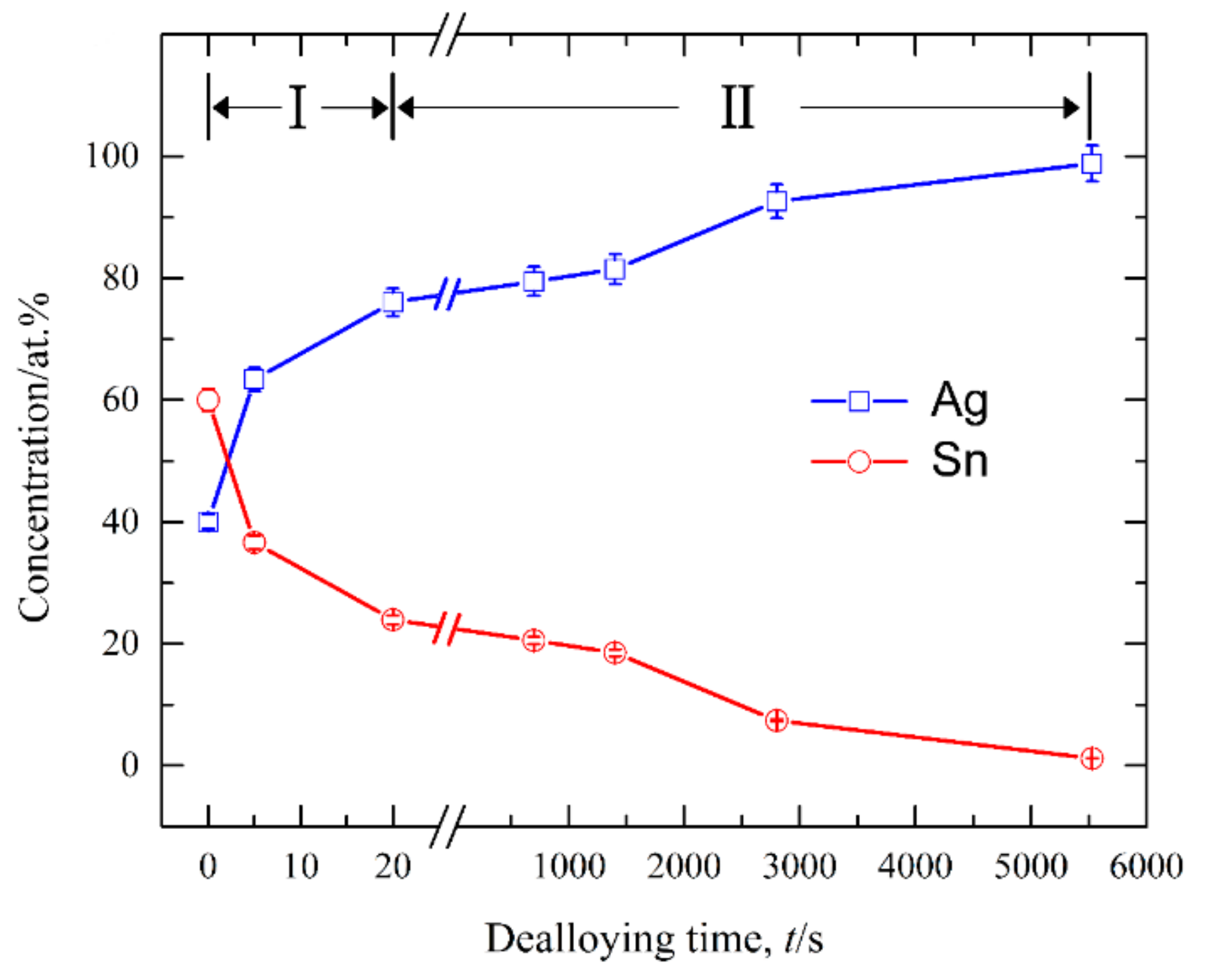
3.1. Effect of Phase Constitutions of Ag–Sn Precursor Alloys on the Electrochemical Behavior
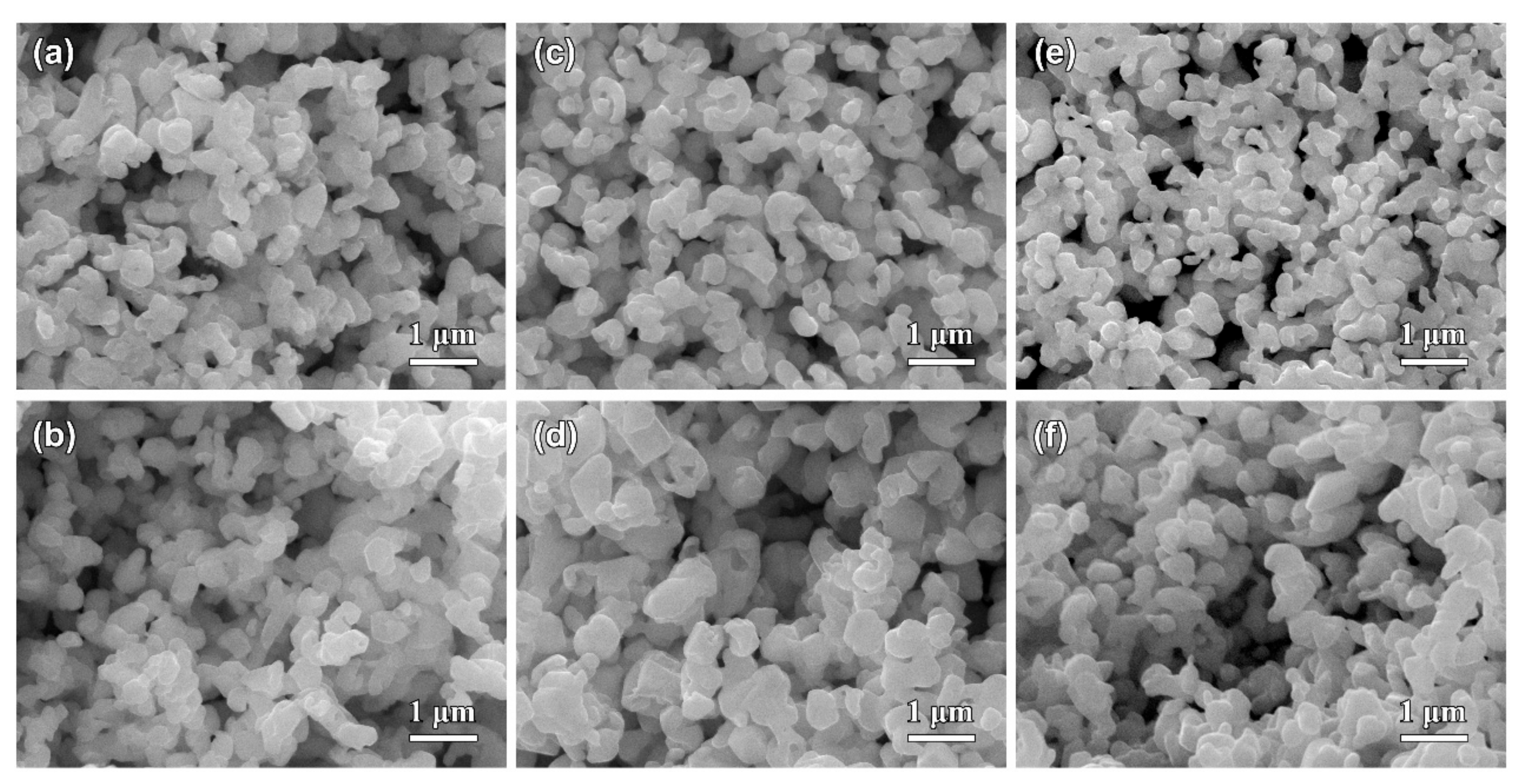
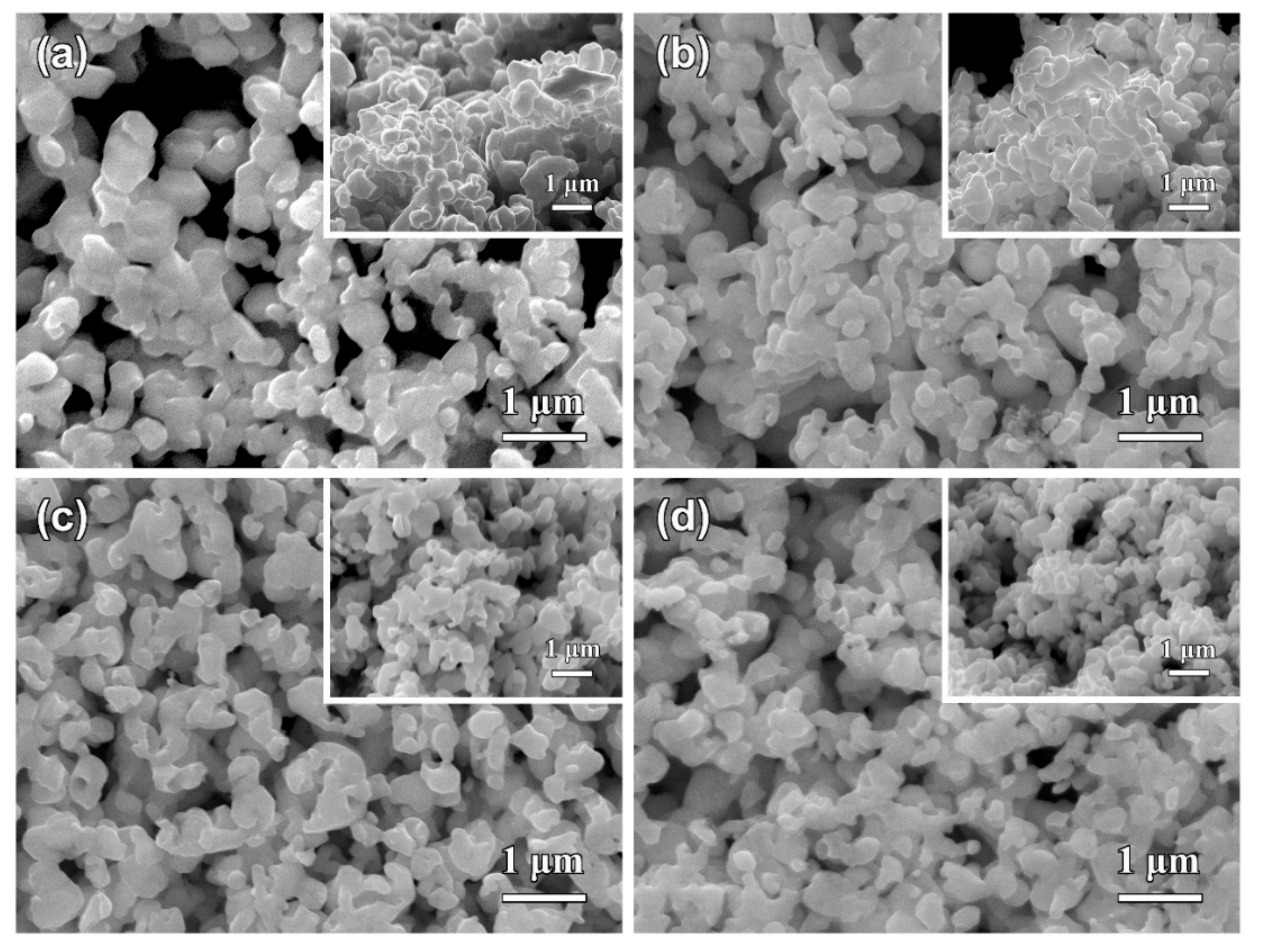
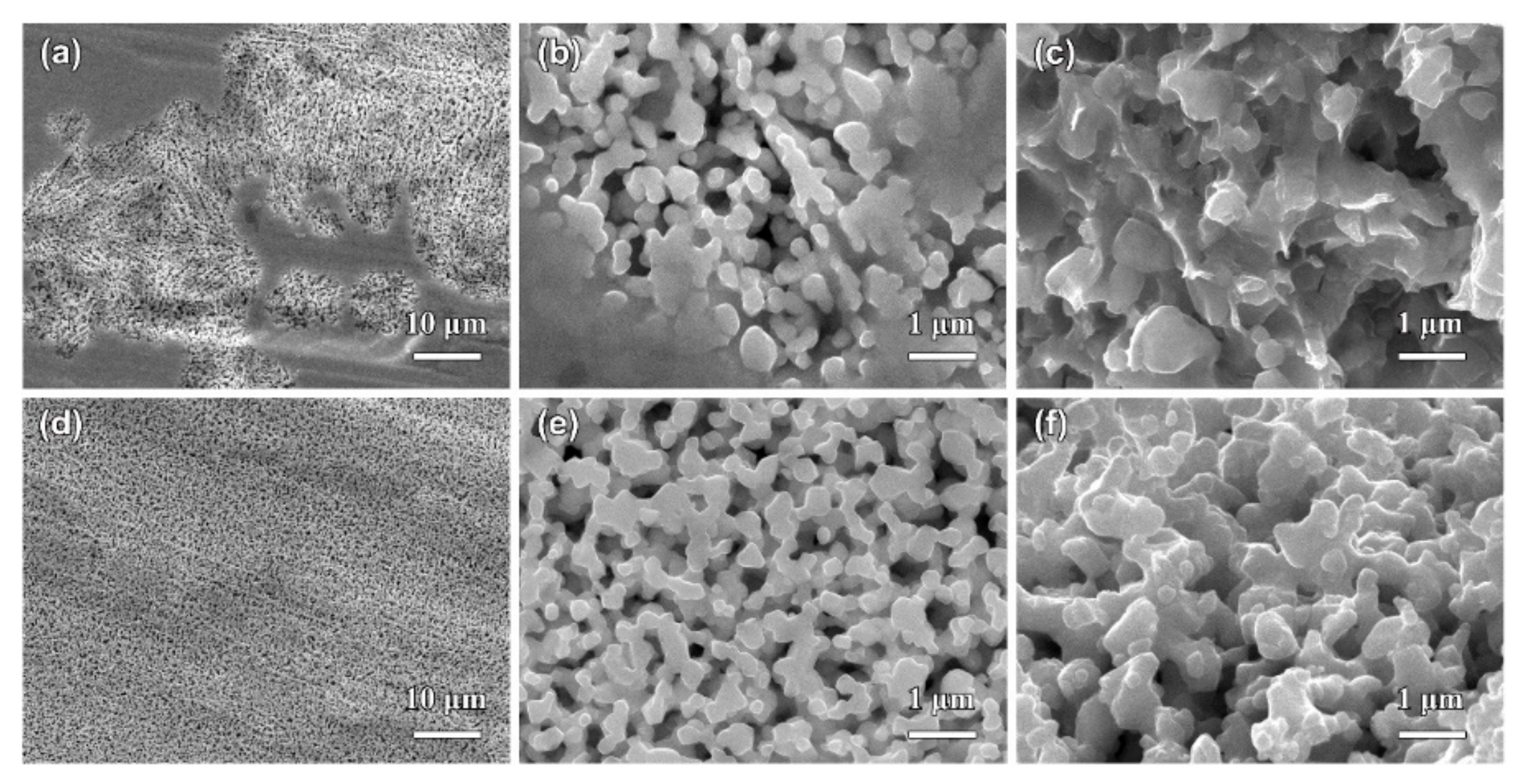
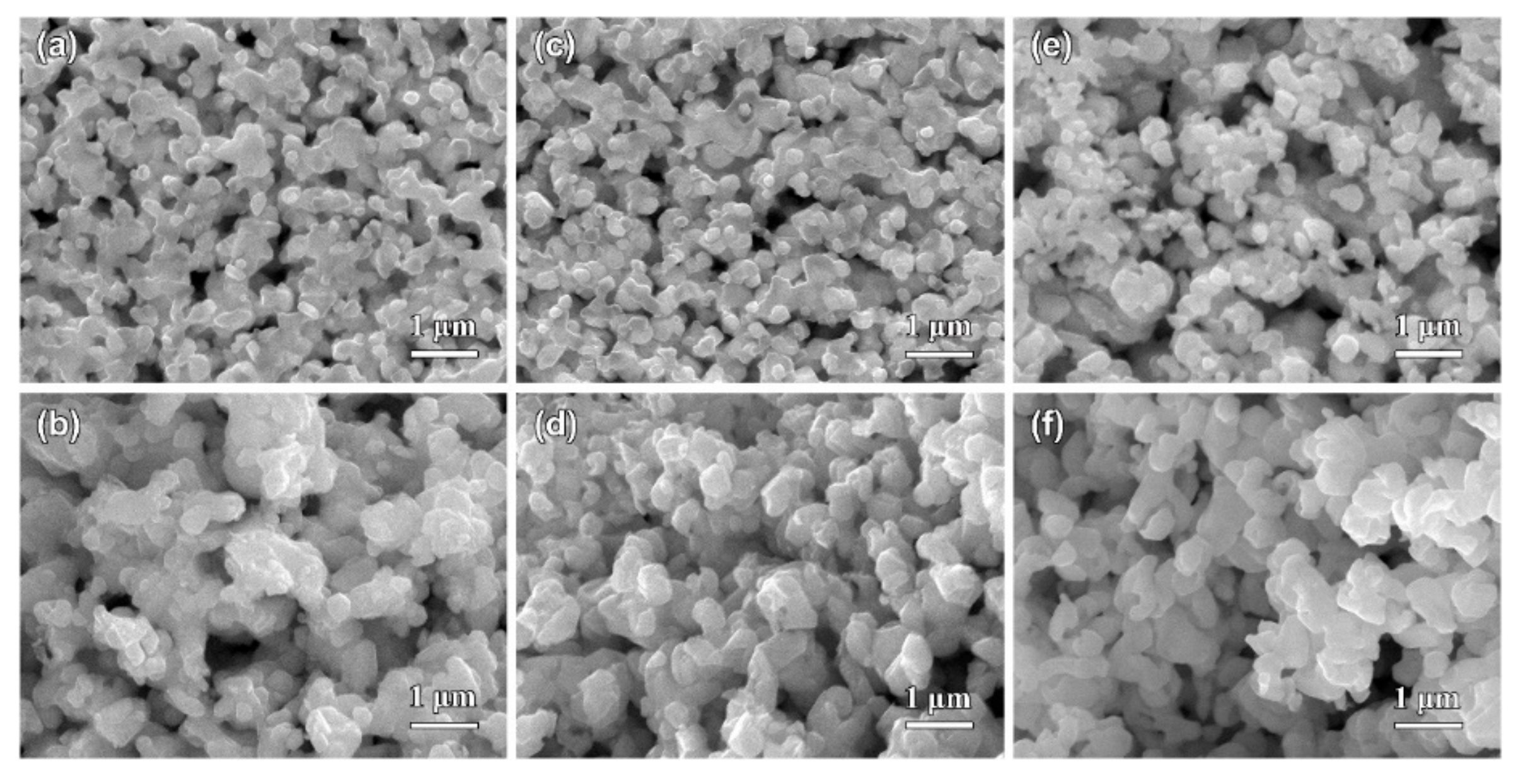
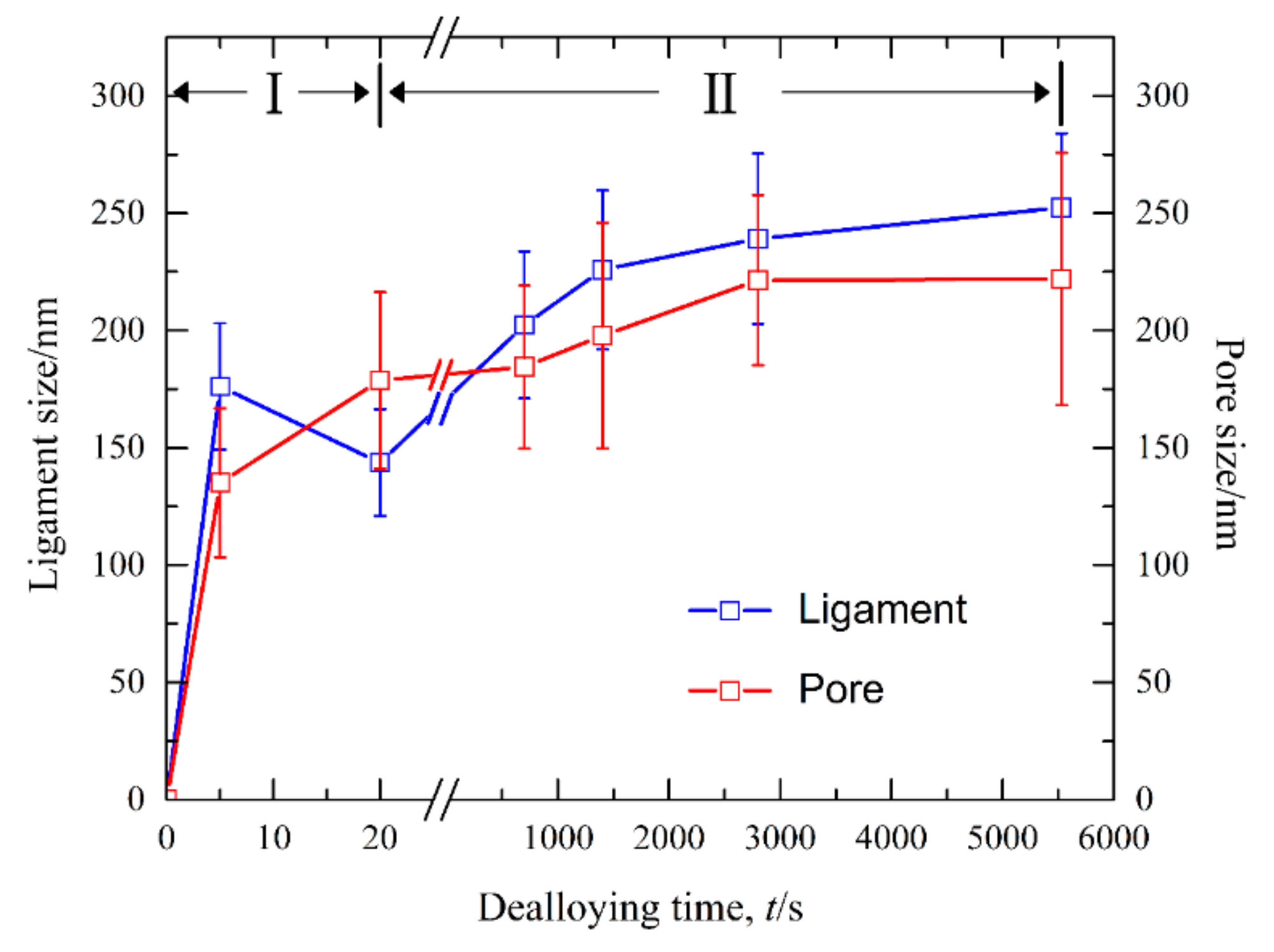
3.2. Morphological Characteristics of Nanoporous Silver after Potentiostatic Dealloying of Dual-Phase Ag–Sn Alloys
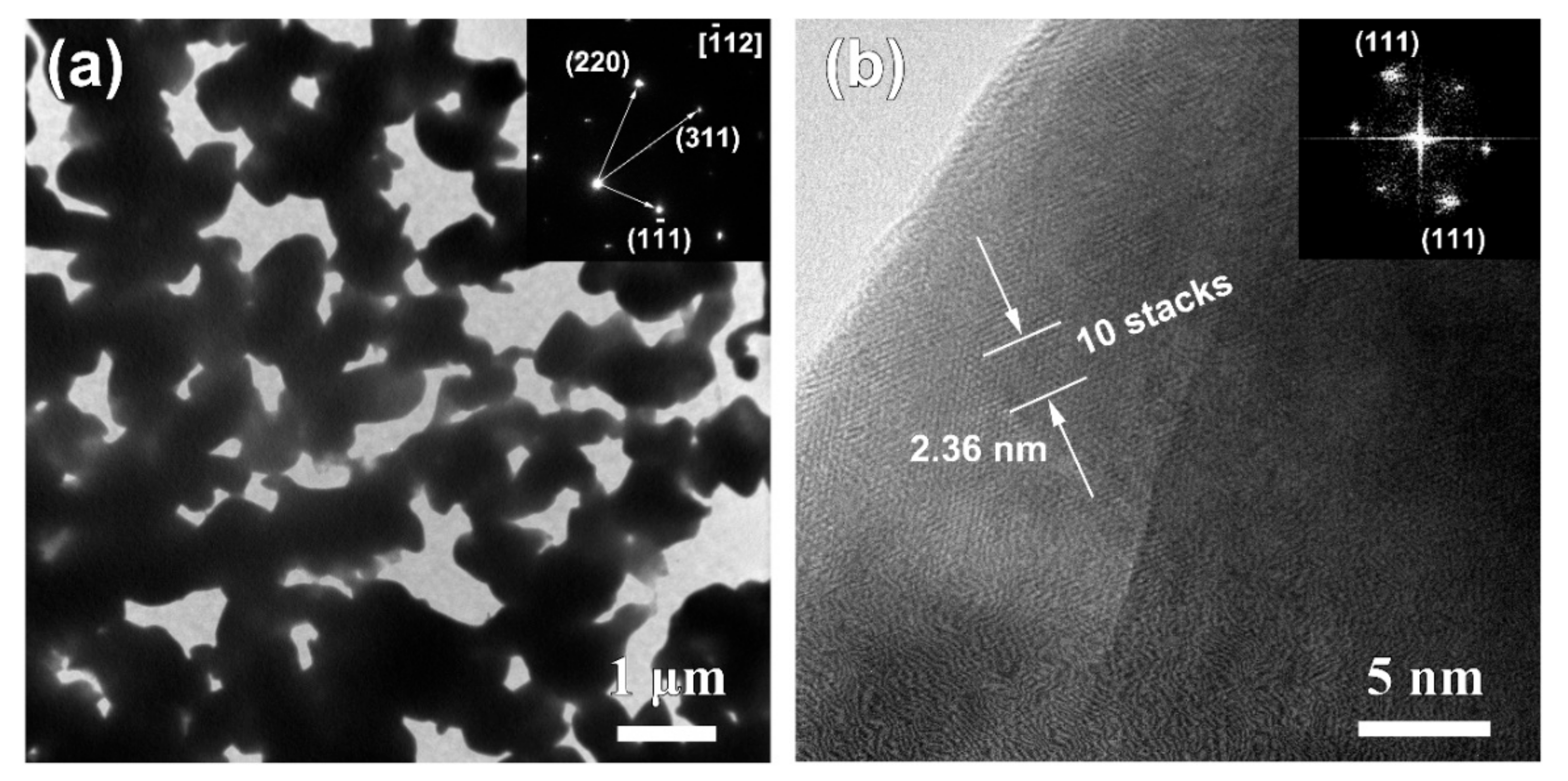
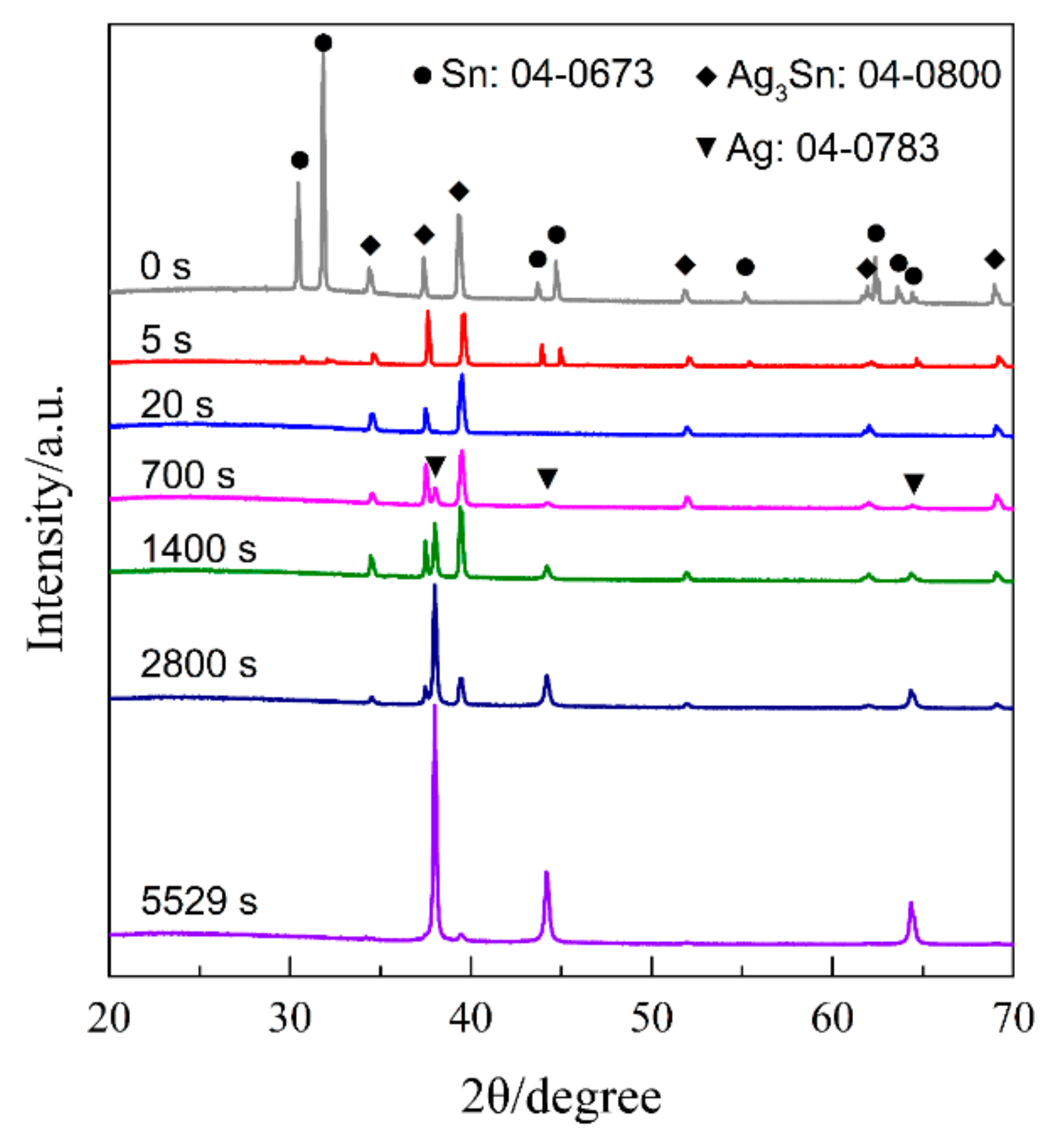
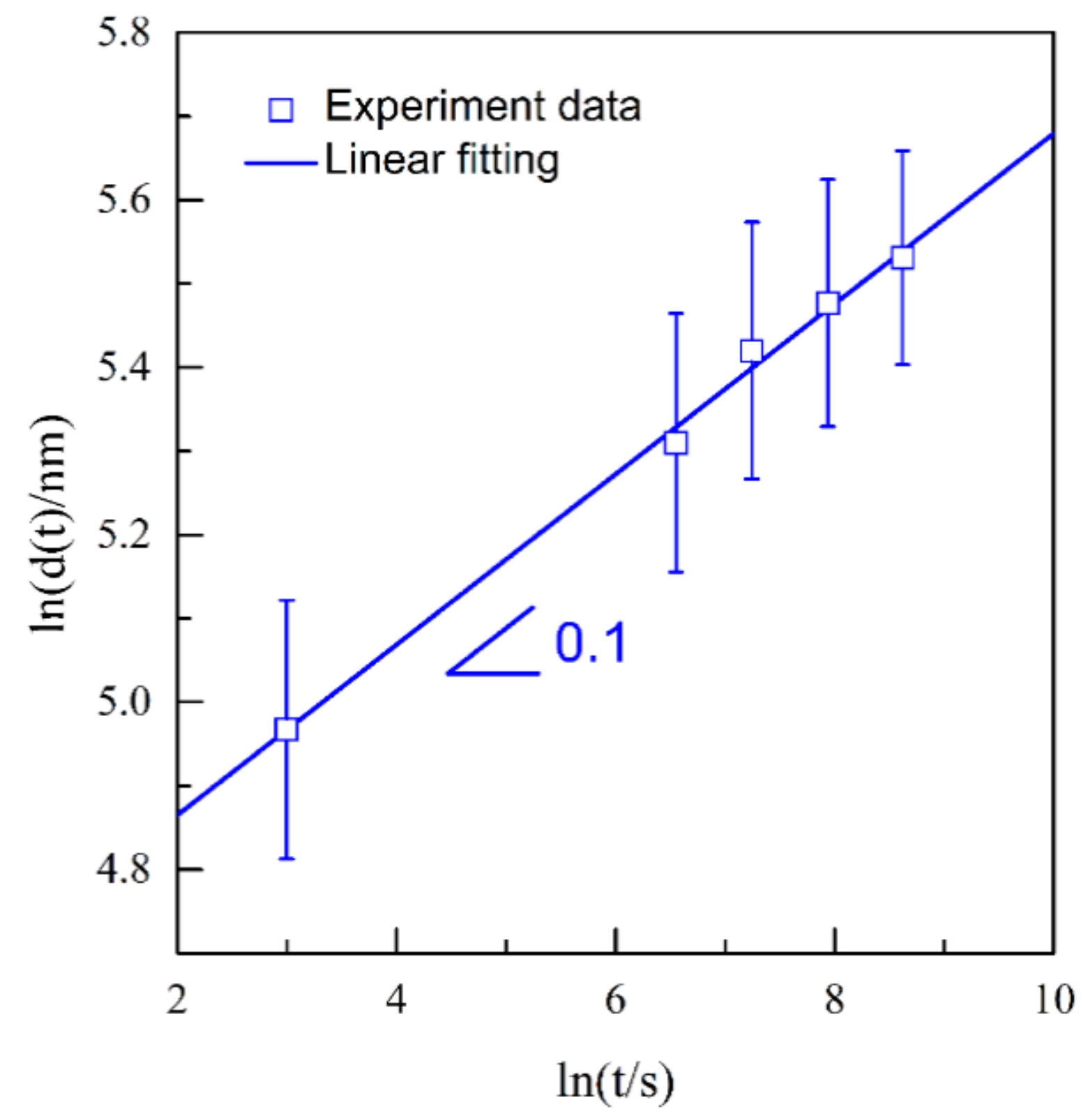
3.3. Nanoporous Structures and Phase Changes during Potentiostatic Dealloying
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev. Sci. Eng. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Wurschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.W.; Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions ofplatinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.; Viswanath, R.N.; Weissmüller, J. Surface-stress induced macroscopic bending of nanoporous gold cantilevers. Nano Lett. 2004, 4, 793–796. [Google Scholar] [CrossRef]
- Ruffato, G.; Romanato, F.; Garoli, D.; Cattarin, S. Nanoporous gold plasmonic structures for sensing applications. Opt. Express 2011, 19, 13164–13170. [Google Scholar] [CrossRef] [PubMed]
- Kucheyev, S.O.; Hayes, J.R.; Biener, J.; Huser, T.; Talley, C.E.; Hamza, A.V. Surface-enhanced Raman scattering on nanoporous Au. Appl. Phys. Lett. 2006, 89, 053102. [Google Scholar] [CrossRef]
- Chen, L.Y.; Lang, X.Y.; Fujita, T.; Chen, M.W. Nanoporous gold for enzyme-free electrochemical glucose sensors. Scr. Mater. 2011, 65, 17–20. [Google Scholar] [CrossRef]
- Ji, H.; Wang, X.G.; Zhao, C.C.; Zhang, C.; Xu, J.L.; Zhang, Z.H. Formation, control and functionalization of nanoporous silver through changing dealloying media and elemental doping. CrystEngComm 2011, 13, 2617–2628. [Google Scholar] [CrossRef]
- Song, T.; Yan, M.; Shi, Z.; Atrens, A.; Qian, M. Creation of bimodal porous copper materials by an annealing-electrochemical dealloying approach. Electrochim. Acta 2015, 164, 288–296. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Yamaura, S.; Xie, G.Q.; Makino, A.; Hara, N. Refinement of nanoporous copper by dealloying MgCuY amorphous alloys in sulfuric acids containing polyvinylpyrrolidone. J. Electrochem. Soc. 2014, 161, C120–C125. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Hara, N. Polyvinylpyrrolidone macromolecules function as a diffusion barrier during dealloying. Mater. Chem. Phys. 2014, 146, 277–282. [Google Scholar] [CrossRef]
- Qin, F.X.; Dan, Z.H.; Hara, N.; Li, W.R.; Li, Y.D. Selective dissolution of an amorphous Mg65Cu25Y10 alloy in organic acids and dilute HCl solution. Mater. Chem. Phys. 2016, 179, 27–34. [Google Scholar] [CrossRef]
- Qian, L.H.; Chen, M.W. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 083105. [Google Scholar] [CrossRef]
- Qi, Z.; Weissmüller, J. Hierarchical nested-network nanostructure by dealloying. ACS Nano 2013, 7, 5948–5954. [Google Scholar] [CrossRef]
- Forty, A.J. Corrosion micromorphology of noble metal alloys and depletion gilding. Nature 1979, 282, 597–598. [Google Scholar] [CrossRef]
- Forty, A.J.; Durkin, P. A micromorphological study of the dissolution ofsilver-gold alloys in nitric acid. Philos. Mag. A 1980, 42, 295–318. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wang, Y.; Qi, Z.; Lin, J.K.; Bian, X.F. Nanoporous gold ribbons with bimodal channel size distributions by chemical dealloying of Al-Au alloys. J. Phys. Chem. C 2009, 113, 1308–1314. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Wada, T.; Yamaura, S.; Xie, G.Q.; Sugawara, Y.; Muto, I.; Makino, A.; Hara, N. Nanoporous palladium fabricated from an amorphous Pd42.5Cu30Ni7.5P20 precursor and its ethanol electro-oxidation performance. Electrochim. Acta 2013, 108, 512–519. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Bimodal nanoporous nickel prepared by dealloying Ni38Mn62 alloys. Intermetallics 2012, 31, 157–164. [Google Scholar] [CrossRef]
- Grillo, R.; Torrisi, V.; Ruffino, F. Nanoporous Au: An experimental study on the porosity of dealloyed AuAg Leafs. Superlattices Microst. 2016, 100, 780–791. [Google Scholar] [CrossRef]
- Ruffino, F.; Torrisi, V.; Grillo, R.; Cacciato, G.; Zimbone, M.; Piccitto, G.; Grimaldi, M.G. Nanoporous Au structures by dealloying Au/Ag thermal-or laser-dewetted bilayers on surfaces. Superlattices Microst. 2017, 103, 28–47. [Google Scholar] [CrossRef]
- Wang, D.; Schaaf, P. Nanoporous gold nanoparticles. J. Mater. Chem. 2012, 22, 5344–5348. [Google Scholar] [CrossRef]
- Keir, D.S.; Pryor, M.J. The dealloying of copper-manganese alloys. J. Electrochem. Soc. 1980, 127, 2138–2144. [Google Scholar] [CrossRef]
- Pugh, D.V.; Dursun, A.; Corcoran, S.G. Electrochemical and morphological characterization of Pt-Cu dealloying. J. Electrochem. Soc. 2005, 152, B455–B459. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.H. On the electrochemical dealloying of Al-based alloys in a NaCl aqueous solution. Phys. Chem. Chem. Phys. 2010, 12, 1453–1472. [Google Scholar] [CrossRef]
- Lin, Y.W.; Tai, C.C.; Sun, I.W. Electrochemical preparation of porous copper surfaces in zinc chloride-1-ethyl-3-methyl imidazolium chloride ionic liquid. J. Electrochem. Soc. 2007, 154, D316–D321. [Google Scholar] [CrossRef][Green Version]
- Liu, W.B.; Zhang, S.C.; Li, N.; Zheng, J.W.; An, S.S.; Xing, Y.L. A general dealloying strategy to nanoporous intermetallics, nanoporous metals with bimodal, and unimodal pore size distributions. Corros. Sci. 2012, 58, 133–138. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, J.Z.; Xu, J.L.; Wang, X.G.; Ji, H.; Zhao, C.C.; Zhang, Z.H. Formation and microstructure of nanoporous silver by dealloying rapidly solidified Zn-Ag alloys. Electrochim. Acta 2012, 63, 302–311. [Google Scholar] [CrossRef]
- Jin, Y.; Li, R.; Zhang, T. Formation of nanoporous silver by dealloying Ca-Ag metallic glassesin water. Intermetallics 2015, 67, 166–170. [Google Scholar] [CrossRef]
- Zhang, M.; Junior, A.M.J.; Pang, S.J.; Zhang, T.; Yavari, A.R. Fabrication of nanoporous silver with open pores. Scr. Mater. 2015, 100, 21–23. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Sn (silver-tin) system. Bull. Alloy Phase Diagr. 1987, 8, 340–347. [Google Scholar] [CrossRef]
- Xu, J.L.; Wang, Y.; Zhang, Z.H. Potential and concentration dependent electrochemical dealloying of Al2Au in sodium chloride solutions. J. Phys. Chem. C 2012, 116, 5689–5699. [Google Scholar] [CrossRef]
- Sieradzki, K.; Corderman, R.R.; Shukla, K. Computer simulations of corrosion: Selective dissolution of binary alloys. Philos. Mag. A 1989, 59, 713–746. [Google Scholar] [CrossRef]
- Dixon, M.C.; Daniel, T.A.; Hieda, M.; Smilgies, D.M.; Chan, M.H.W.; Allara, D.L. Preparation, structure, and optical properties of nanoporous gold thin films. Langmuir 2007, 23, 2414–2422. [Google Scholar] [CrossRef]
- Zhao, C.C.; Wang, X.G.; Qi, Z.; Ji, H.; Zhang, Z.H. On the electrochemical dealloying of Mg-Cu alloys in a NaCl aqueous solution. Corros. Sci. 2010, 52, 3962–3972. [Google Scholar] [CrossRef]
- Straumanis, M.E.; Dutta, M. The divalency of tin ions formed during anodic dissolution and the behavior of the tin anode. Inorg. Chem. 1966, 5, 992–995. [Google Scholar] [CrossRef]
- Mezey, L.Z.; Giber, J. The surface free energies of solid chemical elements: Calculation from internal free enthalpies of atomization. Jpn. J. Appl. Phys. Part 1 1982, 21, 1569–1571. [Google Scholar] [CrossRef]
- Doña, J.M.; González-Velasco, J. The dependence of the surface diffusion coefficients of gold atoms on the potential: Its influence on reconstruction of metal lattices. Surf. Sci. 1992, 274, 205–214. [Google Scholar] [CrossRef]
- Doña, J.M.; González-Velasco, J. Mechanism of surface diffusion of gold adatoms in contact with an electrolytic solution. J. Phys. Chem. 1993, 97, 4714–4719. [Google Scholar] [CrossRef]
| Alloys/Metals | Open-Circuit Potential (Eocp, mV) | Corrosion Potential (Ecorr, mV) | Current Density at −50 mV (j−50mV, mA cm−2) |
|---|---|---|---|
| Ag | −38 | −111 | 0.0126 |
| Ag3Sn | −407 | −456 | 3.43 |
| Ag40Sn60 | −496 | −513 | 7.34 |
| Ag30Sn70 | −498 | −518 | 9.91 |
| Ag20Sn80 | −498 | −515 | 5.00 |
| Sn | −500 | −513 | 397 |
| Alloy | Applied Potential, mV | Dealloying Duration, s | Ligament Size, nm | Pore Size, nm | Surface Diffusivity/Ds, cm2 s−1 |
|---|---|---|---|---|---|
| Ag20Sn80 | −50 | 4673 | 182 ± 38 | 301 ± 49 | 8.3 × 10−12 |
| Ag30Sn70 | −50 | 4979 | 213 ± 33 | 249 ± 39 | 1.5 × 10−11 |
| Ag40Sn60 | 0 | 1924 | 306 ± 50 | 295 ± 42 | 1.6 × 10−10 |
| Ag40Sn60 | −25 | 3611 | 278 ± 55 | 250 ± 34 | 5.8 × 10−11 |
| Ag40Sn60 | −50 | 5529 | 252 ± 32 | 222 ± 54 | 2.6 × 10−11 |
| Ag40Sn60 | −75 | 17,597 | 210 ± 32 | 190 ± 20 | 3.9 × 10−12 |
| Ag40Sn60 | −100 | 36745 | 190 ± 29 | 165 ± 39 | 1.2 × 10−12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Dan, Z.; Liang, Y.; Wang, Y.; Qin, F.; Chang, H. Asynchronous Evolution of Nanoporous Silver on Dual-Phase Ag–Sn Alloys by Potentiostatic Dealloying in Hydrochloric Acid Solution. Nanomaterials 2019, 9, 743. https://doi.org/10.3390/nano9050743
Yang Y, Dan Z, Liang Y, Wang Y, Qin F, Chang H. Asynchronous Evolution of Nanoporous Silver on Dual-Phase Ag–Sn Alloys by Potentiostatic Dealloying in Hydrochloric Acid Solution. Nanomaterials. 2019; 9(5):743. https://doi.org/10.3390/nano9050743
Chicago/Turabian StyleYang, Yulin, Zhenhua Dan, Yongfeng Liang, Ying Wang, Fengxiang Qin, and Hui Chang. 2019. "Asynchronous Evolution of Nanoporous Silver on Dual-Phase Ag–Sn Alloys by Potentiostatic Dealloying in Hydrochloric Acid Solution" Nanomaterials 9, no. 5: 743. https://doi.org/10.3390/nano9050743
APA StyleYang, Y., Dan, Z., Liang, Y., Wang, Y., Qin, F., & Chang, H. (2019). Asynchronous Evolution of Nanoporous Silver on Dual-Phase Ag–Sn Alloys by Potentiostatic Dealloying in Hydrochloric Acid Solution. Nanomaterials, 9(5), 743. https://doi.org/10.3390/nano9050743






