Photothermal Response of Hollow Gold Nanorods under Femtosecond Laser Irradiation
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
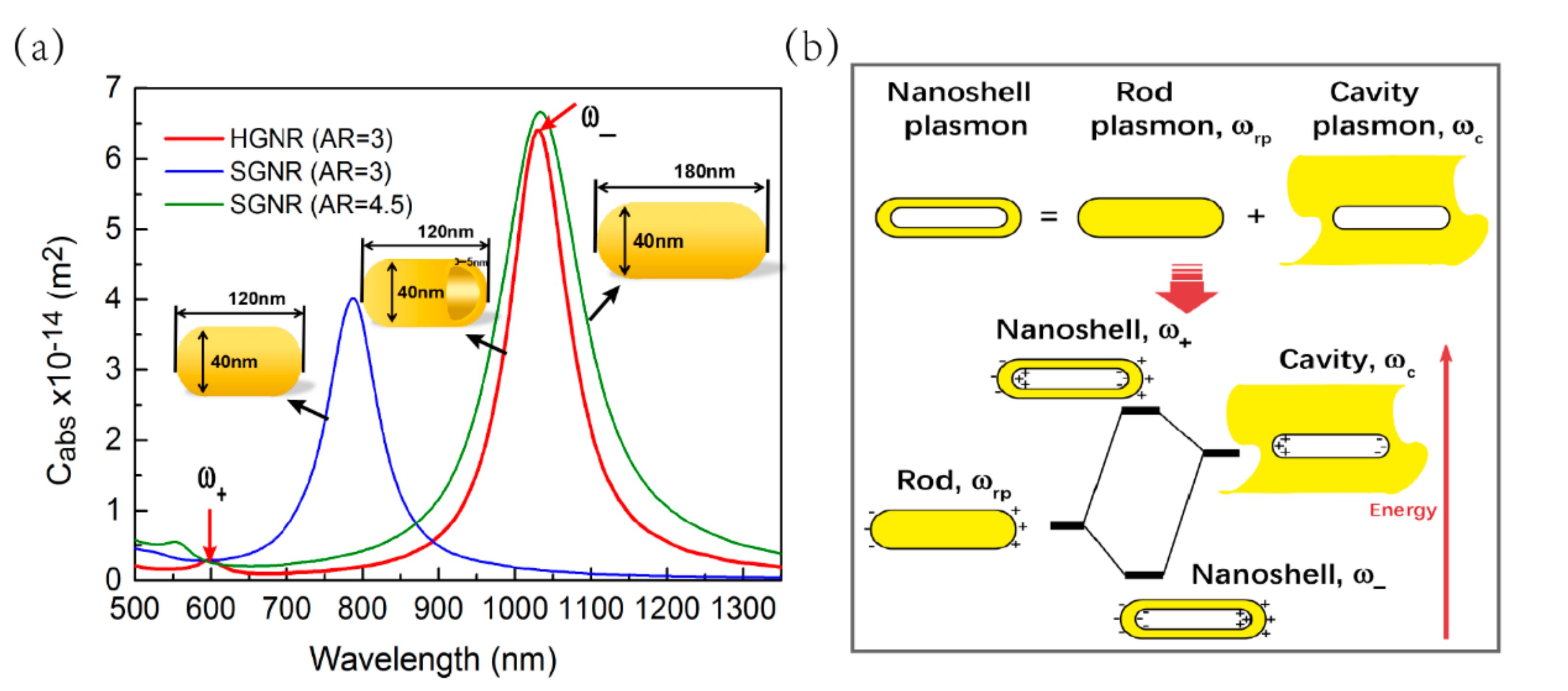
3.1. Longitudinal Absorption Spectra of HGNR and SGNR
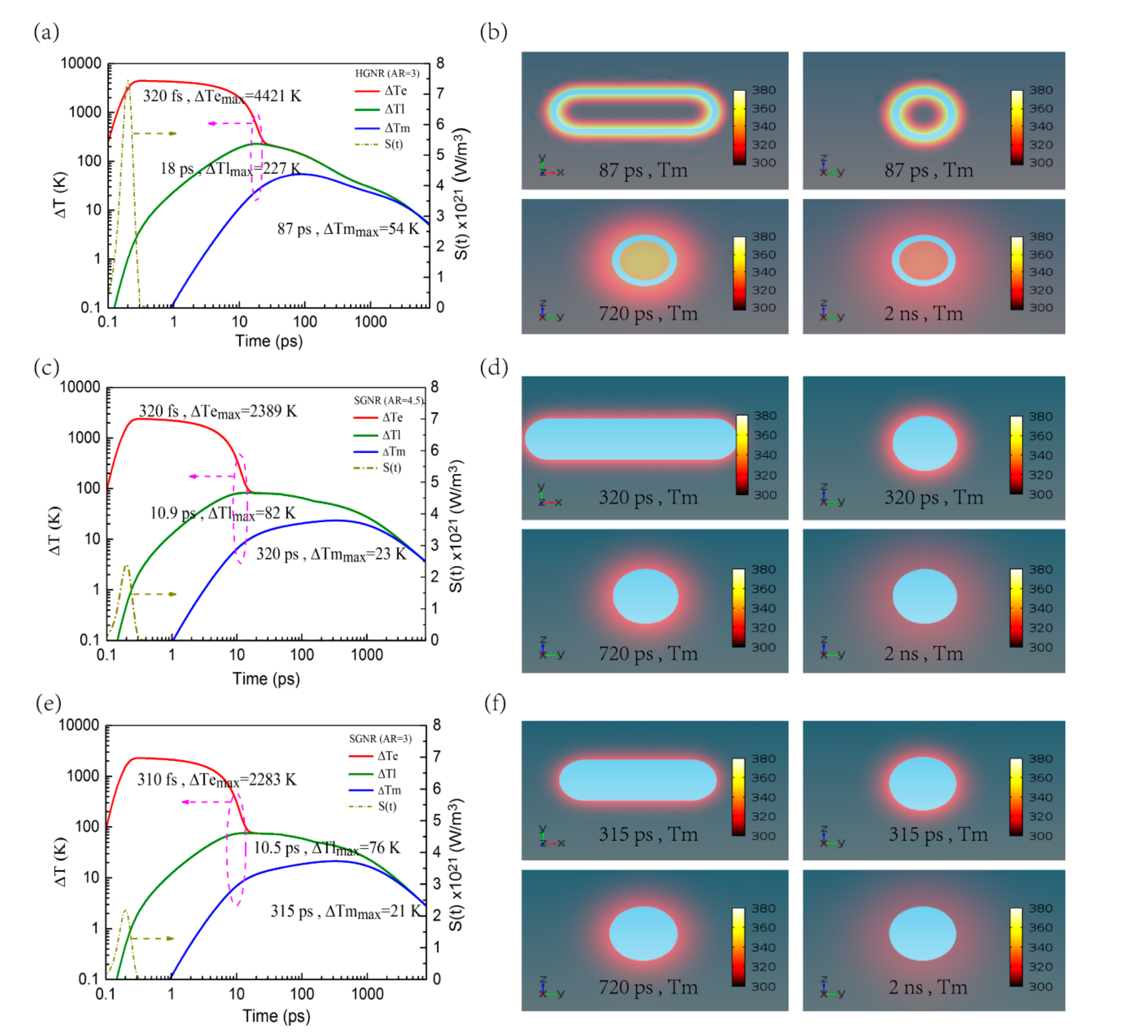
3.2. Photothermal Response of HGNR and SGNR under Femtosecond Laser Irradiation
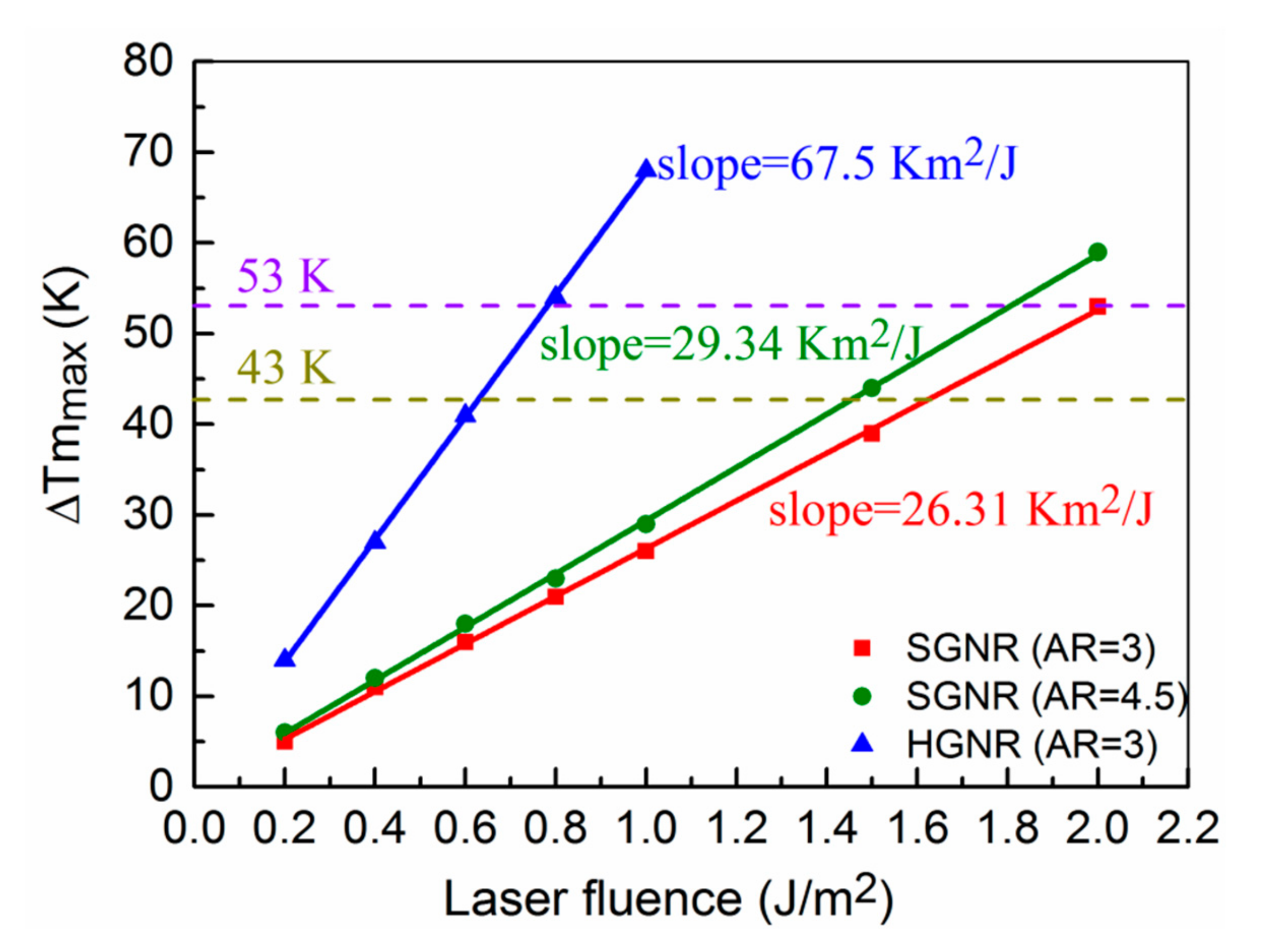
3.3. Energy Threshold Required for Photothermal Destruction of Cancer Cells
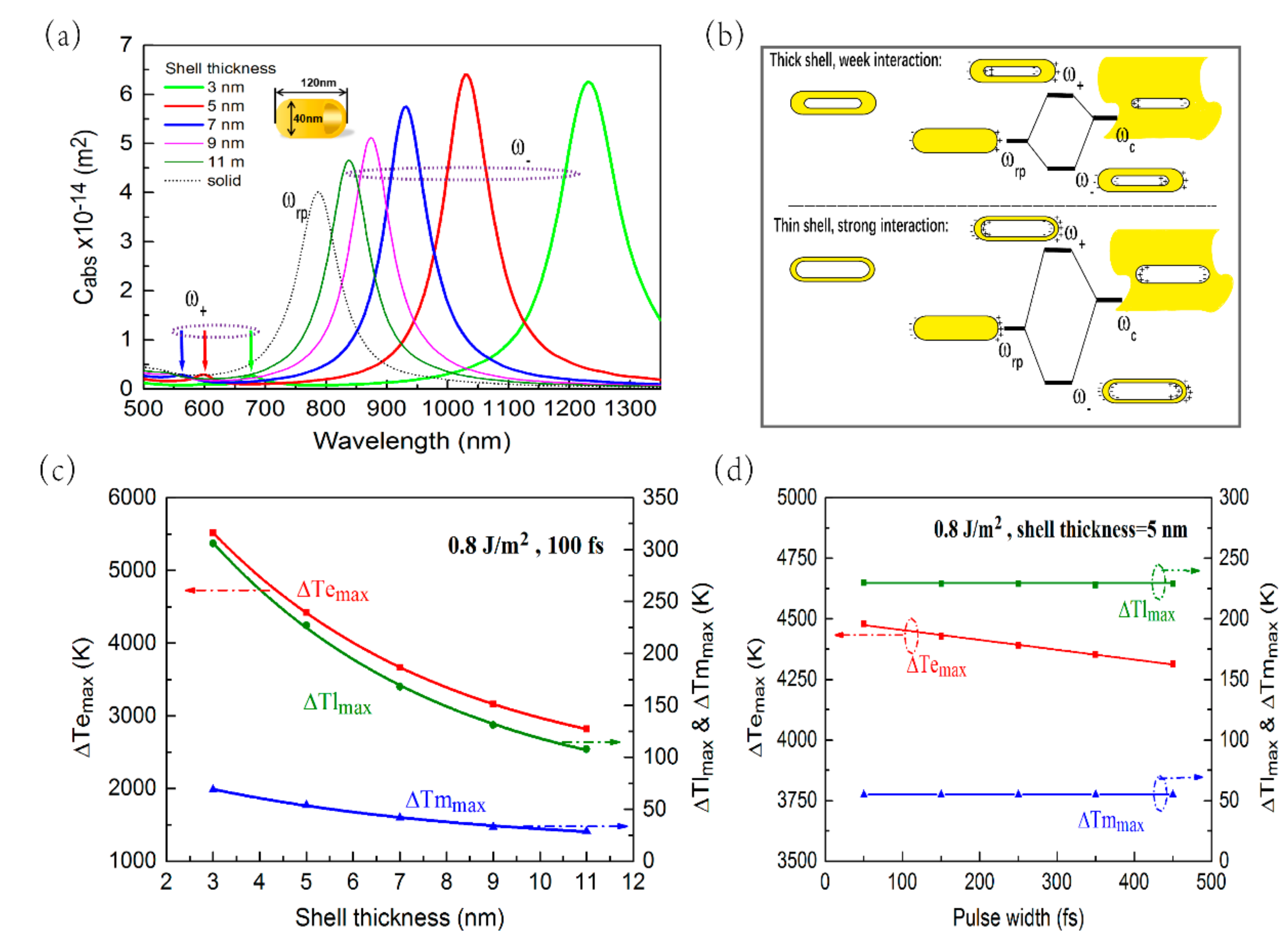
3.4. Photothermal Response of HGNR at Different Shell Thickness or Different Pulse Width
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stockman, M.I. Nanoplasmonics: Past, Present, and Glimpse into Future. Opt. Express 2011, 19, 22029–22106. [Google Scholar] [CrossRef] [PubMed]
- Boulais, E.; Lachaine, R.; Hatef, A.; Meunier, M. Plasmonics for Pulsed-Laser Cell Nanosurgery: Fundamentals and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 26–49. [Google Scholar] [CrossRef]
- Qin, Z.; Bischof, J.C. Thermophysical and Biological Responses of Gold Nanoparticle Laser Heating. Chem. Soc. Rev. 2012, 41, 1191–1217. [Google Scholar] [CrossRef]
- Dai, Q.; Ouyang, M.; Yuan, W.; Li, J.; Guo, B.; Lan, S.; Liu, S.; Zhang, Q.; Lu, G.; Tie, S.; et al. Encoding Random Hot Spots of a Volume Gold Nanorod Assembly for Ultralow Energy Memory. Adv. Mater. 2017, 29, 1701918. [Google Scholar]
- Kumar, A.; Kim, S.; Nam, J.M. Plasmonically Engineered Nanoprobes for Biomedical Applications. J. Am. Chem. Soc. 2016, 138, 14509–14525. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Yu, Y.; Zhang, H.; He, Q. Structural-Engineering Rationales of Gold Nanoparticles for Cancer Theranostics. Adv. Mater. 2016, 28, 8567–8585. [Google Scholar] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [PubMed]
- Huang, X.; El-Sayed, M.A. Plasmonic Photo-Thermal Therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Bayazitoglu, Y.; Kheradmand, S.; Tullius, T.K. An Overview of Nanoparticle Assisted Laser Therapy. Int. J. Heat Mass Transf. 2013, 67, 469–486. [Google Scholar] [CrossRef]
- Tsai, M.F.; Chang, S.H.G.; Cheng, F.Y.; Shanmugam, V.; Cheng, Y.S.; Su, C.H.; Yeh, C.S. Au Nanorod Design as Light-Absorber in the First and Second Biological Near-Infrared Windows for in Vivo Photothermal Therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Sun, T.; Dou, J.H.; Liu, S.; Wang, X.; Zheng, X.; Wang, Y.; Pei, J.; Xie, Z. Second Near-Infrared Conjugated Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7919–7926. [Google Scholar] [CrossRef]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Lyu, Y.; Zhang, L.; Xiong, Q.; Pramanik, M.; Pu, K. Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second Near-Infrared Window. Nano Lett. 2017, 17, 4964–4969. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Jacobson, O.; Huang, P.; Sun, X.; Lin, L.; Yan, X.; Niu, G.; Ma, Q.; Chen, X. Ultrasmall Gold Nanorod Vesicles with Enhanced Tumor Accumulation and Fast Excretion from the Body for Cancer Therapy. Adv. Mater. 2015, 27, 4910–4917. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, M.; Hwang, J.H.; Nam, J.M. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications Based on the Second Near-Infrared Window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef]
- Chang, H.; Murphy, C.J. Mini Gold Nanorods with Tunable Plasmonic Peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Takahata, R.; Yamazoe, S.; Koyasu, K.; Imura, K.; Tsukuda, T. Gold Ultrathin Nanorods with Controlled Aspect Ratios and Surface Modifications: Formation Mechanism and Localized Surface Plasmon Resonance. J. Am. Chem. Soc. 2018, 140, 6640–6647. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, W.; Zhang, J.; Li, H.; Han, H.; Zhai, T. Design of Gold Hollow Nanorods with Controllable Aspect Ratio for Multimodal Imaging and Combined Chemo-Photothermal Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2018, 10, 36703–36710. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, N.; Liu, X.; Dai, Q.; Liu, H.; Wei, Z.; Tie, S.; Li, Y.; Fan, H.; Lan, S. A Novel Fast Photothermal Therapy Using Hot Spots of Gold Nanorods for Malignant Melanoma Cells. Nanomaterials (Basel) 2018, 8, 880. [Google Scholar] [CrossRef]
- Baffou, G.; Rigneault, H. Femtosecond-Pulsed Optical Heating of Gold Nanoparticles. Phys. Rev. B 2011, 84, 664–675. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-Induced Hot Carrier Science and Technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal Analysis of Gold Nanorods Heated with Femtosecond Laser Pulses. J. Phys. D Appl. Phys. 2008, 41, 185501. [Google Scholar] [CrossRef] [Green Version]
- Werner, D.; Hashimoto, S. Improved Working Model for Interpreting the Excitation Wavelength—And Fluence-Dependent Response in Pulsed Laser-Induced Size Reduction of Aqueous Gold Nanoparticles. J. Phys. Chem. C 2010, 115, 5063–5072. [Google Scholar] [CrossRef]
- Hatef, A.; Fortin-Deschênes, S.; Boulais, E.; Lesage, F.; Meunier, M. Photothermal Response of Hollow Gold Nanoshell to Laser Irradiation: Continuous Wave, Short and Ultrashort Pulse. Int. J. Heat Mass Transf. 2015, 89, 866–871. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Halas, N. Playing with Plasmons: Tuning the Optical Resonant Properties of Metallic Nanoshells. MRS Bull. 2005, 30, 362–367. [Google Scholar] [CrossRef]
- Prodan, E.; Radloff, C.; Halas, N.J.; Nordlander, P. A Hybridization Model for the Plasmon Response of Complex Nanostructures. Science 2003, 302, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Genç, A.; Patarroyo, J.; Sancho-Parramon, J.; Bastsús, N.G.; Puntes, V.; Arbiol, J. Hollow Metal Nanostructures for Enhanced Plasmonics: Synthesis, Local Plasmonic Properties and Applications. Nanophotonics 2017, 6, 193–213. [Google Scholar]
- Zhang, J. Biomedical Applications of Shape-Controlled Plasmonic Nanostructures: A Case Study of Hollow Gold Nanospheres for Photothermal Ablation Therapy of Cancer. J. Phys. Chem. Lett. 2010, 1, 686–695. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the Minimum Temperature Required for Selective Photothermal Destruction of Cancer Cells with the Use of Immunotargeted Gold Nanoparticles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef]
| Parameter | Value | Reference |
|---|---|---|
| Gold properties | ||
| The electron heat capacity, Ce (J·m-3·K-1) | 70.0 × Te | [25] |
| Thermal conductivity of electron, ke (W·m-1·K-1) | 300 | [25] |
| The lattice heat capacity, Cl (J·m-3·K-1) | 3 × 106 | [25] |
| Thermal conductivity of lattice, kl (W·m-1·K-1) | 0.001 × ke | [25] |
| Electron-lattice coupling coefficient, g (W·m-3·K-1) | 2 × 1016 | [25] |
| Dielectric function | Johnson and Christy | [26] |
| Water properties | ||
| Density, ρw (kg·m-3) | 1000 | [25] |
| Heat capacity, Cw (J·kg-1·K-1) | 4182 | [25] |
| Thermal conductivity, kw (W·m-1·K-1) | 0.6 | [25] |
| At the GNPs/water interface | ||
| Thermal conductivity, G (W·m-2·K-1) | 105 × 106 | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, R.; Fan, H.; Wei, Z.; Liu, H.; Lan, S.; Dai, Q. Photothermal Response of Hollow Gold Nanorods under Femtosecond Laser Irradiation. Nanomaterials 2019, 9, 711. https://doi.org/10.3390/nano9050711
Gan R, Fan H, Wei Z, Liu H, Lan S, Dai Q. Photothermal Response of Hollow Gold Nanorods under Femtosecond Laser Irradiation. Nanomaterials. 2019; 9(5):711. https://doi.org/10.3390/nano9050711
Chicago/Turabian StyleGan, Rongping, Haihua Fan, Zhongchao Wei, Haiying Liu, Sheng Lan, and Qiaofeng Dai. 2019. "Photothermal Response of Hollow Gold Nanorods under Femtosecond Laser Irradiation" Nanomaterials 9, no. 5: 711. https://doi.org/10.3390/nano9050711






