Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Pre-Treatment of the Working Electrode
2.3. Electrochemical Measurements
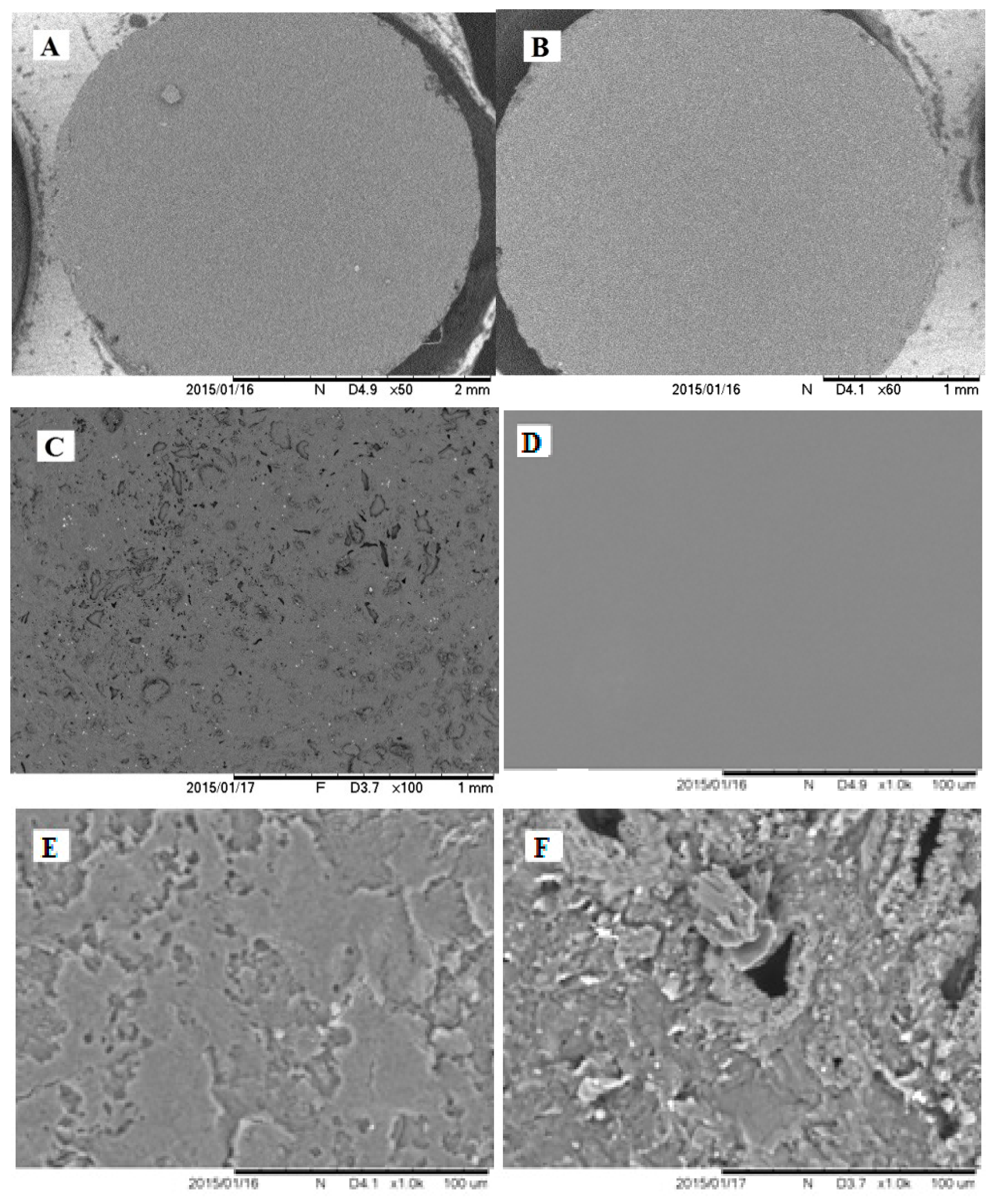
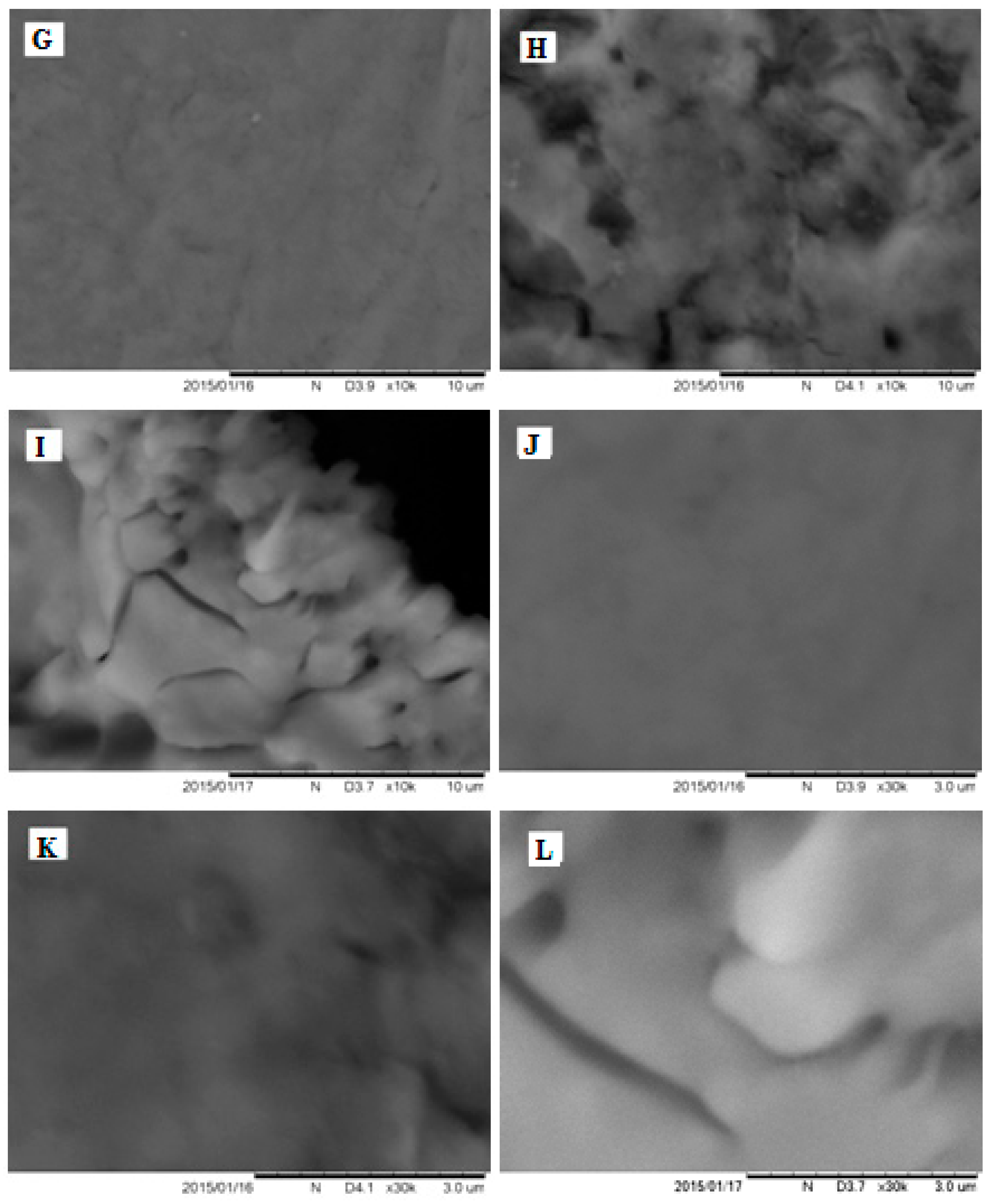
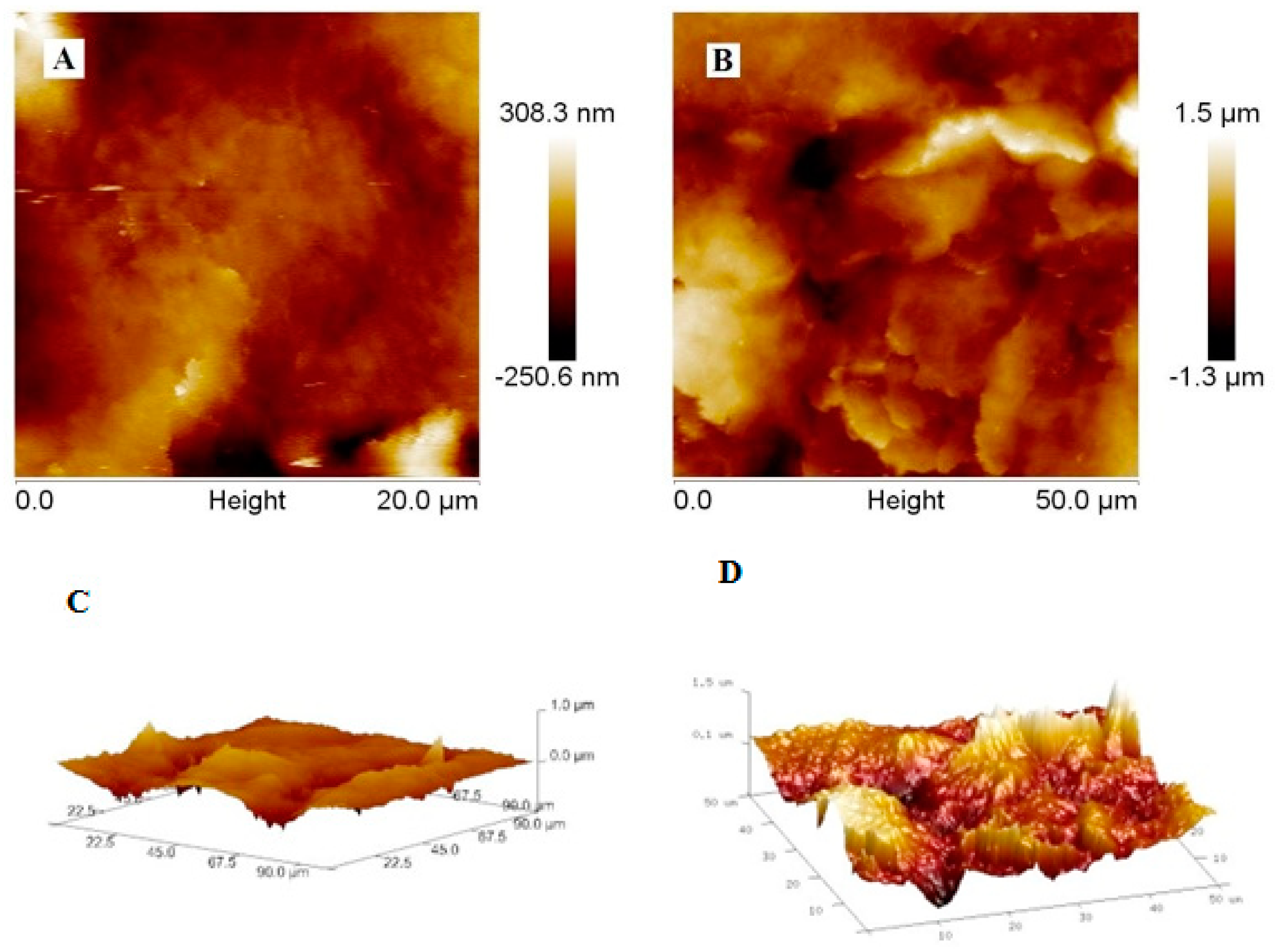
2.4. Imaging of the Electrode Surface by Atomic Force Microscope and Scanning Electron Microscope
3. Results and Discussion
3.1. Investigation of Electrochemical Formation of Poly-PQ Layer
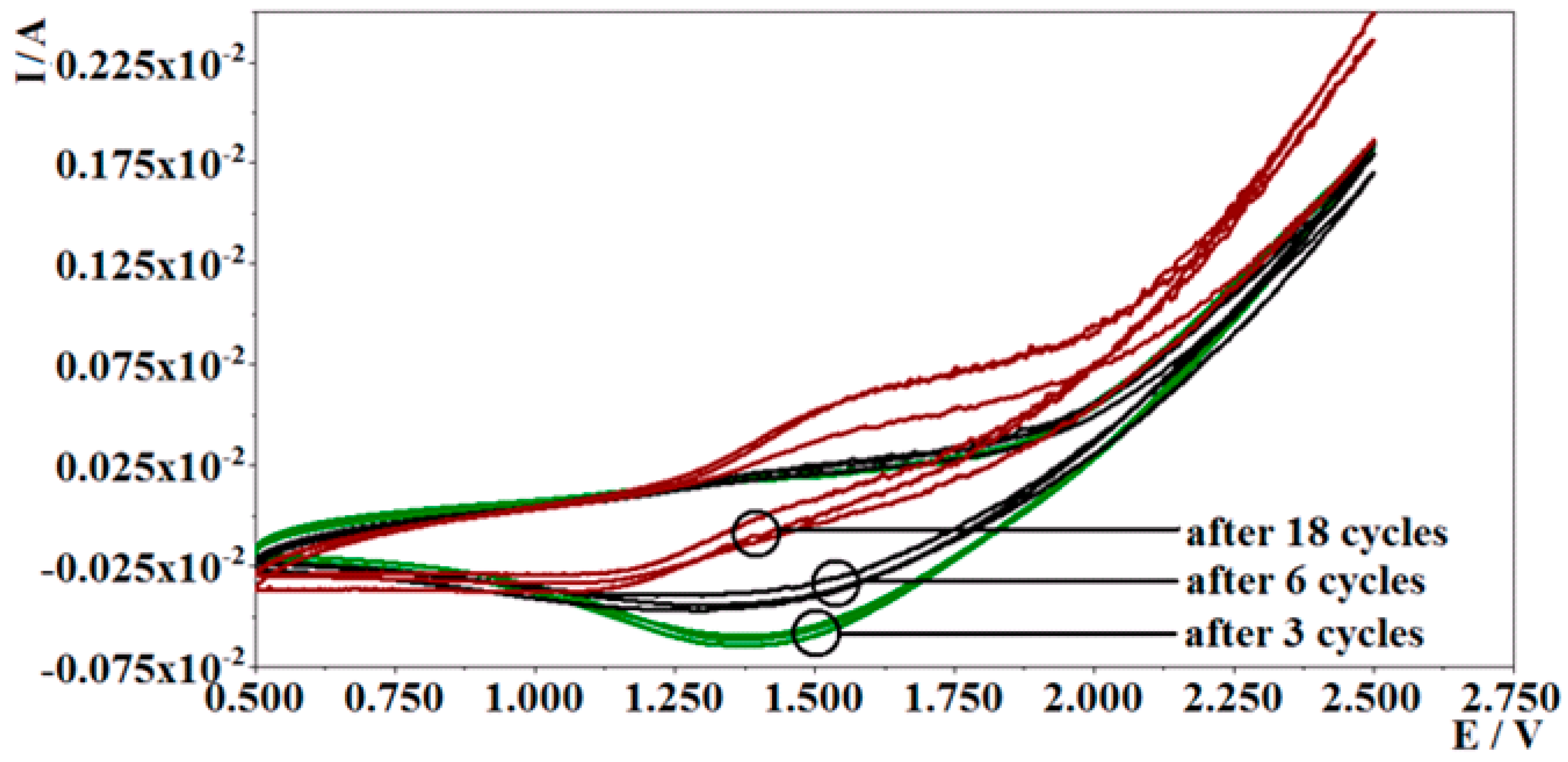
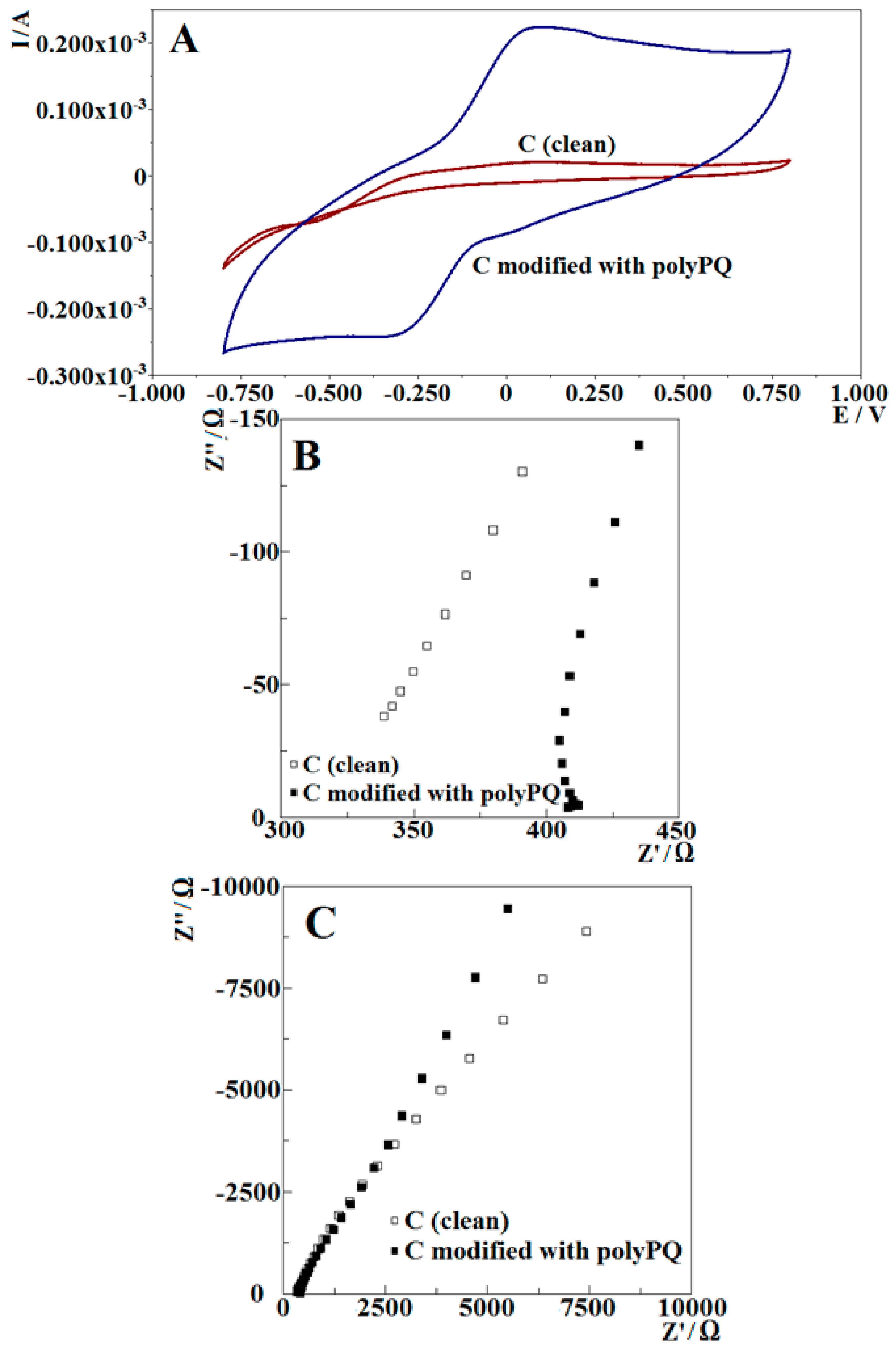
3.1.1. Investigation of Polymerization by Potential Cycling
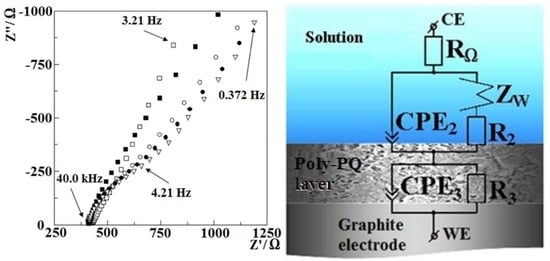
3.1.2. Investigation of Polymerization by Electrochemical Impedance Spectroscopy
3.2. The Evaluation of Formed Poly-PQ Layer Morphology and Some Electrochemical Properties
3.2.1. The Analysis of Poly-PQ Layer Topography
3.2.2. Electrochemical Investigation of Poly-PQ Modified Electrode
Poly-PQ Modified Electrode Analysis by Cyclic Voltamperometry
Poly-PQ Modified Electrode Analysis by Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snook, G.A.; Kao, P.; Best, A.S.J. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Gilissen, K.; Stryckers, J.; Verstappen, P.; Drijkoningen, J.; Heintges, G.H.L.; Lutsen, L.; Manca, J.; Maes, W.; Deferme, W. Ultrasonic spray coating as deposition technique for the light-emitting layer in polymer LEDs. Org. Electron. 2015, 20, 31–35. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Ying, T.; Sun, K.; Qin, Y.; Qi, D. Amperometric biosensor sensitive to glucose and lactose based on co-immobilization of ferrocene, glucose oxidase, β-galactosidase and mutarotase in β-cyclodextrin polymer. Anal. Chim. Acta 1998, 358, 137–144. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Ciui, B.; Hartel, M.C.; Martin, A.; Wang, J. Edible electrochemistry: Food materials based electrochemical sensors. Adv. Healthc. Mater. 2017, 6, 1700770. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Buldu, M.; Karakuscu, A.; Ponzoni, A.; Assefa, D.; Soraru, G.D. High surface area carbonous components from emulsion derived SiOC and their gas sensing behavior. J. Eur. Ceram. Soc. 2015, 16, 4447–4452. [Google Scholar] [CrossRef]
- Chen, C.; Kine, A.; Nelson, R.D.; La Rue, J.C. Impedance spectroscopy study of conducting polymer blends of PEDOT:PSS and PVA. Synth. Met. 2015, 206, 106–114. [Google Scholar] [CrossRef]
- Deen, I.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite chitosan-halloysite nanotube-hydroxyapatite films. Colloid Surf. A 2012, 410, 38–44. [Google Scholar] [CrossRef]
- Li, Z.; Konno, T.; Takai, M.; Ishihara, K. Fabrication of polymeric electron-transfer mediator/enzyme hydrogel multilayer on an Au electrode in a layer-by-layer process. Biosens. Bioelectron. 2012, 34, 191–196. [Google Scholar] [CrossRef]
- Zhai, S.; Hu, E.J.; Zhi, Y.Y.; Shen, Q. Fabrication of highly ordered porous superhydrophobic polystyrene films by electric breath figure and surface chemical modification. Colloid Surf. A 2015, 469, 294–299. [Google Scholar] [CrossRef]
- Patel, A.K. Chitosan: Emergence as potent candidate for green adhesive market. Biochem. Eng. J. 2015, 102, 74–81. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Netti, P.A. Isothermal and non-isothermal polymerization of methyl methacrylate in presence of multiple initiators. Chem. Eng. J. 2010, 162, 776–786. [Google Scholar] [CrossRef]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Properties of experimental urethane dimethacrylate-based dental resin composite blocks obtained via thermo-polymerization under high pressure. Dent. Mater. 2013, 29, 535–541. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Medany, S.S. Optimization of the electropolymerization of 1-amino-9,10-anthraquinone conducting films from aqueous media. Electrochim. Acta 2006, 51, 6353–6360. [Google Scholar] [CrossRef]
- Oztekin, Y.; Tok, M.; Nalvuran, H.; Kiyak, S.; Gover, T.; Yazicigil, Z.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical modification of glassy carbon electrode by poly-4-nitroaniline and its application for determination of copper (II). Electrochim. Acta 2010, 56, 387–395. [Google Scholar] [CrossRef]
- Ohshita, J.; Tada, Y.; Kunai, A.; Harima, Y.; Kunugi, Y. Hole-injection properties of annealed polythiophene films to replace PEDOT–PSS in multilayered OLED systems. Synth. Met. 2009, 159, 214–217. [Google Scholar] [CrossRef]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly imprinted polypyrrole based impedimentric sensor for theophylline determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Simbirtseva, G.V.; Nazarov, V.G.; Stolyarov, V.P.; Dubois, M.; Peyroux, J. Enhanced anti-graffiti or adhesion properties of polymers using versatile combination of fluorination and polymer grafting. Prog. Org. Coat. 2015, 88, 127–136. [Google Scholar] [CrossRef]
- Cousseau, R.; Patin, N.; Forgez, C.; Monmasson, E.; Idkhajine, L. Improved electrical model of aluminum electrolytic capacitor with anomalous diffusion for health monitoring. Math. Comput. Simul. 2017, 131, 268–282. [Google Scholar] [CrossRef]
- Oztekin, Y.; Krikstolaityte, V.; Ramanaviciene, A.; Yazicigil, Z.; Ramanavicius, A. 1,10-Phenanthroline derivatives as mediators for glucose oxidase. Biosens. Bioelectron. 2010, 26, 267–270. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Li, X.; Yan, S.; Ren, Y.; Wang, M.; Liu, P.; Dong, Y.; Zhang, C. [Cu(phen)2](2+) acts as electrochemical indicator and anchor to immobilize probe DNA in electrochemical DNA biosensor. Anal. Biochem. 2016, 492, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Wang, C.H. Electrocatalytic properties of guanine, adenine, guanosine-5′-monophosphate, and ssDNA by Fe(II) bis(2,2′:6′,2′′-terpyridine), Fe(II) tris(1,10-phenanthroline), and poly-Fe(II) tris(5-amino-1,10-phenanthroline). Bioelectrochemistry 2006, 70, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Pinto, E.M.; Brett, C.M.A. Electrosynthesis and electrochemical characterisation of phenazine polymers for application in biosensors. Electrochim. Acta 2008, 53, 3973–3982. [Google Scholar] [CrossRef] [Green Version]
- Gligor, D.; Dilgin, Y.; Popescu, I.C.; Gorton, L. Poly-phenothiazine derivative-modified glassy carbon electrode for NADH electrocatalytic oxidation. Electrochim. Acta 2009, 54, 3124–3128. [Google Scholar] [CrossRef]
- Lapenaite, I.; Ramanaviciene, A.; Ramanavicius, A. Current trends in enzymatic determination of glycerol. Crit. Rev. Anal. Chem. 2006, 36, 13–25. [Google Scholar] [CrossRef]
- Muddasir, H.; Lu, P.; Gu, C.; Wang, Z.; Yang, S.; Yang, B.; Wang, C.; Ma, Y. Molecular Properties of 9,10-Phenanthrenequinone and Benzil. Chem. Res. Chin. Univ. 2009, 25, 950–956. [Google Scholar]
- Togashi, D.M.; Nicodem, D.E. Photophysical studies of 9,10-phenanthrenequinones. Spectrochim. Acta A 2004, 60, 3205–3212. [Google Scholar] [CrossRef]
- Vaik, K.; Sarapuu, A.; Tammeveski, K.; Mirkhala, F.; Schiffrin, D.J. Oxygen reduction on phenanthrenequinone-modified glassy carbon electrodes in 0.1 M KOH. J. Electroanal. Chem. 2004, 564, 159–166. [Google Scholar] [CrossRef]
- Arashti, M.G.; Sadeghzadeh, M.A. Electrical characterization of p-Si/SiGe/Si (100) surface covered by 9, 10 Phenanthrenequinone over layer. Vacuum 2014, 101, 267–270. [Google Scholar] [CrossRef]
- Tian, G.; Li, H.; Ma, W.; Wang, Y. Substituent Effects in π-Stacking of Histidine on Functionalized-SWNT and Graphene. Comput. Theor. Chem. 2015, 1062, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Atkinson, R.; Arey, J. Formation of 9,10-phenanthrenequinone by atmospheric gas-phase reactions of phenanthrene. Atmos. Environ. 2007, 41, 2025–2035. [Google Scholar] [CrossRef]
- Oztekin, Y.; Ramanaviciene, A.; Solak, A.O.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline modified-glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546. [Google Scholar] [CrossRef]
- Ratautaite, V.; Ramanaviciene, A.; Oztekin, Y.; Voronovic, J.; Balevicius, Z.; Mikoliunaite, L.; Ramanavicius, A. Electrochemical stability and repulsion of polypyrrole film. Colloids Surf. A 2013, 418, 16–21. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Harrington, D.A.; Driessche, P. Mechanism and equivalent circuits in electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 8005–8013. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Genys, P.; Ramanaviciene, A. Electrochemical impedance spectroscopy based evaluation of 1,10-phenanthroline-5,6-dione and glucose oxidase modified graphite electrode. Electrochim. Acta 2014, 146, 659–665. [Google Scholar] [CrossRef]
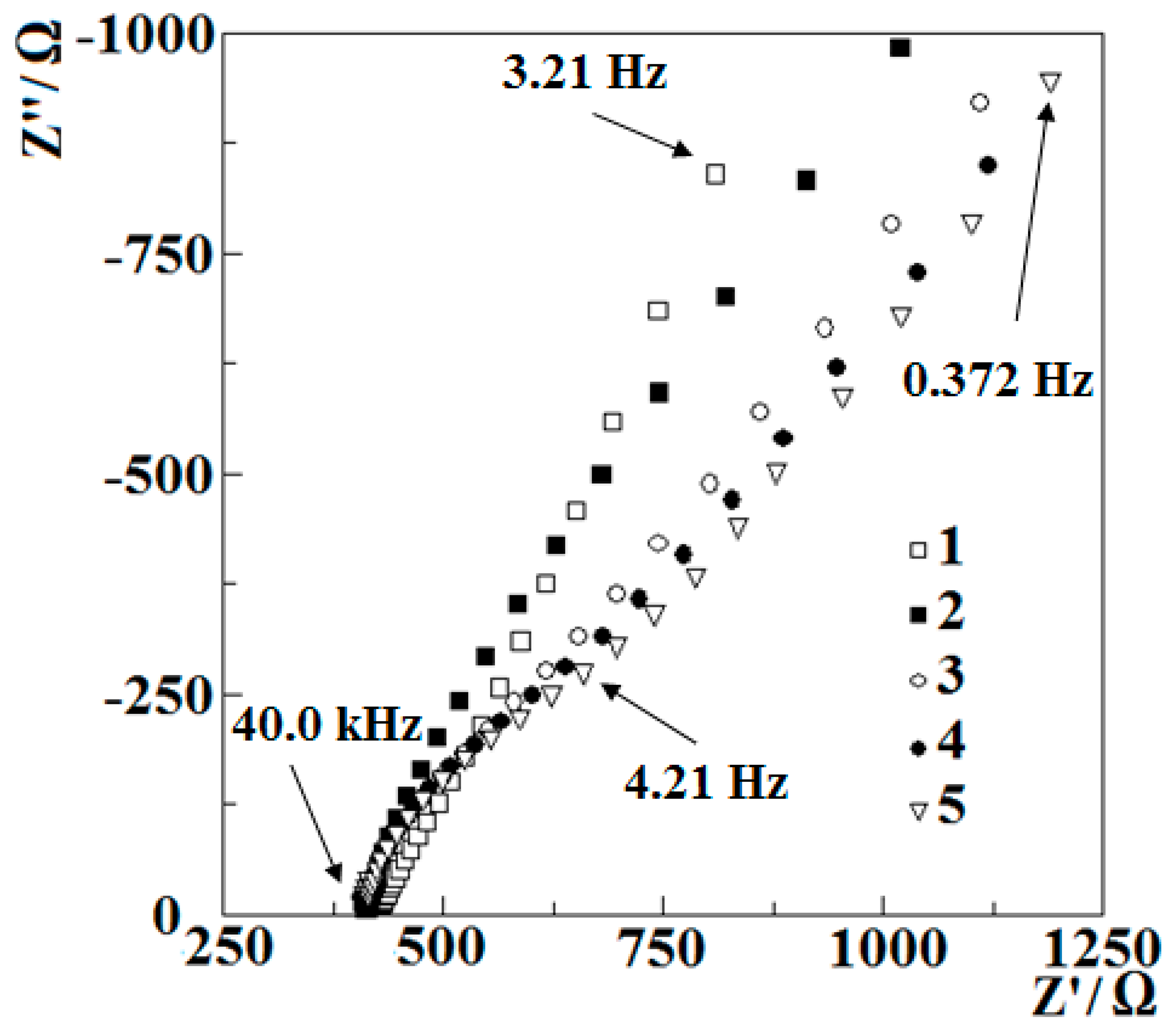
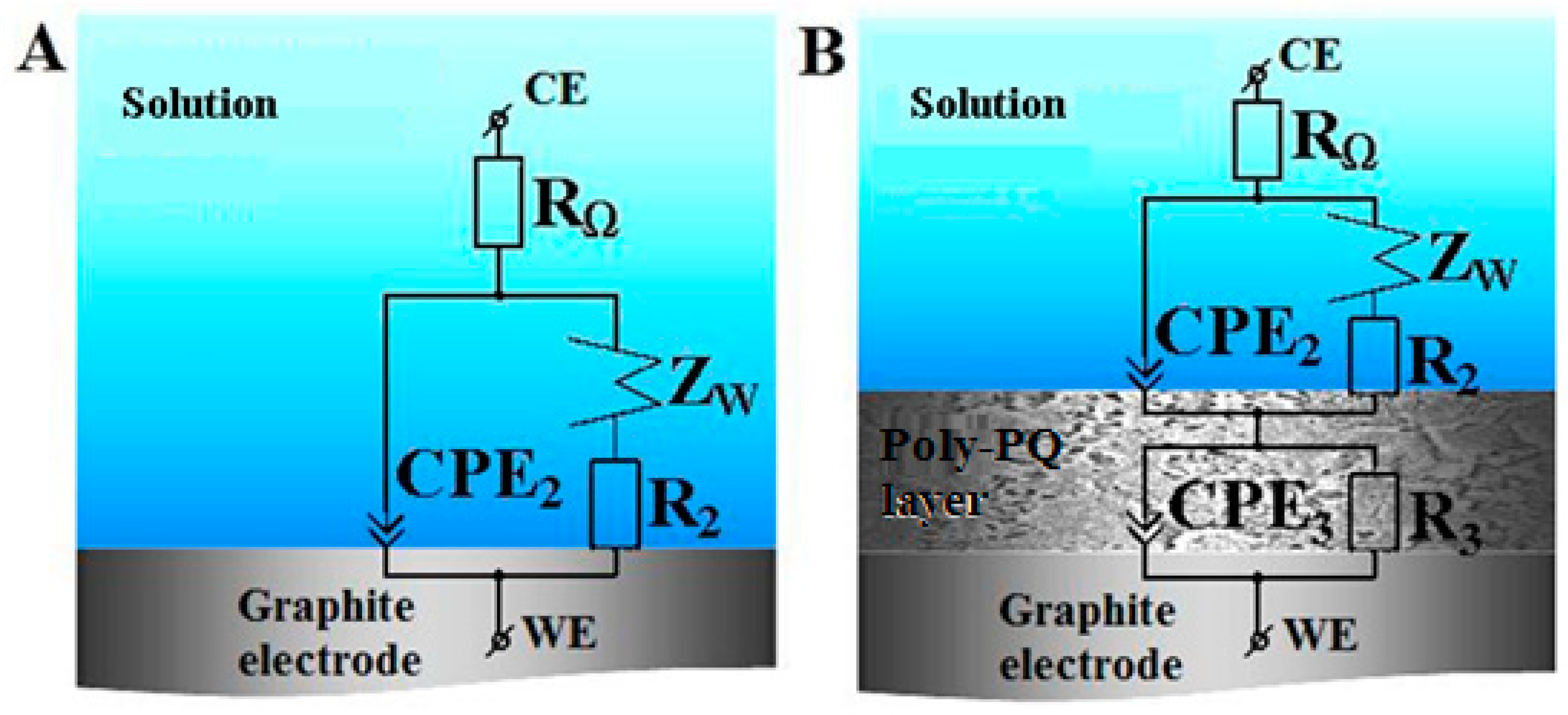
| RΩ, Ω | CPE2, μF | n2 | R2, Ω | R3, Ω | CPE3, μF | n3 | WR, Ω | WT, F | |
|---|---|---|---|---|---|---|---|---|---|
| Before cyclic polymerization | 479 | 126.1 | 0.7195 | 281,900 | 47,860 | 15 | |||
| After 3 cycles | 414.0 | 130.0 | 0.8622 | 679.8 | 27.93 | 1052 | 0.9857 | 16,570 | 11.3 |
| After 6 cycles | 412.4 | 225.0 | 0.8195 | 581.7 | 115.4 | 1812 | 0.9672 | 12,310 | 10.3 |
| After 9 cycles | 411.5 | 321.8 | 0.7910 | 1465.0 | 207.4 | 163.6 | 0.9011 | 12,320 | 9.8 |
| After 18 cycles | 406.7 | 404.9 | 0.6887 | 713.9 | 187.3 | 137.8 | 0.9500 | 25,260 | 10.3 |
| RΩ, Ω | CPE2, μF | n2 | R2, Ω | R3, Ω | CPE3, μF | n3 | WR, Ω | WT, F | |
|---|---|---|---|---|---|---|---|---|---|
| GR (bare) | 457 | 6.203 | 0.7700 | 7918 | 1,475,000 | 713.5 | |||
| CR\poly-PQ (18 cycles) | 385.9 | 578 | 0.7294 | 149,200 | 530 | 2068 | 0.9242 | 52,470 | 4.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genys, P.; Aksun, E.; Tereshchenko, A.; Valiūnienė, A.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials 2019, 9, 702. https://doi.org/10.3390/nano9050702
Genys P, Aksun E, Tereshchenko A, Valiūnienė A, Ramanaviciene A, Ramanavicius A. Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials. 2019; 9(5):702. https://doi.org/10.3390/nano9050702
Chicago/Turabian StyleGenys, Povilas, Elif Aksun, Alla Tereshchenko, Aušra Valiūnienė, Almira Ramanaviciene, and Arunas Ramanavicius. 2019. "Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer" Nanomaterials 9, no. 5: 702. https://doi.org/10.3390/nano9050702
APA StyleGenys, P., Aksun, E., Tereshchenko, A., Valiūnienė, A., Ramanaviciene, A., & Ramanavicius, A. (2019). Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials, 9(5), 702. https://doi.org/10.3390/nano9050702