As(III) Removal from Aqueous Solution by Calcium Titanate Nanoparticles Prepared by the Sol Gel Method
Abstract
1. Introduction
2. Materials and Methods
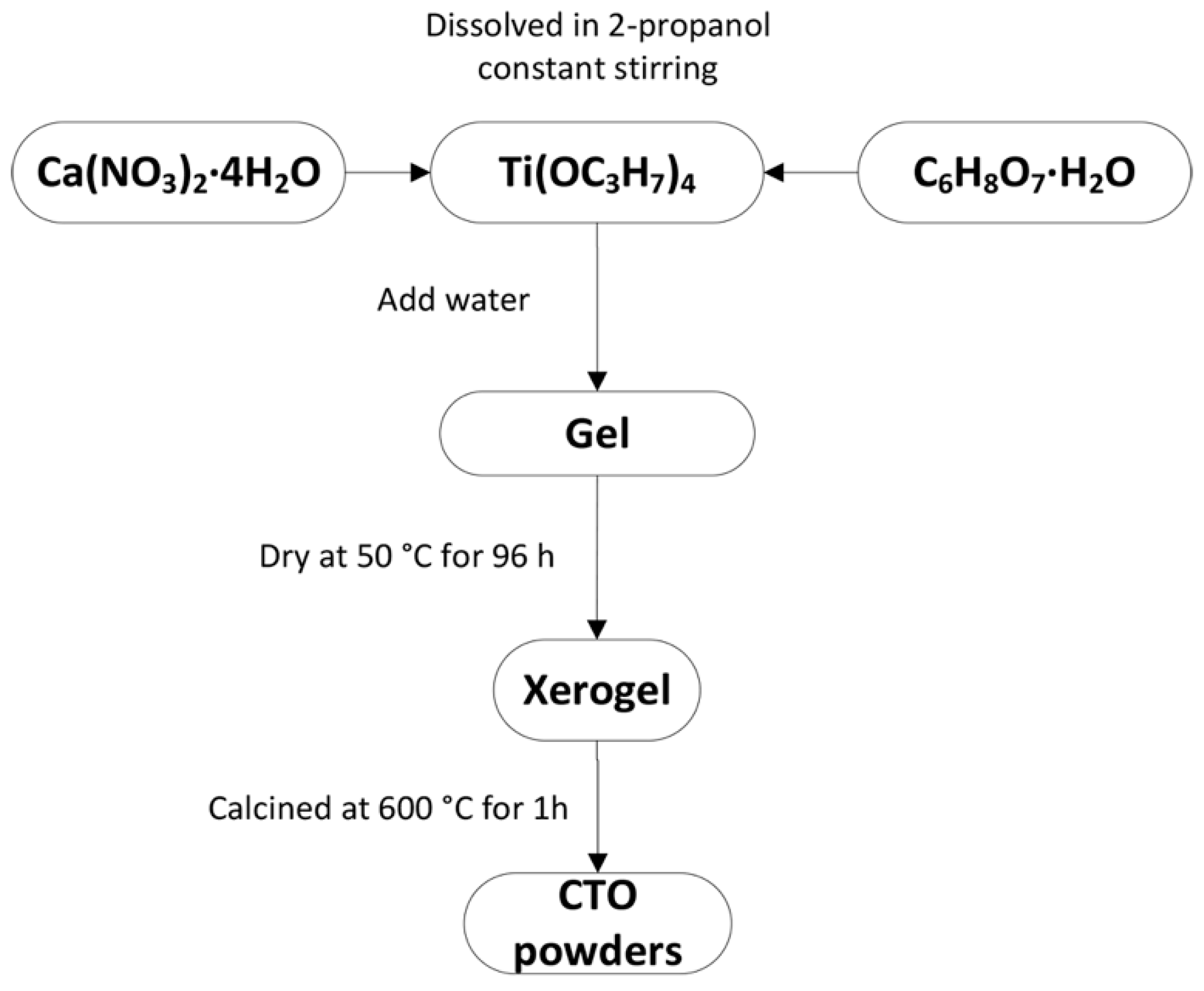
2.1. Synthesis of CaTiO3 Nanopowder
2.2. Characterization
2.3. Batch Adsorption Experiments
2.4. Ion Effect and Adsorption/Desorption Cycles
3. Results
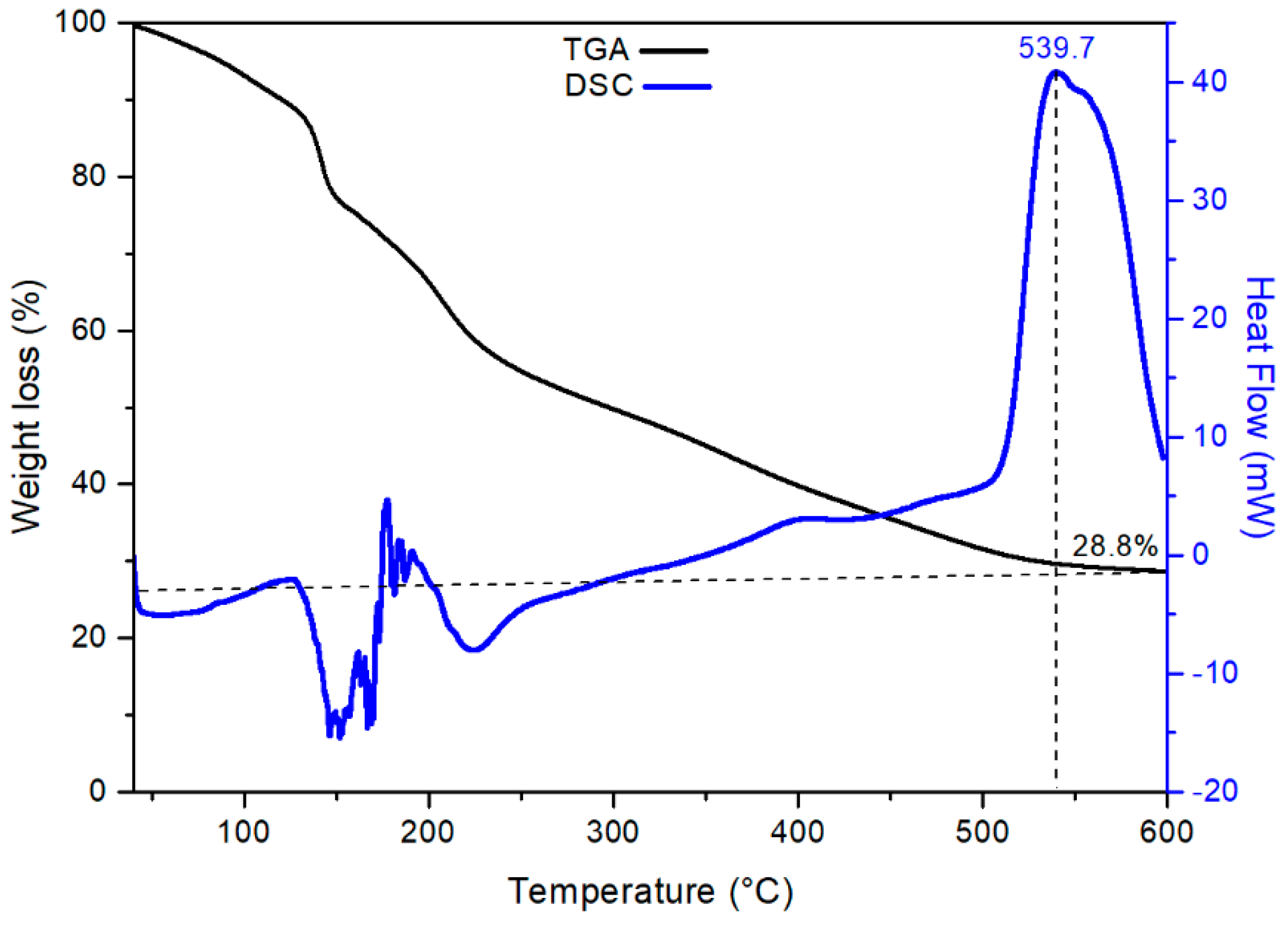
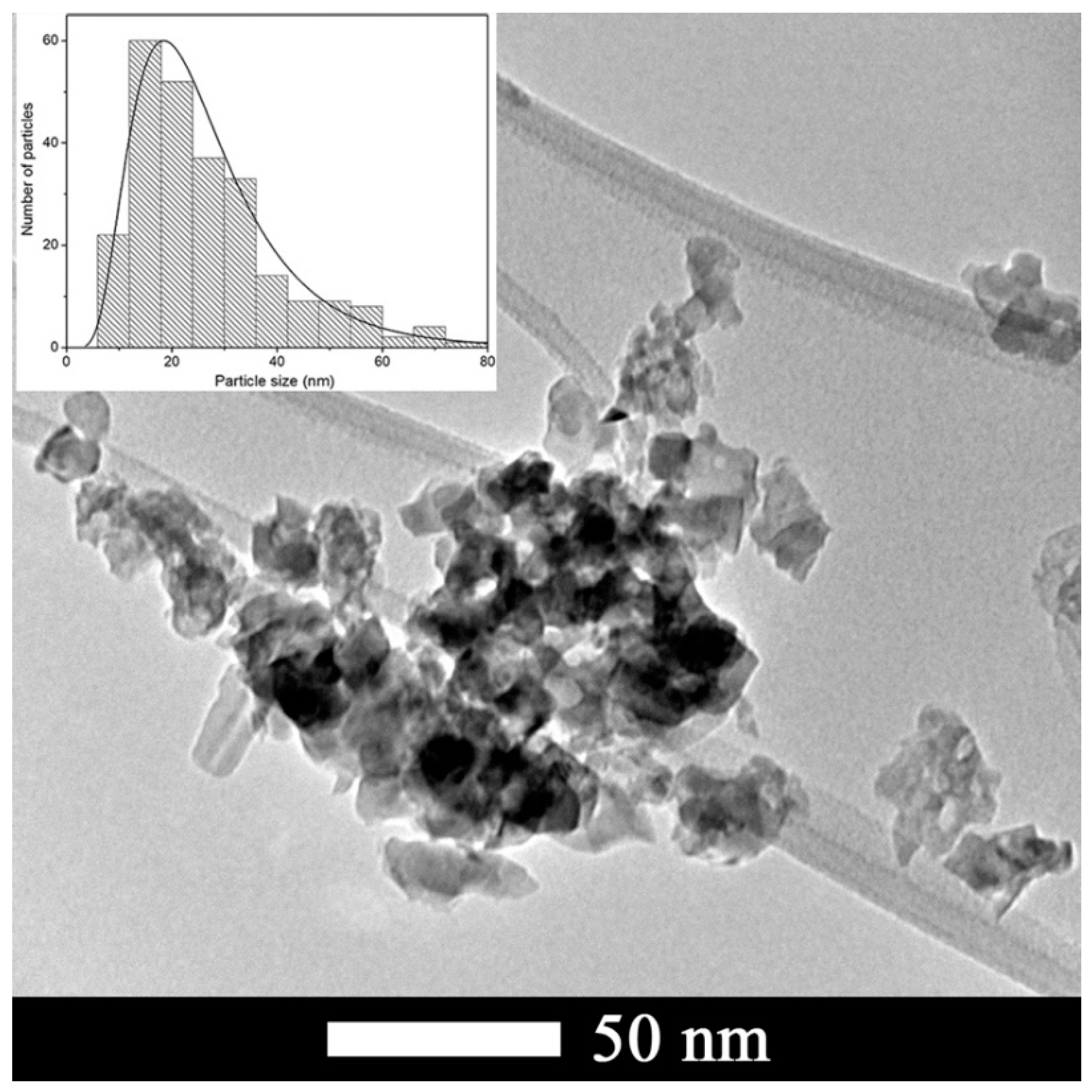
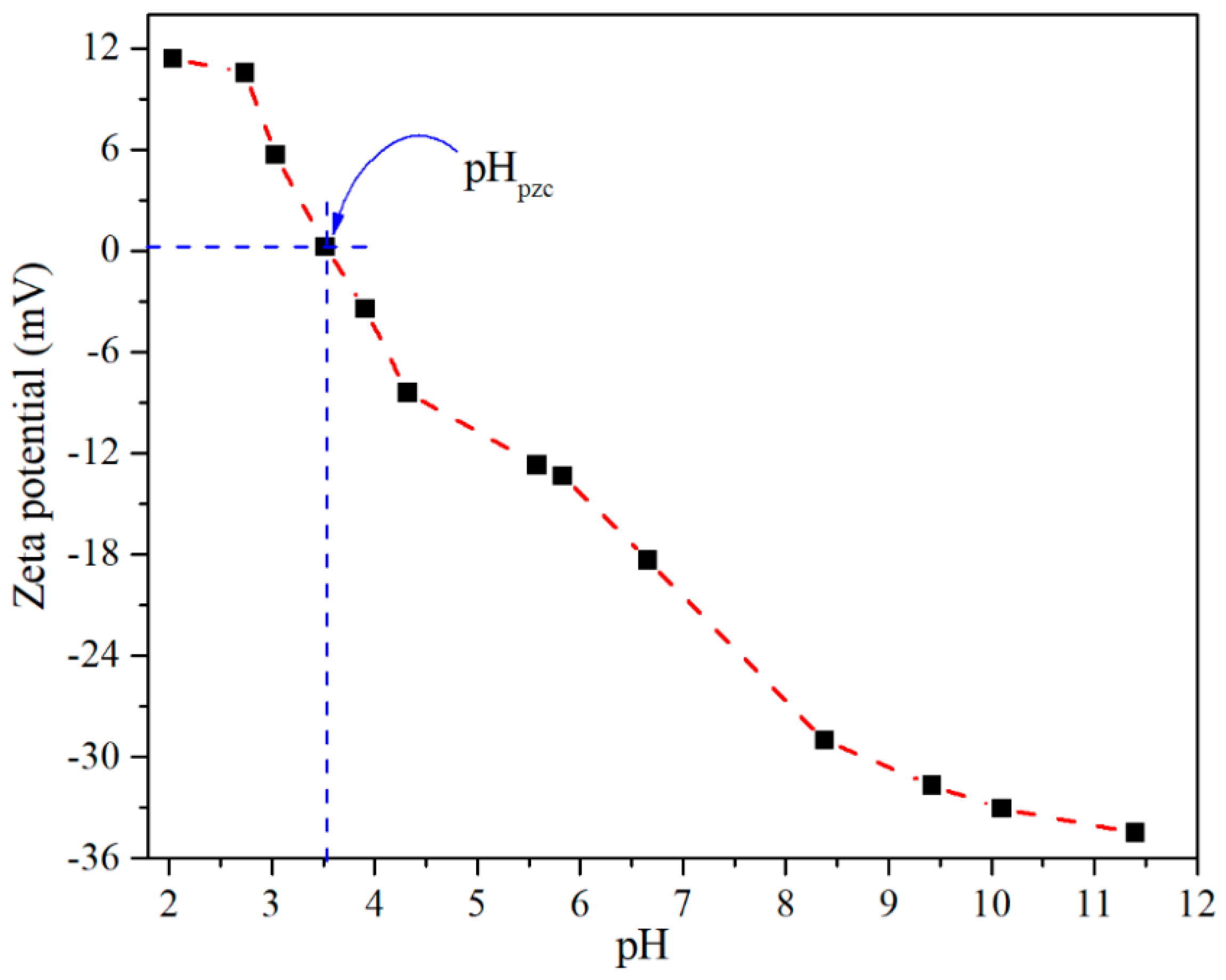
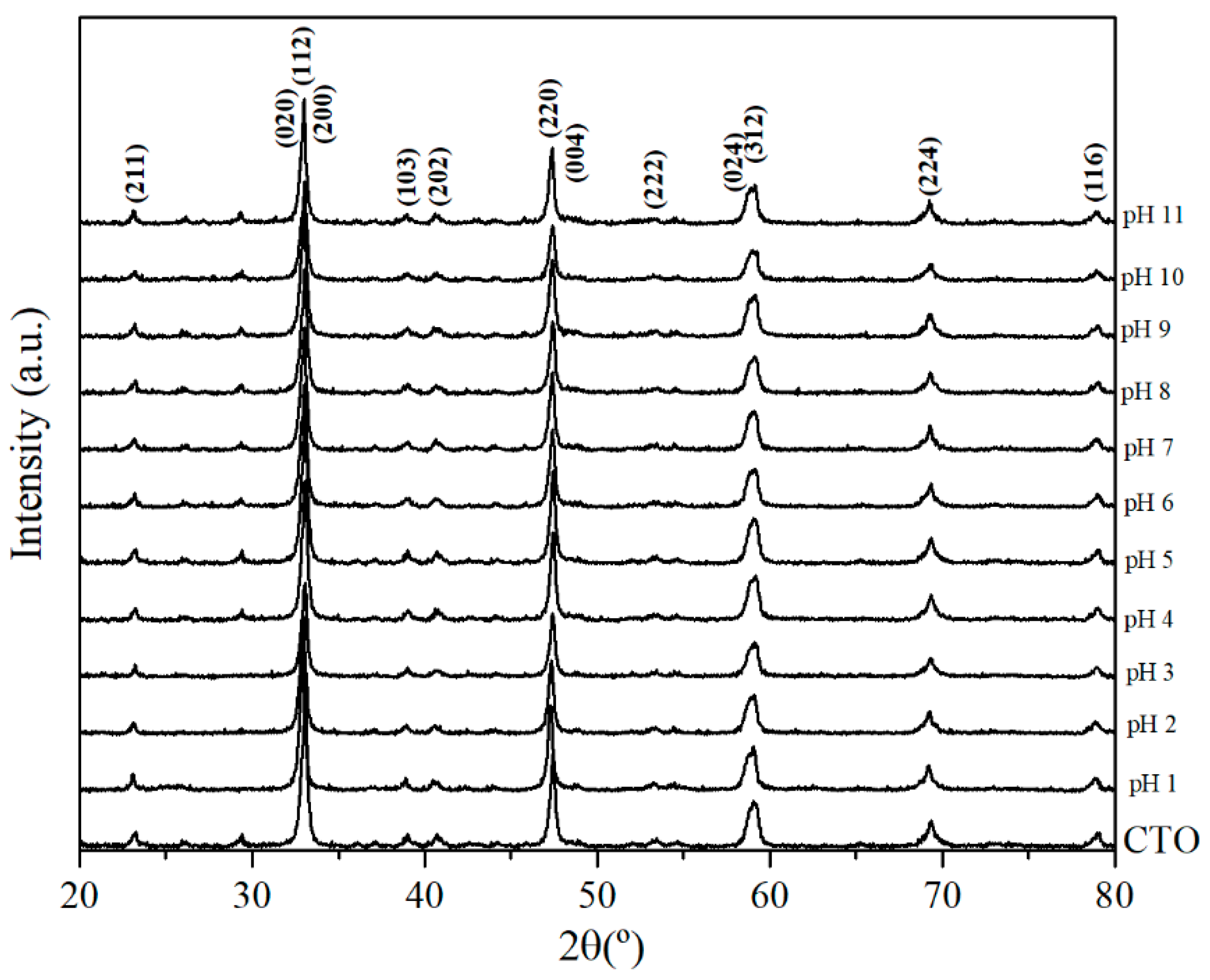
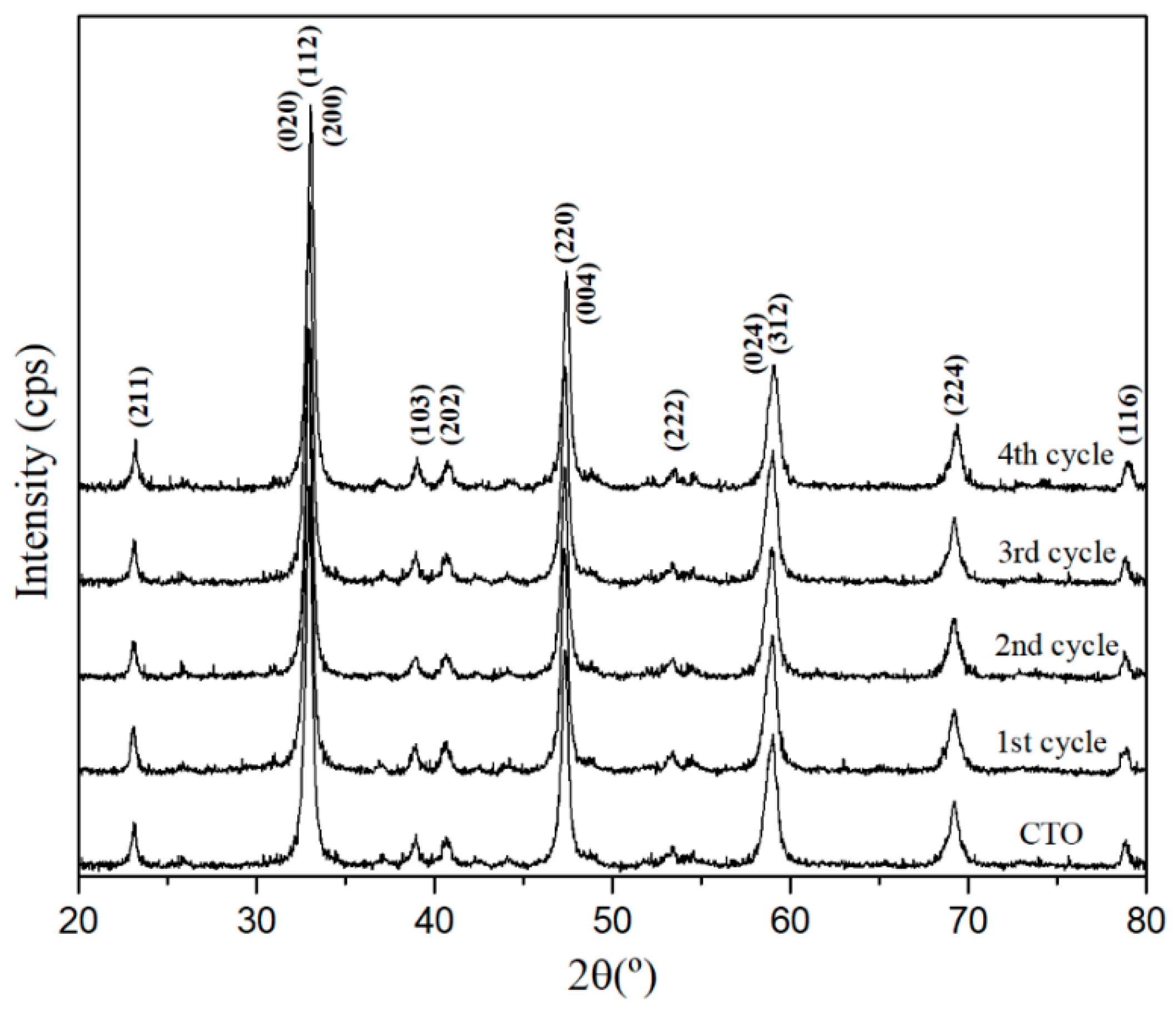
3.1. Characterization of CTO Nanopowders
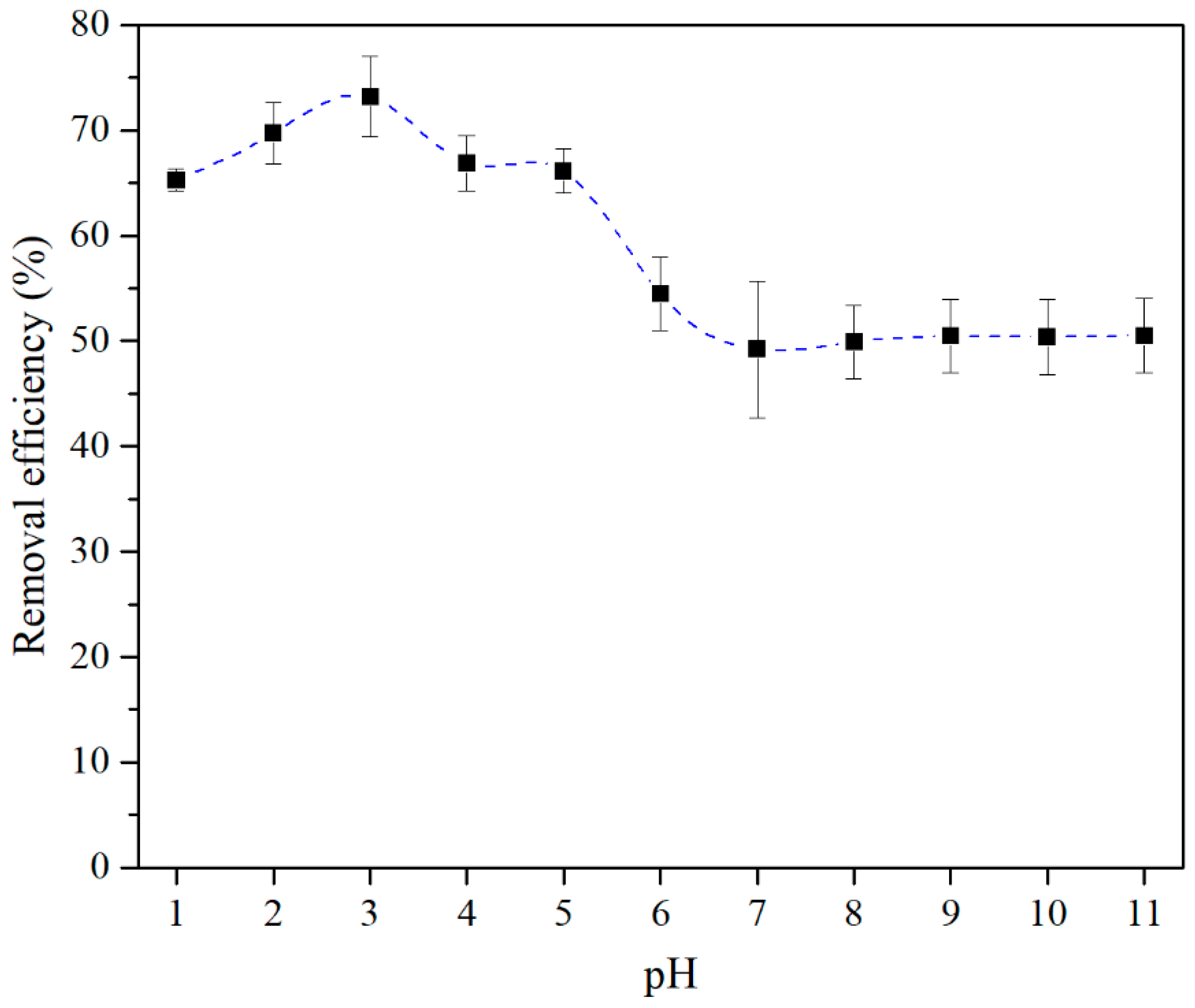
3.2. Batch Adsorption Experiments
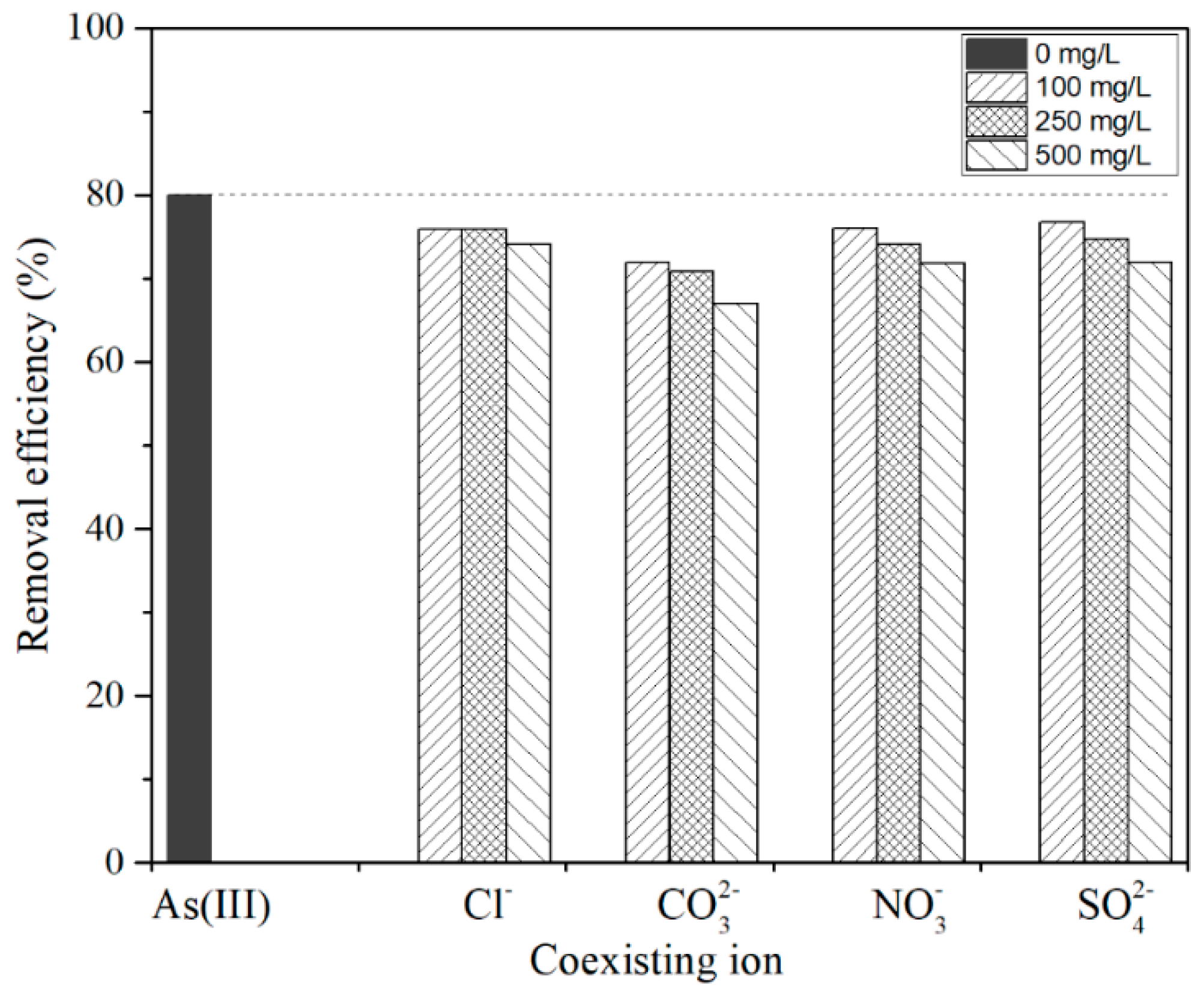
3.3. Effect of Coexisting Anions
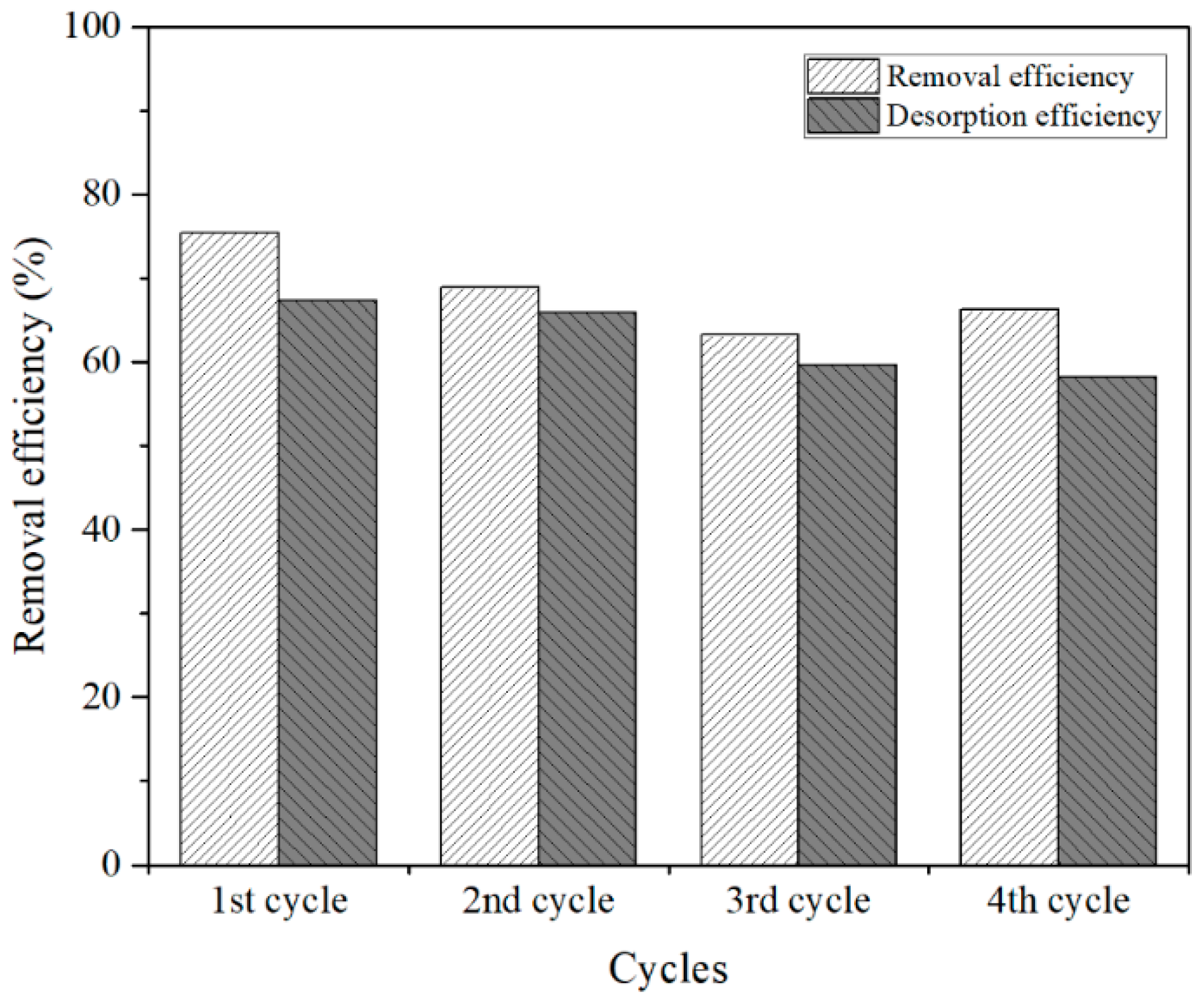
3.4. Recycle and Stability
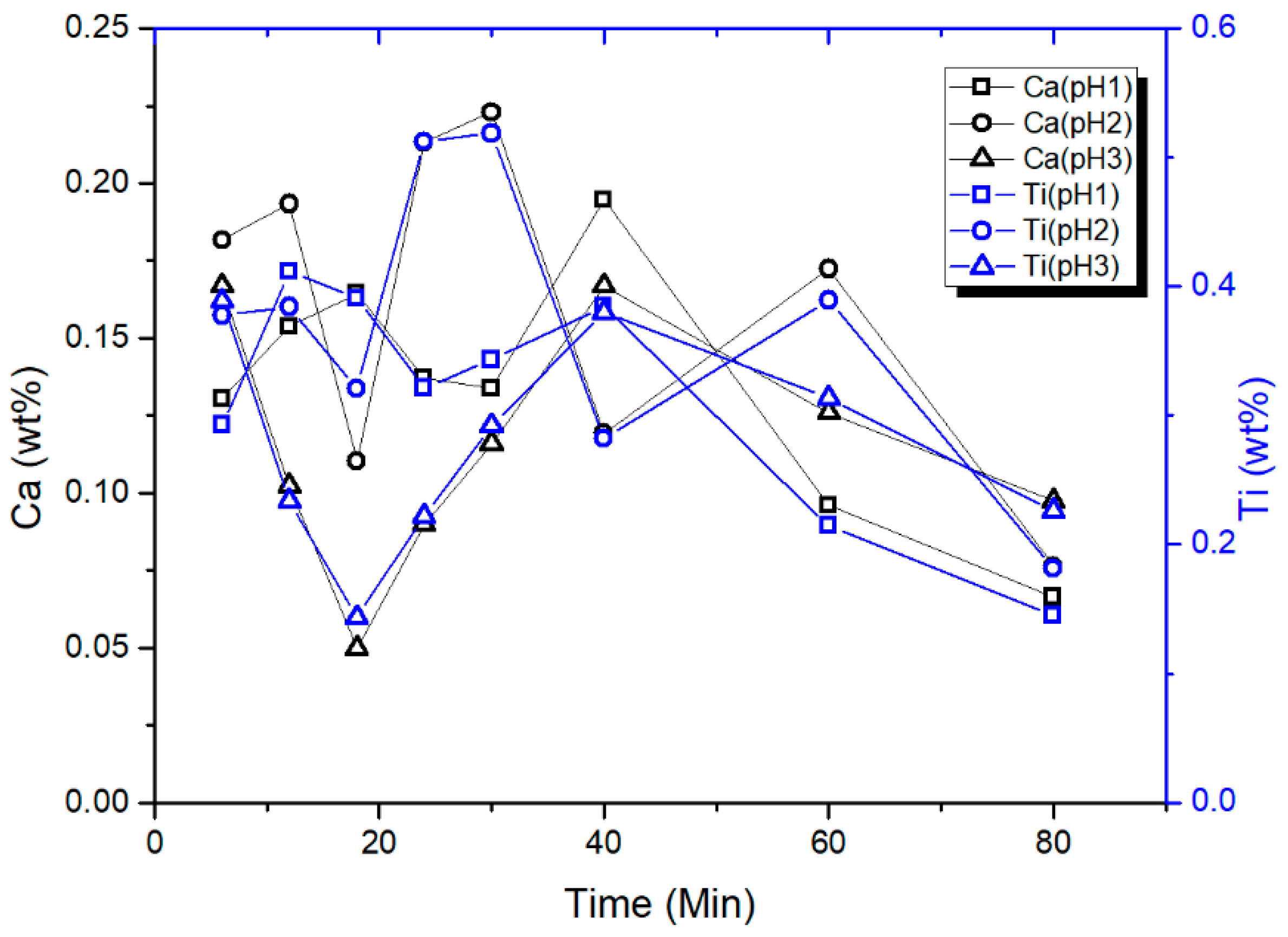
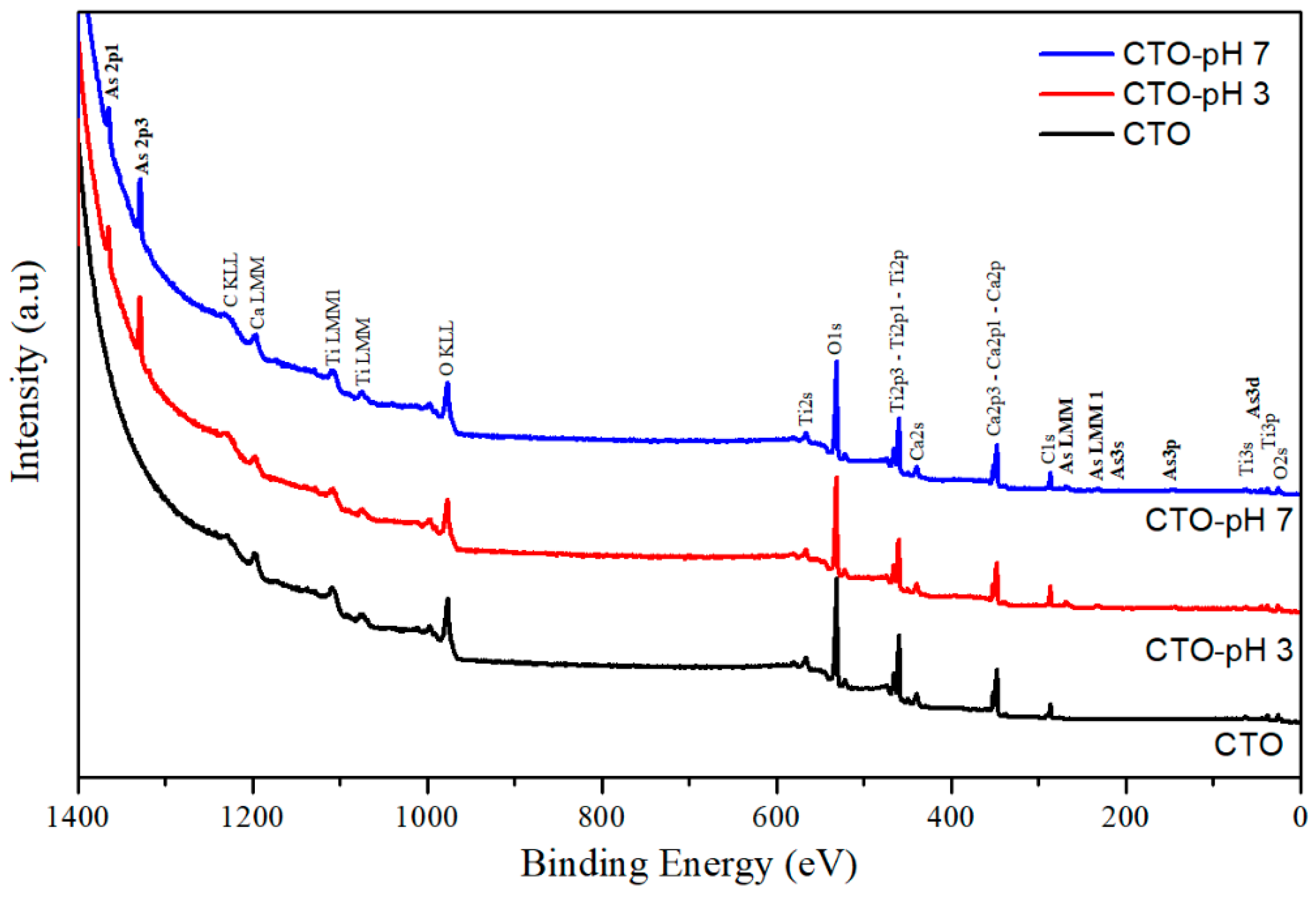
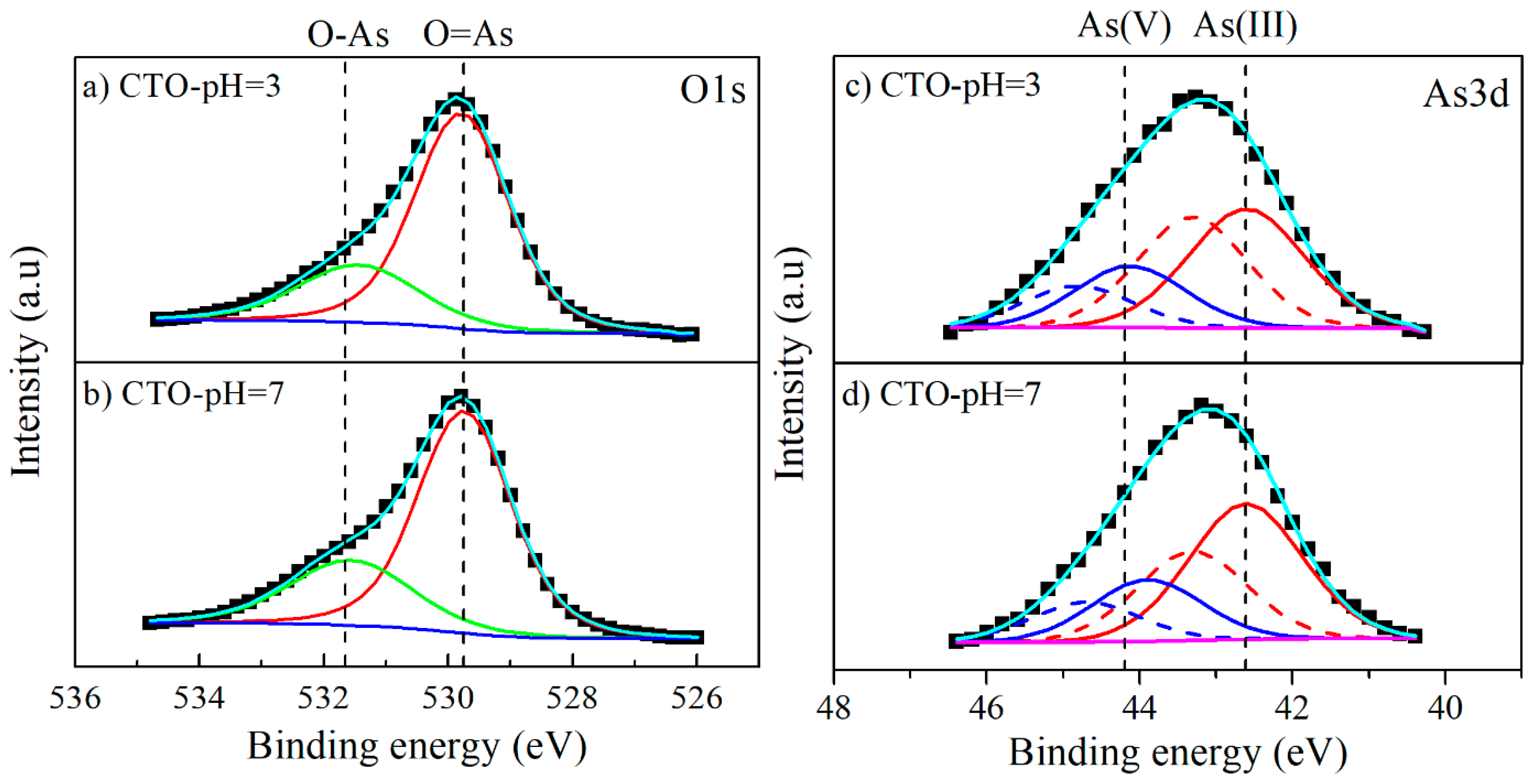
3.5. Characterization of CTO Surface after Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Majumder, A.; Banerjee, G.; Roy, M.B.; Pal, S.; Mazumdar, A. Removal of arsenic from drinking water using dual treatment process. Clean Technol. Environ. Policy 2015, 17, 1065–1076. [Google Scholar] [CrossRef]
- German, M.; Seingheng, H.; SenGupta, A.K. Mitigating arsenic crisis in the developing world: Role of robust, reusable and selective hybrid anion exchanger (HAIX). Sci. Total Environ. 2014, 488–489, 547–553. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Arsenic in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; López, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef]
- Byrne, S.; Amarasiriwardena, D.; Bandak, B.; Bartkus, L.; Kane, J.; Jones, J.; Yañez, J.; Arriaza, B.; Cornejo, L. Were Chinchorros exposed to arsenic? Arsenic determination in Chinchorro mummies’ hair by laser ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS). Microchem. J. 2010, 94, 28–35. [Google Scholar]
- Mandal, B.; Suzuki, K. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Cullen, K.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H.; Ag, C. Arsenic—A Review. Part I: Occurrence, Toxicity, Speciation, Mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Chemical and Electrochemical Equilibria in the Presence of a Gaseous Phase; National Assn of Corrosion: Houston, TX, USA, 1997; ISBN 9782960013405. [Google Scholar]
- Manning, B. Surface Structures and Stability of Arsenic(III) on Goethite: Spectroscopic Evidence for Inner-Sphere Complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Jain, A.; Raven, K.P.; Loeppert, R.H. Arsenite and Arsenate adsorption on ferrihydrite: Surface charge reduction and net OH- release stoichiometry. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Chen, G.; Qu, J. Effect of aluminum speciation on arsenic removal during coagulation process. Sep. Purif. Technol. 2012, 86, 35–40. [Google Scholar] [CrossRef]
- Lacasa, E.; Cañizares, P.; Sáez, C.; Fernández, F.J.; Rodrigo, M.A. Removal of arsenic by iron and aluminium electrochemically assisted coagulation. Sep. Purif. Technol. 2011, 79, 15–19. [Google Scholar] [CrossRef]
- Mohora, E.; Rončević, S.; Agbaba, J.; Tubić, A.; Mitić, M.; Klašnja, M.; Dalmacija, B. Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF). Sep. Purif. Technol. 2014, 136, 150–156. [Google Scholar] [CrossRef]
- Zavareh, S.; Zarei, M.; Darvishi, F.; Azizi, H. As(III) adsorption and antimicrobial properties of Cu—chitosan/alumina nanocomposite. Chem. Eng. J. 2015, 273, 610–621. [Google Scholar] [CrossRef]
- Hlavay, J.; Polyák, K. Determination of surface properties of iron hydroxide-coated alumina adsorbent prepared for removal of arsenic from drinking water. J. Colloid Interface Sci. 2005, 284, 71–77. [Google Scholar] [CrossRef]
- Vitela-Rodriguez, A.V.; Rangel-mendez, J.R. Arsenic removal by modified activated carbons with iron hydro (oxide) nanoparticles. J. Environ. Manag. 2013, 114, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-G.; Gong, X.-J.; Wang, K.; Zhang, X.-R.; Fan, W.-B. Adsorption characteristics of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon. Bioresour. Technol. 2014, 165, 166–173. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Lin, L.; Khan, Z.; Qiu, W.; Song, Z. Synthesis and Characterization of Novel Fe-Mn-Ce Ternary Oxide–Biochar Composites as Highly Efficient Adsorbents for As (III) Removal from Aqueous Solutions. Materials 2018, 11, 2445. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of Arsenic(III) from Aqueous Solution Using Metal Organic Framework-Graphene Oxide Nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Contreras, D.; Yáñez, J.; Mansilla, H.D. In field arsenic removal from natural water by zero-valent iron assisted by solar radiation. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef]
- Thanawatpoontawee, S.; Imyim, A.; Praphairaksit, N. Iron-loaded zein beads as a biocompatible adsorbent for arsenic(V) removal. J. Ind. Eng. Chem. 2016, 43, 127–132. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Y.; Mo, F.; Zhou, L.; Li, B. Synthesis and luminescent properties of CaTiO3:Eu3+, Al3+ phosphors. Ceram. Int. 2014, 40, 10887–10892. [Google Scholar] [CrossRef]
- Oliveira, L.H.; De Moura, A.P.; La Porta, F.A.; Nogueira, I.C.; Aguiar, E.C.; Sequinel, T.; Rosa, I.L.V.; Longo, E.; Varela, J.A. Influence of Cu-doping on the structural and optical properties of CaTiO3 powders. Mater. Res. Bull. 2016, 81, 1–9. [Google Scholar]
- Manik, S.K.; Pradhan, S.K.; Pal, M. Nanocrystalline CaTiO3 prepared by soft-chemical route. Physica E 2005, 25, 421–424. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Huang, M.; Zhang, S.; Li, L. Synthesis and characterization of CaTiO3-(Sm,Nd)AlO3 microwave ceramics via sol-gel method. J. Sol-Gel Sci. Technol. 2014, 69, 61–66. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, G.; Zhou, J.; Zhou, H.; Zhan, J.; Yang, Z. Facile sol-gel combustion synthesis and photoluminescence enhancement of CaZrO3:Sm3+ nanophosphors via Gd3+ doping. J. Rare Earths 2012, 30, 1000–1004. [Google Scholar] [CrossRef]
- Jia, C.; Gao, J.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel catalysts supported on calcium titanate for enhanced CO methanation. Catal. Sci. Technol. 2013, 3, 490–499. [Google Scholar] [CrossRef]
- Zhuang, J.; Tian, Q.; Lin, S.; Yang, W.; Chen, L.; Liu, P. Applied Catalysis B: Environmental Precursor morphology-controlled formation of perovskites CaTiO3 and their photo-activity for As(III) removal. Appl. Catal. B Environ. 2014, 156–157, 108–115. [Google Scholar] [CrossRef]
- Coreño, J.; Coreño, O. Evaluation of calcium titanate as apatite growth promoter. J. Biomed. Mater. Res. Part A 2005, 75, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ray, A.K.; Sharma, V.K.; Millero, F.J. Adsorption of arsenate and arsenite on titanium dioxide suspensions. J. Colloid Interface Sci. 2004, 278, 270–275. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, K.; Wu, Y.; Zou, Y.; Zuo, J.; Arriagada, D.C.; Pan, Z.; Hu, G. The effect of pH on the adsorption of arsenic(III) and arsenic(V) at the TiO2 anatase [101] surface. J. Colloid Interface Sci. 2016, 462, 252–259. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Fyfe, W.S.; Karkhanis, S.N.; Nishijima, A.; Shin, S. Thermodynamic stability and kinetics of perovskite dissolution. Nature 1981, 289, 358–362. [Google Scholar] [CrossRef]
- Blesa, M.A.; Morando, P.J.; Regazzoni, A.E. Chemical Dissolution of Metal Oxides, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 1994; Chapter 17. [Google Scholar]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Li, Y.; Cai, X.; Guo, J.; Na, P. UV-induced photoactive adsorption mechanism of arsenite by anatase TiO2 with high surface hydroxyl group density. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 202–210. [Google Scholar] [CrossRef]
- Pena, M.E.; Korfiatis, G.P.; Patel, M.; Lippincott, L.; Meng, X. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2005, 39, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Q.; Gao, S.; Shang, J.K. High efficient As(III) removal by self-assembled zinc oxide micro-tubes synthesized by a simple precipitation process. J. Mater. Sci. 2011, 46, 5851–5858. [Google Scholar] [CrossRef]
- Cho, D.-W.; Chon, C.-M.; Yang, H.; Tsang, Y.F.; Song, H. Effect of Mn substitution on the oxidation/adsorption abilities of iron(III) oxyhydroxides. Clean Technol. Environ. Policy 2018, 20, 2201–2208. [Google Scholar] [CrossRef]
- Su, H.; Lv, X.; Zhang, Z.; Yu, J.; Wang, T. Arsenic removal from water by photocatalytic functional Fe2O3–TiO2 porous ceramic. J. Porous Mater. 2017, 24, 1227–1235. [Google Scholar] [CrossRef]
- Deng, M.; Wu, X.; Zhu, A.; Zhang, Q.; Liu, Q. Well-dispersed TiO2 nanoparticles anchored on Fe3O4 magnetic nanosheets for efficient arsenic removal. J. Environ. Manag. 2019, 237, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, J.; Yang, W.; Wu, Q.; Yang, H.; Xue, X. Enhancement of photocatalytic activity of CaTiO3 through HNO3 acidification. J. Photochem. Photobiol. A Chem. 2016, 323, 1–9. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, B.; Gao, L. X-ray photoemission spectroscopy investigation of CaTiO3:Eu for luminescence property: Effect of Eu3+ ion. Mater. Res. Bull. 2016, 78, 31–35. [Google Scholar] [CrossRef]
- Yan, W.; Ramos, M.A.V.; Koel, B.E.; Zhang, W. Multi-tiered distributions of arsenic in iron nanoparticles: Observation of dual redox functionality enabled by a core–shell structure. Chem. Commun. 2010, 46, 6995. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Li, W.; Beecham, S.; Mulcahy, D. Aqueous arsenite removal by simultaneous ultraviolet photocatalytic oxidation-coagulation of titanium sulfate. J. Hazard. Mater. 2016, 303, 162–170. [Google Scholar] [CrossRef]
- Han, Y.S.; Jeong, H.Y.; Demond, A.H.; Hayes, K.F. X-ray absorption and photoelectron spectroscopic study of the association of As(III) with nanoparticulate FeS and FeS-coated sand. Water Res. 2011, 45, 5727–5735. [Google Scholar] [CrossRef]
- Jegadeesan, G.; Al-Abed, S.R.; Sundaram, V.; Choi, H.; Scheckel, K.G.; Dionysiou, D.D. Arsenic sorption on TiO2 nanoparticles: Size and crystallinity effects. Water Res. 2010, 44, 965–973. [Google Scholar] [CrossRef]

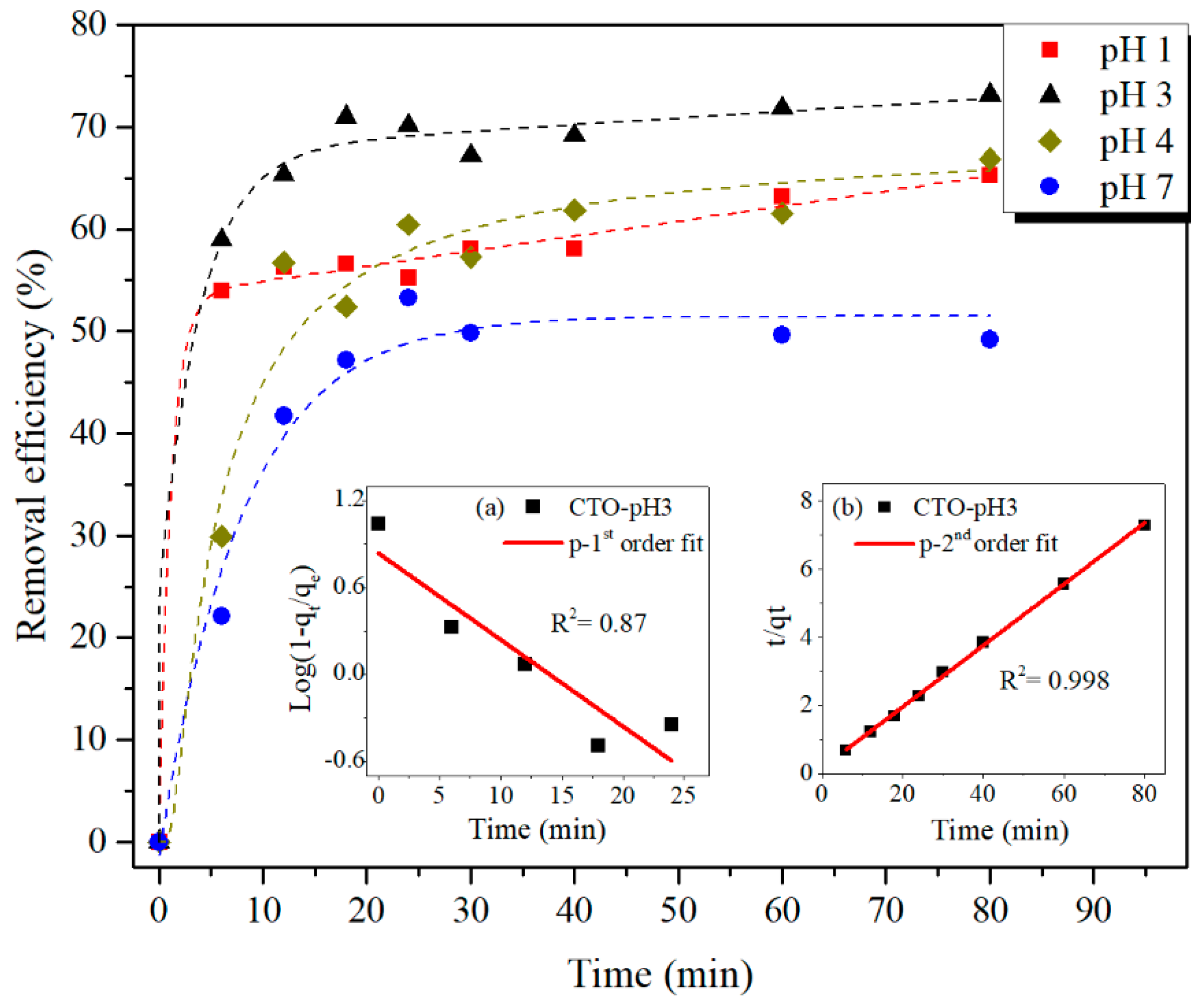
| Pseudo-Second Order Kinetic Parameters | k2 [g mg−1 min−1] | qe [mg g−1] | R2 |
|---|---|---|---|
| pH 1 | 0.0296 | 10.00 | 0.996 |
| pH 2 | 0.0374 | 10.43 | 0.994 |
| pH 3 | 0.0508 | 11.12 | 0.999 |
| pH 4 | 0.0406 | 9.65 | 0.996 |
| pH 5 | 0.0211 | 8.37 | 0.975 |
| pH 7 | 0.0250 | 8.89 | 0.974 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamayo, R.; Espinoza-González, R.; Gracia, F.; Rodrigues-Filho, U.P.; Flores, M.; Sacari, E. As(III) Removal from Aqueous Solution by Calcium Titanate Nanoparticles Prepared by the Sol Gel Method. Nanomaterials 2019, 9, 733. https://doi.org/10.3390/nano9050733
Tamayo R, Espinoza-González R, Gracia F, Rodrigues-Filho UP, Flores M, Sacari E. As(III) Removal from Aqueous Solution by Calcium Titanate Nanoparticles Prepared by the Sol Gel Method. Nanomaterials. 2019; 9(5):733. https://doi.org/10.3390/nano9050733
Chicago/Turabian StyleTamayo, Rocío, Rodrigo Espinoza-González, Francisco Gracia, Ubirajara Pereira Rodrigues-Filho, Marcos Flores, and Elisban Sacari. 2019. "As(III) Removal from Aqueous Solution by Calcium Titanate Nanoparticles Prepared by the Sol Gel Method" Nanomaterials 9, no. 5: 733. https://doi.org/10.3390/nano9050733
APA StyleTamayo, R., Espinoza-González, R., Gracia, F., Rodrigues-Filho, U. P., Flores, M., & Sacari, E. (2019). As(III) Removal from Aqueous Solution by Calcium Titanate Nanoparticles Prepared by the Sol Gel Method. Nanomaterials, 9(5), 733. https://doi.org/10.3390/nano9050733







