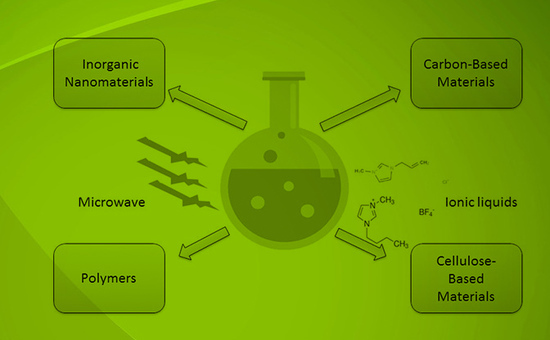
New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation
Abstract
1. Introduction
2. Preparation of Inorganic Nanomaterials
2.1. Metal Nanostructures
2.2. Metal Oxide Nanostructures
2.2.1. Zinc Oxide (ZnO)
2.2.2. Titanium Dioxide (TiO2)
2.2.3. Other Metal Oxide Nanomaterials
2.3.4. Other Complex Metal Structures
3. Polymers Preparation
3.1. Ring Opening Polymerization
3.2. Polycondensation
3.3. Free Radical Polymerization
3.4. Copolymers
3.4.1. Cellulose Graft Copolymers
3.4.2. Copolyurethanes
3.5. Poly(ionic Liquid)s
4. Preparation of Carbon-Based Composites
4.1. Carbon Dots/Nanodots
4.2. Carbon Nanoparticles
4.3. Other Carbon Materials
5. Preparation of Biomass-Based Materials
5.1. Cellulose-Based Composites
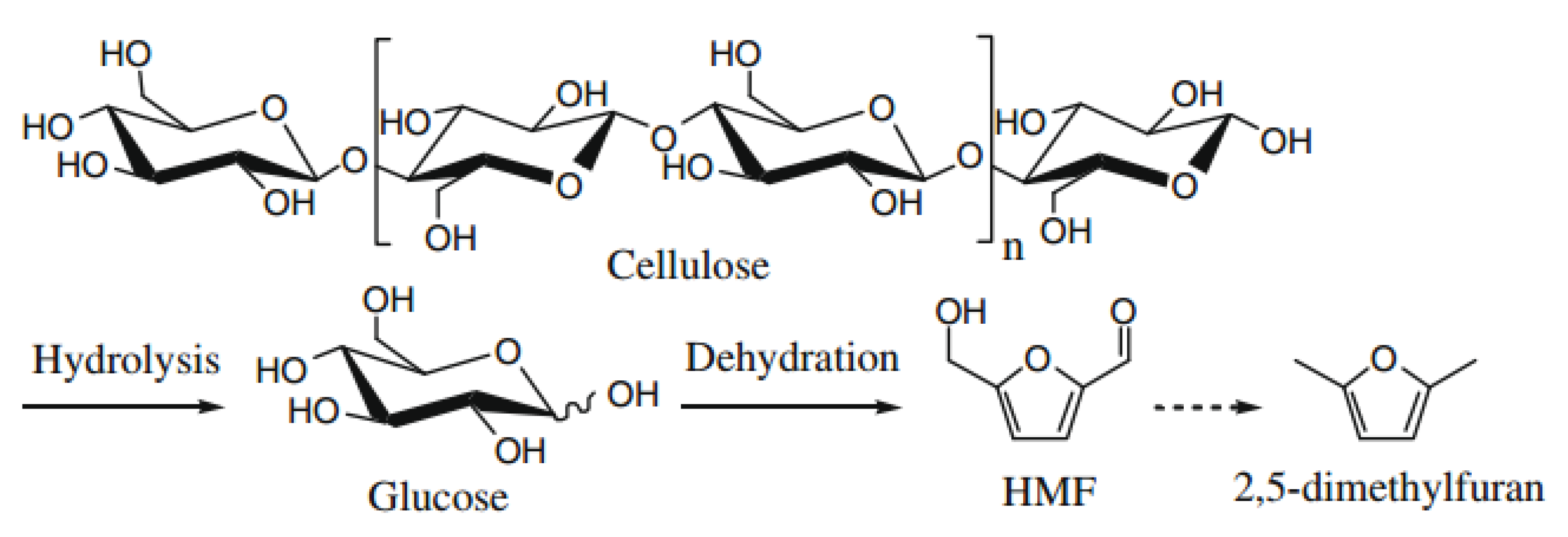
5.2. Production of 5-Hydroxymethylfurfural
5.3. Production of Furfural
5.4. Production of Reducing Sugar
6. Mechanisms Involved in MAIL Methods
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, R.D. CHEMISTRY: Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, T.; Tsuda, T.; Okazaki, K.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef]
- Hapiot, P.; Lagrost, C. Electrochemical reactivity in room-temperature ionic liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Dupont, J. From molten salts to ionic liquids: A “nano” journey. Acc. Chem. Res. 2011, 44, 1223–1231. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Jinghong, L. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal synthesis of zeolites, metal-organic frameworks, and inorganic-organic hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Nockemann, P.; Lagunas, M.C. Crystal engineering with ionic liquids. CrystEngComm 2012, 14, 4873. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, H.; Zhao, B.; Liang, Z.; Zhang, L.; Zhang, Y. Advances of ionic liquids-based methods for protein analysis. TrAC Trends Anal. Chem. 2018, 108, 239–246. [Google Scholar] [CrossRef]
- Morris, R.E. Ionothermal synthesis—Ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 0, 2990–2998. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, V. Current status and future challenges in ionic liquids, functionalized ionic liquids and deep eutectic solvent-mediated synthesis of nanostructured TiO2: A review. New J. Chem. 2017, 41, 2844–2868. [Google Scholar] [CrossRef]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; You, T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Holbrey, J.D.; Rogers, R.D.; Dixon, D.A. Prediction of the formation and stabilities of energetic salts and ionic liquids based on ab initio electronic structure calculations. J. Phys. Chem. B 2005, 109, 49, 23196–23208. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Varma, R.S. Solvent-free sonochemical preparation of ionic liquids. Org. Lett. 2002, 4, 3161–3163. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Zhang, S.; Xuan, X. Structural effects of anions and cations on the aggregation behavior of ionic liquids in aqueous solutions. J. Phys. Chem. B 2008, 112, 16682–16689. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, Y.; Choi, B.; Choi, Y.; Yang, J.; Hong, W. Green one-pot assembly of iron-based nanomaterials for the rational design of structure. Chem. Commun. 2009, 27, 4058–4060. [Google Scholar] [CrossRef]
- Li, M.; Dong, W.; Liu, C.; Liu, Z.; Lin, F. Ionic liquid-assisted synthesis of copper oxalate nanowires and their conversion to copper oxide nanowires. J. Cryst. Growth 2008, 310, 4628–4634. [Google Scholar] [CrossRef]
- Swadźba-Kwaśny, M.; Chancelier, L.; Ng, S.; Manyar, H.G.; Hardacre, C.; Nockemann, P. Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles. Dalton Trans. 2012, 41, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, D. Ionic liquid based approaches to carbon materials synthesis. Eur. J. Inorg. Chem. 2015, 7, 1137–1147. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Zhang, J.; Su, D.; Thomas, A.; Antonietti, M. Ionic liquids as precursors for nitrogen-doped graphitic carbon. Adv. Mater. 2010, 22, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, B.; Ma, M.; Lin, K. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1, 63–83. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis. Tetrahedron 2005, 57, 9225–9283. [Google Scholar] [CrossRef]
- Wiesbrock, F.; Hoogenboom, R.; Schubert, U.S. Microwave-assisted polymer synthesis: State-of-the-art and future perspectives. Macromol. Rapid Commun. 2010, 25, 1739–1764. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F.; Ruoho, A.E. A rapid and convenient method for the synthesis of aldoximes under microwave irradiation using in situ generated ionic liquids. J. Iran. Chem. Soc. 2010, 7, 114–118. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, L.; Gong, S. Recent developments in microwave-assisted polymerization with a focus on ring-opening polymerization. Green Chem. 2007, 9, 303–314. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Ma, M. Microwave-assisted preparation of calcium sulfate nanowires. Mater. Lett. 2008, 62, 4552–4554. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Nadagouda, M.N.; Varma, R.S. The synthesis and applications of a micro-pine-structured nanocatalyst. Chem. Commun. 2008, 47, 6318–6320. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-assembly of metal oxides into three-dimensional nanostructures: Synthesis and application in catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef]
- Butt, F.K.; Bandarenka, A.S. Microwave-assisted synthesis of functional electrode materials for energy applications. J. Solid State Electrochem. 2016, 20, 2915–2928. [Google Scholar] [CrossRef]
- Manzoli, M.; Gaudino, C.E.; Cravotto, G.; Tabasso, S.; Baig, R.B.N.; Colacino, E.; Varma, R.S. Microwave-assisted reductive amination with aqueous ammonia: Sustainable pathway using recyclable magnetic nickel-based nano-catalyst. ACS Sustain. Chem. Eng. 2019, 7, 5936–5974. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Varma, R.S.; Namboodiri, V.V. An expeditious solvent-free route to ionic liquids using microwaves. Chem. Commun. 2001, 7, 643–644. [Google Scholar] [CrossRef]
- Lévêque, J.M.; Cravotto, G. Microwave, power ultrasound and ionic liquids: A new synergy in green organic synthesis. CHIMIA Int. J. Chem. 2010, 60, 613–620. [Google Scholar] [CrossRef]
- Duan, X.; Ma, J.; Lian, J.; Zheng, W. The art of using ionic liquids in the synthesis of inorganic nanomaterials. CrystEngComm 2014, 16, 2550–2559. [Google Scholar] [CrossRef]
- Ahmed, E.; Breternitz, J.; Groh, M.F.; Ruck, M. Ionic liquids as crystallisation media for inorganic materials. CrystEngComm 2012, 14, 4874–4885. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Qi, R.; Xi, H. Microwave-assisted synthesis of single-crystalline tellurium nanorods and nanowires in ionic liquids. Angew. Chem. Int. Ed. Engl. 2004, 43, 1410–1414. [Google Scholar] [CrossRef]
- Richter, K.; Campbell, P.S.; Baecker, T.; Schimitzek, A.; Yaprak, D.; Mudring, A.V. Ionic liquids for the synthesis of metal nanoparticles. Phys. Status Solidi B 2013, 250, 1152–1164. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Zhang, J.; Han, B.; Du, J.; Gao, Y.; Jiang, T. Synthesis of single-crystal gold nanosheets of large size in ionic liquids. J. Phys. Chem. B 2005, 109, 14445–14448. [Google Scholar] [CrossRef]
- Bhawawet, N.; Essner, J.B.; Atwood, J.L.; Baker, G.A. On the non-innocence of the imidazolium cation in a rapid microwave synthesis of oleylamine-capped gold nanoparticles in an ionic liquid. Chem. Commun. 2018, 54, 7523–7526. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Cheng, H.; Wang, Q.; Miao, C.; Ju, S.; Liu, F. Development of ionic liquid assisted-synthesized nano-silver combined with vascular endothelial growth factor as wound healing in the care of femoral fracture in the children after surgery. J Photochem. Photobiol. B 2018, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Tohidi, M. Microwave-assisted synthesis of gold, silver, platinum and palladium nanostructures and their use in electrocatalytic applications. J. Nanosci. Nano. 2014, 14, 7189–7198. [Google Scholar] [CrossRef]
- Schütte, K.; Meyer, H.; Gemel, C.; Barthel, J.; Fischer, R.; Janiak, C. Synthesis of Cu, Zn and Cu/Zn brass alloy nanoparticles from metal amidinate precursors in ionic liquids or propylene carbonate with relevance to methanol synthesis. Nanoscale 2014, 6, 3116–3126. [Google Scholar] [CrossRef]
- Schütte, K.; Barthel, J.; Endres, M.; Siebels, M.; Smarsly, B.M.; Yue, J.; Janiak, C. Synthesis of metal nanoparticles and metal fluoride nanoparticles from metal amidinate precursors in 1-butyl-3-methylimidazolium ionic liquids and propylene carbonate. ChemistryOpen 2017, 6, 137–148. [Google Scholar] [CrossRef]
- Choi, S.M.; Seo, M.H.; Kim, H.J.; Kim, W.B. Synthesis and characterization of graphene-supported metal nanoparticles by impregnation method with heat treatment in H2 atmosphere. Synth. Met. 2011, 161, 2405–2411. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yung, T.Y.; Liu, L.K. The microwave-assisted ionic liquid nanocomposite synthesis: Platinum nanoparticles on graphene and the application on hydrogenation of styrene. Nanoscale Res. Lett. 2013, 8, 414. [Google Scholar] [CrossRef]
- Esteban, R.M.; Schütte, K.; Brandt, P.; Marquardt, D.; Meyer, H.; Beckert, F.; Mülhaupt, R.; Kölling, H.; Janiak, C. Iridium@graphene composite nanomaterials synthesized in ionic liquid as re-usable catalysts for solvent-free hydrogenation of benzene and cyclohexene. Nano-Struct. Nano-Objects 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y. Shape-controlled synthesis of zinc oxide by microwave heating using an imidazolium salt. Inorg. Chem. Commun. 2004, 7, 1003–1005. [Google Scholar] [CrossRef]
- Wang, J.; Cao, J.; Fang, B.; Lu, P.; Deng, S.; Wang, H. Synthesis and characterization of multipod, flower-like, and shuttle-like Zno frameworks in ionic liquids. Mate. Lett. 2005, 59, 1405–1408. [Google Scholar] [CrossRef]
- Rabieh, S.; Bagheri, M.; Heydari, M.; Badiei, E. Microwave assisted synthesis of ZnO nanoparticles in ionic liquid [Bmim]Cl and their photocatalytic investigation. Mater. Sci. Semicond. Process. 2014, 26, 244–250. [Google Scholar] [CrossRef]
- Esmaili, M.; Habibi-Yangjeh, A. Preparation and characterization of ZnO nanocrystallines in the presence of an ionic liquid using microwave irradiation and photocatalytic activity. J. Iran. Chem. Soc. 2010, 7, S70–S82. [Google Scholar] [CrossRef]
- da Trindade, L.G.; Minervino, G.B.; Trench, A.B.; Carvalho, M.H.; Assis, M.; Li, M.S.; de Oliveira, A.J.A.; Pereira, E.C.; Mazzo, T.M.; Longo, E. Influence of ionic liquid on the photoelectrochemical properties of ZnO particles. Ceram. Int. 2018, 44, 10393–10401. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Macak, J.M.; Schmidt-Stein, F.; Hahn, R.; Mierke, C.T.; Fabry, B.; Schmuki, P. Magnetically guided titania nanotubes for site-selective photocatalysis and drug release. Angew. Chem. Int. Ed. Engl. 2010, 121, 969–972. [Google Scholar]
- Chen, C.; Hu, X.; Hu, P.; Qiao, Y.; Qie, L.; Huang, Y. Ionic-liquid-assisted synthesis of self-assembled TiO2-B nanosheets under microwave irradiation and their enhanced lithium storage properties. Eur. J. Inorg. Chem. 2013, 30, 5320–5328. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhao, Y.; Liu, J.; Hao, Y.; Liu, R.; Zhao, D. Ionic-liquid-assisted synthesis of high-visible-light-activated N–B–F-tri-doped mesoporous TiO2, via a microwave route. Appl. Catal. B 2014, 144, 442–453. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Yang, X.; Yu, J. A micrometer-size TiO2 single-crystal photocatalyst with remarkable 80% level of reactive facets. Chem. Commun. 2009, 29, 4381–4383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lü, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured “black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef]
- Xiao, S.; Zhu, W.; Liu, P.; Liu, F.; Dai, W.; Zhang, D.; Chen, W.; Li, H. CNTs threaded (001) exposed TiO2 with high activity in photocatalytic NO oxidation. Nanoscale 2016, 8, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.O.; Akomolafe, T.; Carter, M.J. Production of cuprous oxide, a solar cell material, by thermal oxidation and a study of its physical and electrical properties. Sol. Energy Mater. Sol. Cells 1998, 51, 305–316. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Cheng, G.; Huang, Y. Microwave-assisted synthesis of cupric oxide nanosheets and nanowhiskers. Mater. Lett. 2006, 60, 609–612. [Google Scholar] [CrossRef]
- Xia, J.; Li, H.; Luo, Z.; Shi, H.; Wang, K.; Shu, H.; Yan, Y. Microwave-assisted synthesis of flower-like and leaf-like CuO nanostructures via room-temperature ionic liquids. J. Phys. Chem. Solids 2009, 70, 1461–1464. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.; Zhang, M. Microwave-assisted synthesis and characterization of CuO nanocrystals. J. Dispersion Sci. Technol. 2008, 29, 508–513. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Abu-Hani, A.F.S.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B 2016, 231, 593–600. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Sun, Y.; Cui, Y.; Liang, M.; Zhao, J.; Li, N. Phase-manipulable synthesis of Cu-based nanomaterials using ionic liquid 1-butyl-3-methyl-imidazole tetrafluoroborate. Cryst. Res. Technol. 2010, 45, 398–404. [Google Scholar] [CrossRef]
- Li, S.; Guo, X.; Wang, Y.; Huang, F.; Shen, Y.; Wang, X.; Xie, A. Rapid synthesis of flower-like Cu2O architectures in ionic liquids by the assistance of microwave irradiation with high photochemical activity. Dalton Trans. 2011, 40, 6745–6750. [Google Scholar] [CrossRef]
- Bowers, J.; Butts, C.P.; Martin, P.J.; Vergara-Gutierrez, M.; Heenan, R.K. Aggregation behavior of aqueous solutions of ionic liquids. Langmuir 2004, 20, 2191–2198. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Anwar, M.S.; Danish, R.; Koo, B. Quantum-confinement induced enhancement in photocatalytic properties of iron oxide nanoparticles prepared by Ionic liquid. Ceram. Int. 2014, 40, 15743–15751. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, Y. Iron oxide hollow spheres: Microwave–hydrothermal ionic liquid preparation, formation mechanism, crystal phase and morphology control and properties. Acta Mater. 2009, 57, 2154–2165. [Google Scholar] [CrossRef]
- Yin, S.; Luo, Z.; Xia, J.; Li, H. Microwave-assisted synthesis of Fe3O4 nanorods and nanowires in an ionic liquid. J. Phys. Chem. Solids 2010, 71, 1785–1788. [Google Scholar] [CrossRef]
- Jadhav, A.H.; Lim, A.C.; Thorat, G.M.; Jadhav, H.; Seo, J.G. Green solvent ionic liquids: Structural directing pioneers for microwave-assisted synthesis of controlled MgO nanostructures. RSC Adv. 2016, 6, 31675–31686. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, H.; Diemant, T.; Geiger, D.; Raccichini, R.; Behm, R.J.; Kaiser, U.; Varzi, A.; Passerini, S. Ultrafast ionic liquid-assisted microwave synthesis of SnO microflowers and their superior sodium-Ion storage performance. ACS Appl. Mater. Interfaces 2017, 9, 26797–26804. [Google Scholar] [CrossRef] [PubMed]
- Cybinska, J.; Lorbeer, C.; Mudring, A.V. Ionic liquid assisted microwave synthesis route towards color-tunable luminescence of lanthanide-doped BiPO4. J. Lumin. 2016, 170, 641–647. [Google Scholar] [CrossRef]
- Siramdas, R.; Mclaurin, E.J. InP nanocrystals with color-tunable luminescence by microwave-assisted ionic-liquid etching. Chem. Mater. 2017, 29, 2101–2109. [Google Scholar] [CrossRef]
- Olchowka, J.; Suta, M.; Wickleder, C. Green synthesis of small nanoparticles of A2SiF6 (A = Li - Cs) using ionic liquids as solvent and fluorine source: A novel simple approach without HF. Chemistry 2017, 23, 12092–12095. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Hamm, I.; Grasmik, V.; Wark, M.; Mudring, A.V. Microwave-assisted synthesis of perovskite SrSnO3 nanocrystals in ionic liquids for photocatalytic applications. Inorg. Chem. 2017, 56, 6920–6932. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.J. Microwave-assisted synthesis of porous nickel cobaltite with different morphologies in ionic liquid and their application in supercapacitors. Mater. Chem. Phys. 2016, 176, 6–11. [Google Scholar] [CrossRef]
- Schmitz, A.; Schütte, K.; Ilievski, V.; Barthel, J.; Burk, L.; Mülhaupt, R.; Yue, J.; Smarsly, B.; Janiak, C. Synthesis of metal-fluoride nanoparticles supported on thermally reduced graphite oxide. Beilstein J. Nanotechnol. 2017, 8, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, J.; Loor, M.; Ünal, D.; Mudring, A.; Heimann, S.; Hagemann, U.; Schulz, S.; Maculewicz, F.; Schierning, G. Improving the zT value of thermoelectrics by nanostructuring: Tuning the nanoparticle morphology of Sb2Te3 by using ionic liquids. Dalton Trans. 2017, 46, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lian, Z.; Huo, Y.; Li, H. Microwave-assisted ionothermal synthesis of hierarchical microcube-like BiOBr with enhanced photocatalytic activity. Chin. J. Catal. 2018, 39, 1411–1417. [Google Scholar] [CrossRef]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.V. Ionic liquid assisted microwave synthesis of solid solutions of Sr1−xBaxSnO3 perovskite for photocatalytic applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tang, D.; Li, C. Microwave-assisted synthesis of cobalt phosphide using ionic liquid as Co and P dual-source for hydrogen evolution reaction. Electrochim. Acta 2019, 295, 1027–1033. [Google Scholar] [CrossRef]
- Du, C.; Li, J.; Huang, X. Microwave-assisted ionothermal synthesis of SnSex nanodots: A facile precursor approach towards SnSe2 nanodots/graphene nanocomposite. RSC Adv. 2016, 6, 9835–9842. [Google Scholar] [CrossRef]
- Xia, J.; Ge, Y.; Zhao, D.; Di, J.; Ji, M.; Yin, S.; Li, H.; Chen, R. Microwave-assisted synthesis of few-layered MoS2/BiOBr hollow microspheres with superior visible-light-response photocatalytic activity for ciprofloxacin removal. CrystEngComm 2015, 17, 3645–3651. [Google Scholar] [CrossRef]
- Guerrero-Sánchez, C.; Saldívar, E.; Hernández, M.; Arturo, J. A practical, systematic approach for the scaling-up and modeling of industrial copolymerization reactors. Polym. React. Eng. 2003, 11, 457–506. [Google Scholar] [CrossRef]
- Nising, P.; Zeilmann, T.; Meyer, T. On the degradation and stabilization of poly(methyl methacrylate) in a continuous process. Chem. Eng. Technol. 2003, 26, 599–604. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. New developments in polymer science and technology using combination of ionic liquids and microwave irradiation. Prog. Polym. Sci. 2011, 36, 1754–1765. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Jordan, R. Click chemistry with poly(2-oxazoline)s. Macromolecules 2006, 39, 3509–3516. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Luef, K.P.; Petit, C.; Ottersböck, B.; Ehrenfeld, F.; Grassl, B.; Reynaud, S.; Wiesbrock, F. UV-mediated thiol-ene click reactions for the synthesis of drug-loadable and degradable gels based on copoly(2-oxazoline)s. Eur. Polym. J. 2016, 88, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Fijten, M.W.M.; Brändli, C.; Schroer, J.; Schubert, U.S. Automated parallel temperature optimization and determination of activation energy for the living cationic polymerization of 2-ethyl-2-oxazoline. Macromol. Rapid Commun. 2013, 24, 98–103. [Google Scholar] [CrossRef]
- Park, J.S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Petit, C.; Grassl, B.; Mignard, E. Living cationic ring-opening polymerization of 2-ethyl-2-oxazoline following sustainable concepts: Microwave-assisted and droplet-based millifluidic processes in an ionic liquid medium. Polym. Chem. 2017, 8, 5910–5917. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Fast and “green” living cationic ring opening polymerization of 2-ethyl-2-oxazoline in ionic liquids under microwave irradiation. Chem. Commun. 2006, 28, 3797–3799. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Lobert, M.; Hoogenboom, R. Microwave-assisted homogeneous polymerizations in water-soluble ionic liquids: An alternative and green approach for polymer synthesis. Macromol. Rapid Commun. 2007, 28, 456–464. [Google Scholar] [CrossRef]
- Tan, Y.; Cai, S.; Liao, L.; Wang, Q.; Liu, L. Microwave-assisted ring-opening polymerization of ε-Caprolactone in the presence of ionic liquid. Polym. J. 2009, 41, 849–854. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, C.; Gong, S. Microwave-assisted ring-opening polymerization of trimethylene carbonate in the presence of ionic liquid. J. Polym. Sci. 2007, 45, 5857–5863. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. A study of the ionic liquid mediated microwave heating for the synthesis of new thermally stable and optically active aromatic polyamides under green procedure. Macromol. Res. 2010, 18, 129–136. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Fast synthesis, using microwave induction heating in ionic liquid and characterization of optically active aromatic polyamides. J. Macromol. Sci. A 2009, 46, 783–789. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Microwave-assisted synthesis and morphological characterization of ciral poly(amide-imide) nanostructures in molten ionic liquid salt. Adv. Polym. Technol. 2013, 32, 21333. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. Use of ionic liquid and microwave irradiation as a convenient, rapid and eco-friendly method for synthesis of novel optically active and thermally stable aromatic polyamides containing N-phthaloyl-l-alanine pendent group. Polym. Degrad. Stab. 2008, 93, 753–759. [Google Scholar] [CrossRef]
- Schmidt-Naake, G.; Woecht, I.; Schmalfub, A.; Glück, T.V. polymer reaction engineering free radical polymerization in ionic liquids - Solvent influence of new dimension. Macromol. Symp. 2009, 275, 204–218. [Google Scholar] [CrossRef]
- Glück, T.; Woecht, I.; Schmalfub, A.; Schmidt-Naake, G. Modification of the solvent influence on the free radical polymerization in ionic liquids by microwave irradiation. Macromol. Symp. 2009, 275, 230–241. [Google Scholar] [CrossRef]
- Wei, X.; Chang, G.; Li, J.; Wang, F.; Cui, L.; Fu, T.; Kong, L. Preparation of pH- and salinity-responsive cellulose copolymer in ionic liquid. J. Polym. Res. 2014, 21, 535. [Google Scholar] [CrossRef]
- Lin, C.; Zhan, H.; Liu, M.; Fu, S.; Huang, L. Rapid homogeneous preparation of cellulose graft copolymer in BMIMCl under microwave irradiation. J. Appl. Polym. Sci. 2010, 118, 399–404. [Google Scholar] [CrossRef]
- Rafiemanzelat, F.; Abdollahi, E. Rapid synthesis of new block copolyurethanes derived froml-leucine cyclodipeptide in reusable molten ammonium salts: Novel and efficient green media for the synthesis of new hydrolysable and biodegradable copolyurethanes. Amino Acids 2012, 42, 2177–2186. [Google Scholar] [CrossRef]
- Döbbelin, M.; Azcune, I.; Bedu, M.; Luzuriaga, A.R.; Genua, A.; Jovanovski, V.; Cabañero, G.; Odriozola, I. Synthesis of pyrrolidinium-based poly(ionic liquid) electrolytes with poly(ethylene glycol) side chains. Chem. Mater. 2012, 24, 1583–1590. [Google Scholar] [CrossRef]
- Tang, J.; Radosz, M.; Shen, Y. Poly(ionic liquid)s as optically transparent microwave-absorbing materials. Macromolecules 2008, 41, 493–496. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, J.; Zhao, X. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. A 2014, 2, 9812–9819. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, J.; Yuan, J.; Zhao, X. Microwave-assisted synthesis and high-performance anhydrous electrorheological characteristic of monodisperse poly(ionic liquid) particles with different size of cation/anion parts. Ploymer 2016, 97, 408–471. [Google Scholar] [CrossRef]
- Liu, G.; Su, P.; Zhou, L.; Yang, Y. Microwave-assisted preparation of poly(ionic liquid)-modified polystyrene magnetic nanospheres for phthalate esters extraction from beverages. J. Sep. Sci. 2017, 40, 2603–2611. [Google Scholar] [CrossRef]
- Zhang, R.; Su, P.; Yang, L.; Yang, Y. Microwave-assisted preparation of poly(ionic liquids)-modified magnetic nanoparticles for pesticide extraction. J. Sep. Sci. 2014, 37, 1503–1510. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Tunckol, M.; Durand, J.; Serp, P. Carbon nanomaterial-ionic liquid hybrids. Carbon 2012, 50, 4303–4334. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Functional carbon materials from ionic liquid precursors. Macromol. Chem. Phys. 2012, 213, 1132–1145. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Zhang, Y.; Thomas, A. Synthesis of mesoporous composite materials of nitrogen-doped carbon and silica using a reactive surfactant approach. J. Mater. Chem. 2011, 21, 15537–15543. [Google Scholar] [CrossRef][Green Version]
- Xie, Z.; White, R.J.; Weber, J.; Taubert, A.; Titirici, M.M. Hierarchical porous carbonaceous materials via ionothermal carbonization of carbohydrates. J. Mater. Chem. 2011, 21, 7434–7442. [Google Scholar] [CrossRef]
- Lee, J.; Wang, X.; Luo, H.; Baker, G.A.; Dai, S. Facile ionothermal synthesis of microporous and mesoporous carbons from task specific ionic liquids. J. Am. Chem. Soc. 2009, 131, 4596–4597. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbondots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Sofia, P.; Emilio, P.; Eugenia, M.F. Graphene and carbon quantum dot-based materials in photovoltaic devices: From synthesis to applications. Nanomaterials 2016, 6, 157. [Google Scholar]
- Pham-Truong, T.N.; Petenzi, T.; Ranjan, C.; Randriamahazaka, H.; Ghilane, J. Microwave assisted synthesis of carbon dots in ionic liquid as metal free catalyst for highly selective production of hydrogen peroxide. Carbon 2018, 130, 544–552. [Google Scholar] [CrossRef]
- Jeong, Y.; Moon, K.; Jeong, S.; Koh, W.; Lee, K. Converting waste papers to fluorescent carbon dots in the recycling process without loss of ionic liquids and bio-imaging applications. ACS Sustainable Chem. Eng. 2018, 6, 4510–4515. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Zhang, J.; Li, Z.; Chen, J.; Liu, P.; Wu, D. An ionic liquid promoted microwave-hydrothermal route towards highly photoluminescent carbon dots for sensitive and selective detection of iron. RSC Adv. 2015, 5, 24205–24209. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wu, Q.; Yan, X.; Wu, C.; Chen, X.; Zhang, G. Carbon nanodots as a fluorescence sensor for rapid and sensitive detection of Cr(VI) and their multifunctional applications. Talanta 2017, 165, 216–222. [Google Scholar] [CrossRef]
- Safavi, A.; Sedaghati, F.; Shahbaazi, H.; Farjami, E. Facile approach to the synthesis of carbon nanodots and their peroxidase mimetic function in azo dyes degradation. RSC Adv. 2012, 2, 7367–7370. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, D.; He, H.; Gao, M. Microwave assisted one-step green synthesis of fluorescent carbon nanoparticles from ionic liquids and their application as novel fluorescence probe for quercetin determination. J. Lumin. 2013, 140, 120–125. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Li, H.; Zhang, Q.; Kang, Z.; Lee, S.T. Carbon nanoparticle ionic liquid hybrids and their photoluminescence properties. J. Colloid Sci. 2011, 358, 146–150. [Google Scholar] [CrossRef]
- Meng, Y.; Han, W.; Zhang, Z.; Zhu, F.; Zhang, Y.; Wang, D. LiFePO4 particles coated with N-doped carbon membrane. J. Nanosci. Nanotechnol. 2017, 17, 2000–2005. [Google Scholar] [CrossRef]
- Vadahanambi, S.; Chun, H.H.; Jung, K.H.; Park, H. Nitrogen doped holey carbon nano-sheets as anodes in sodium ion battery. RSC Adv. 2016, 6, 38112–38116. [Google Scholar] [CrossRef]
- Beggs, K.M.; Perus, M.D.; Servinis, L.; O’Dell, L.A.; Fox, B.L.; Gengenbach, T.R.; Henderson, L.C. Rapid surface functionalization of carbon fibres using microwave irradiation in an ionic liquid. RSC Adv. 2016, 6, 32480–32483. [Google Scholar] [CrossRef]
- Myriam, B.; Zois, S.; Maurizio, P. Ionic liquids plus microwave irradiation: A general methodology for the retro-functionalization of single-walled carbon nanotubes. Nanoscale 2018, 10, 15782–15787. [Google Scholar]
- Li, S.; Jia, N.; Zhu, J.; Ma, M.; Sun, R. Synthesis of cellulose-calcium silicate nanocomposites in ethanol/water mixed solvents and their characterization. Carbohydr. Polym. 2010, 80, 270–275. [Google Scholar] [CrossRef]
- Jia, N.; Li, S.; Ma, M.; Sun, R.; Zhu, L. Green microwave-assisted synthesis of cellulose/calcium silicate nanocomposites in ionic liquids and recycled ionic liquids. Carbohydr. Res. 2011, 346, 2970–2974. [Google Scholar] [CrossRef]
- Ma, M.; Qing, S.; Li, S.; Zhu, J.; Sun, R. Microwave synthesis of cellulose/CuO nanocomposites in ionic liquid and its thermal transformation to CuO. Carbohydr. Polym. 2013, 91, 162–168. [Google Scholar] [CrossRef]
- Ma, M.; Dong, Y.; Fu, L.; Li, S.; Sun, R. Cellulose/CaCO3 nanocomposites: Microwave ionic liquid synthesis, characterization, and biological activity. Carbohydr. Polym. 2013, 92, 1669–1676. [Google Scholar] [CrossRef]
- Sharma, M.; Bajpai, A. Superabsorbent nanocomposite from sugarcane bagasse, chitin and clay: Synthesis, characterization and swelling behaviour. Carbohydr. Polym. 2018, 193, 281–288. [Google Scholar] [CrossRef]
- Lu, P.; Cheng, F.; Ou, Y.; Lin, M.; Su, L.; Chen, S.; Yao, X.; Liu, D. Rapid fabrication of transparent film directly from wood fibers with microwave-assisted ionic liquids technology. Carbohydr. Polym. 2017, 174, 330–336. [Google Scholar] [CrossRef]
- Bagheri, M.; Rabieh, S. Preparation and characterization of cellulose-ZnO nanocomposite based on ionic liquid ([C4mim]Cl). Cellulose 2013, 20, 699–705. [Google Scholar] [CrossRef]
- Lin, C.; Zhu, M.; Zhang, T.; Liu, Y.; Lv, Y.; Li, X.; Liu, M. Cellulose/SnS2 composite with enhanced visible-light photocatalytic activity prepared by microwave-assisted ionic liquid method. RSC Adv. 2017, 7, 12255–12264. [Google Scholar] [CrossRef]
- Deng, F.; Fu, L.; Ma, M. Microwave-assisted rapid synthesis and characterization of CaF2 particles-filled cellulose nanocomposites in ionic liquid. Carbohydr. Polym. 2015, 121, 163–168. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L.J. Fast transformation of glucose and di-/polysaccharides into 5-Hydroxymethylfurfural by microwave heating in an ionic liquid/catalyst system. ChemSusChem. 2010, 3, 1071–1077. [Google Scholar] [CrossRef]
- Qu, Y.; Wei, Q.; Li, H.; Oleskowicz-Popiel, P.; Huang, C.; Xu, J. Microwave-assisted conversion of microcrystalline cellulose to 5-hydroxymethylfurfural catalyzed by ionic liquids. Bioresour. Technol. 2014, 162, 358–364. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Fu, Z.; Liu, Y.; Yi, X.; Zheng, A.; Kirk, S.R.; Yin, D. Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 2017, 24, 95–106. [Google Scholar] [CrossRef]
- Xing, R.; Subrahmanyam, A.V.; Olcay, H.; Qi, W.; Van Walsum, G.P.; Pendse, H.; Huber, G.W. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 2010, 12, 1933–1946. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Campelo, J.; Francavilla, M.; Romero, A.A.; Luque, R.; Menéndez-Vázquez, C.; García, A.B.; García-Suárez, E.J. Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalysed by Brönsted acidic ionic liquids. Catal. Sci. Technol. 2012, 2, 1828–1832. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Dong, H.; Peng, X. Conversion of xylan, d-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid. Bioresour. Technol. 2013, 130, 110–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. 2010, 101, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lacerda, V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Li, M.; Chen, J.; Wang, Y.; Zhou, F. Microwave-enhanced sub-critical hydrolysis of rice straw to produce reducing sugar catalyzed by ionic liquid. J. Mater. Cycles Waste Manag. 2018, 20, 1364–1370. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Sun, J.; Li, M.; Wang, S.; Chen, K.; Gao, Z. A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of Eucalyptus and its mechanism. Bioresour. Technol. 2019, 272, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nawshad, M.; Zakaria, M.; Mohamad, A. Ionic liquid—A future solvent for the enhanced uses of wood biomass. Eur. J. Wood Wood Prod. 2011, 70, 125–133. [Google Scholar]
- Zhou, Y.; Schattka, J.H.; Antonietti, M. Room-temperature ionic liquids as template to monolithic mesoporous silica with wormlike pores via a sol-gel nanocasting technique. Nano Lett. 2004, 4, 477–481. [Google Scholar] [CrossRef]
- Pang, J.; Luan, Y.; Wang, Q.; Du, J.; Cai, X.; Li, Z. Microwave-assistant synthesis of inorganic particles from ionic liquid precursors. Colloids Surf. A 2010, 360, 6–12. [Google Scholar] [CrossRef]
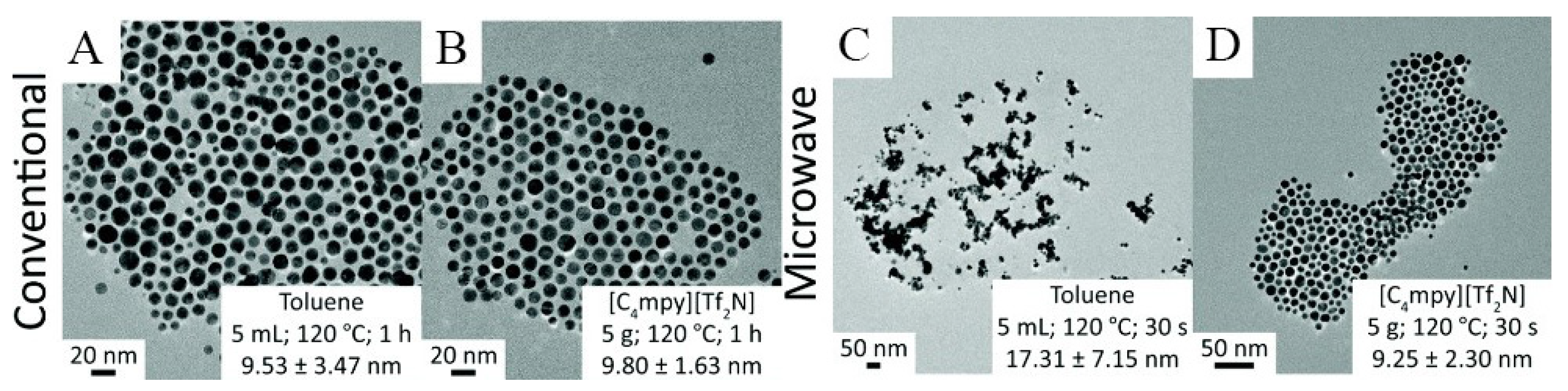
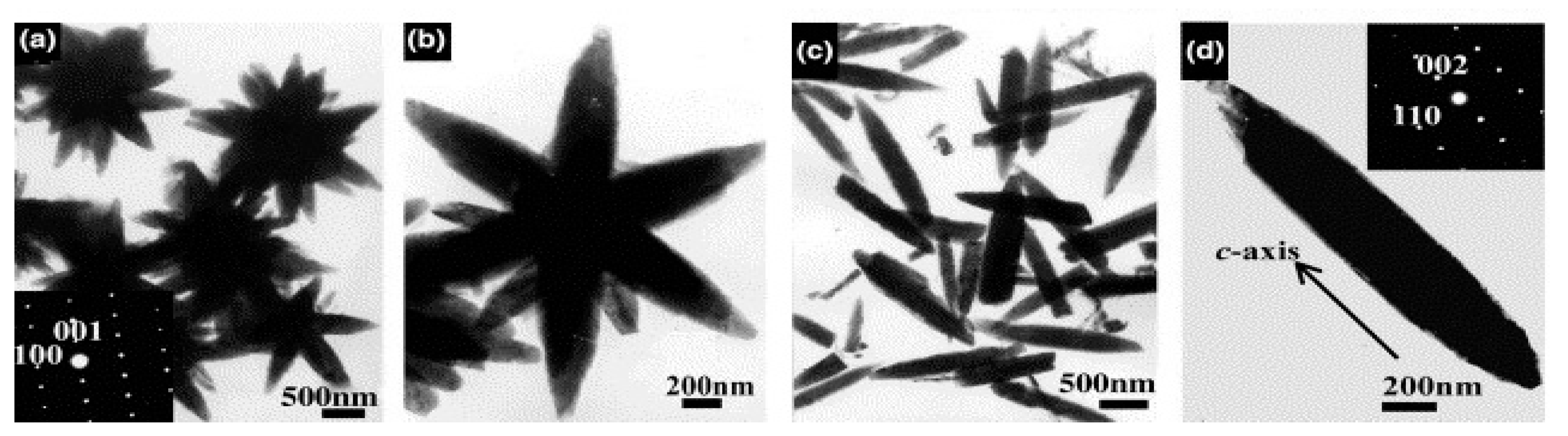
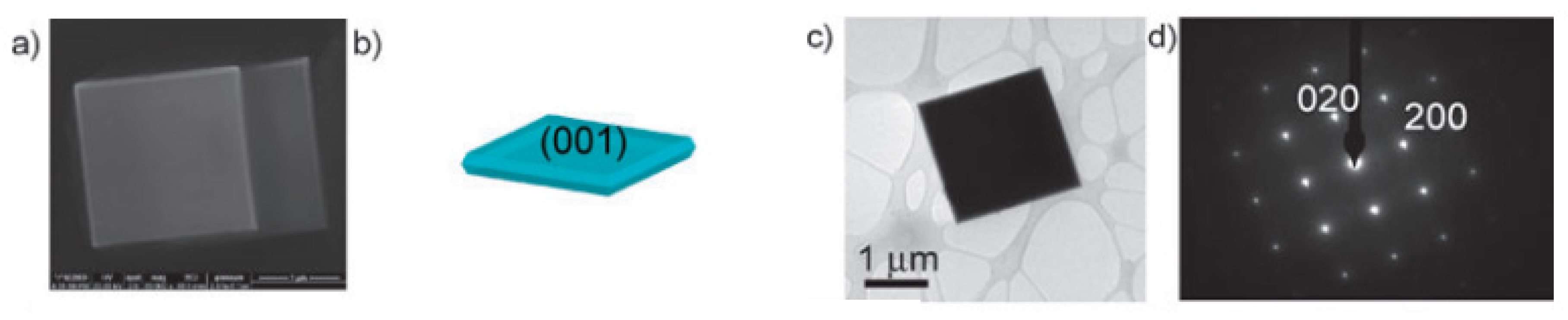
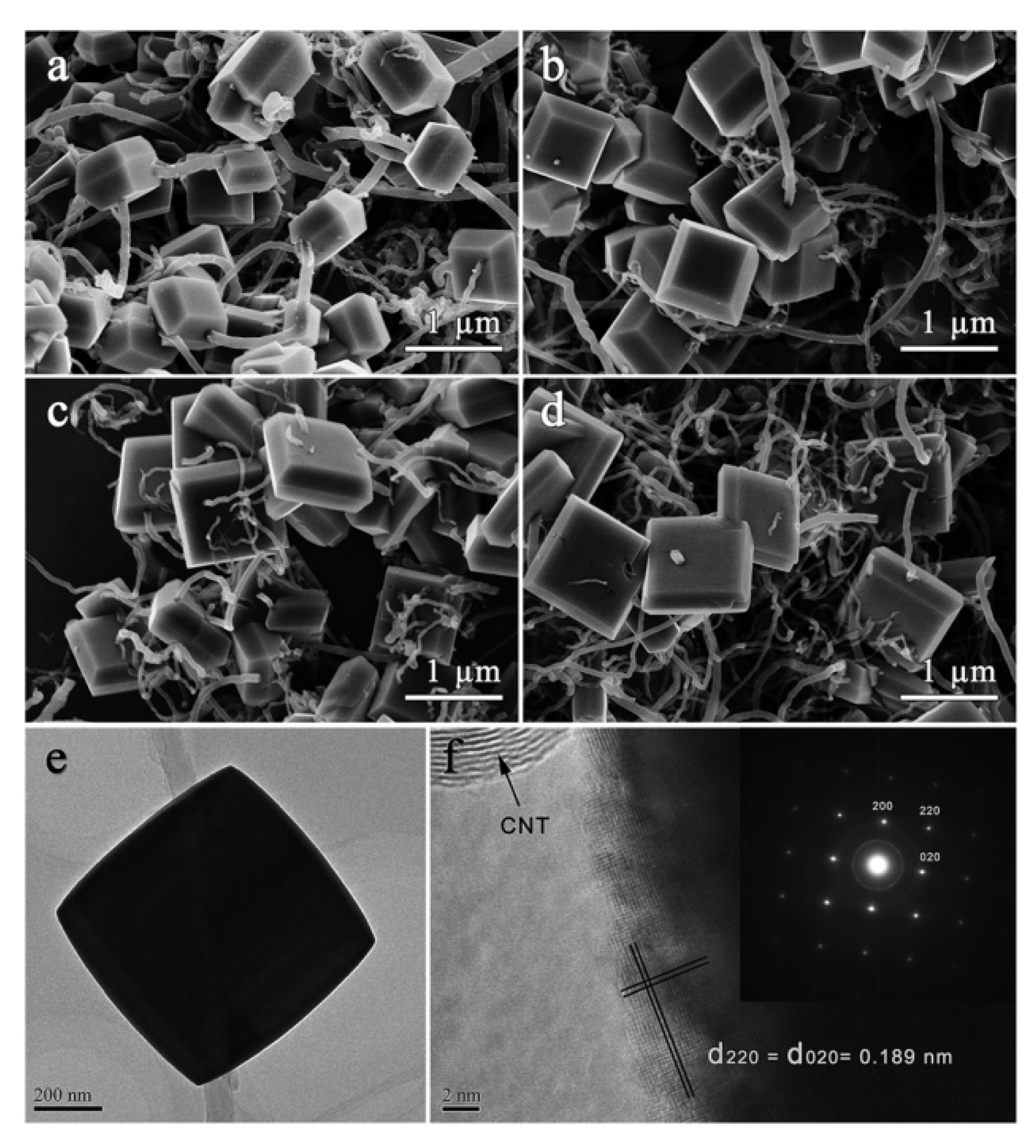
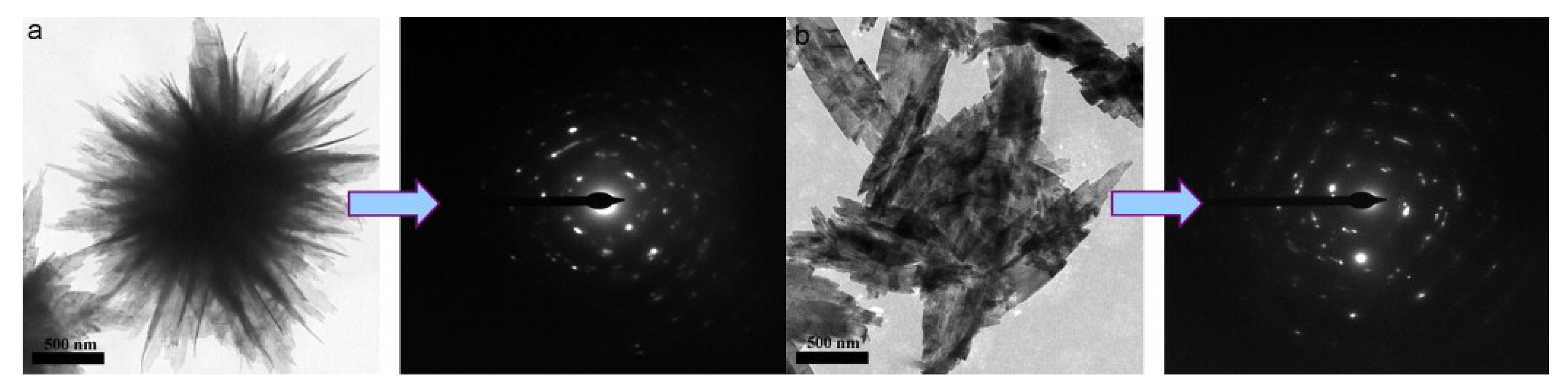
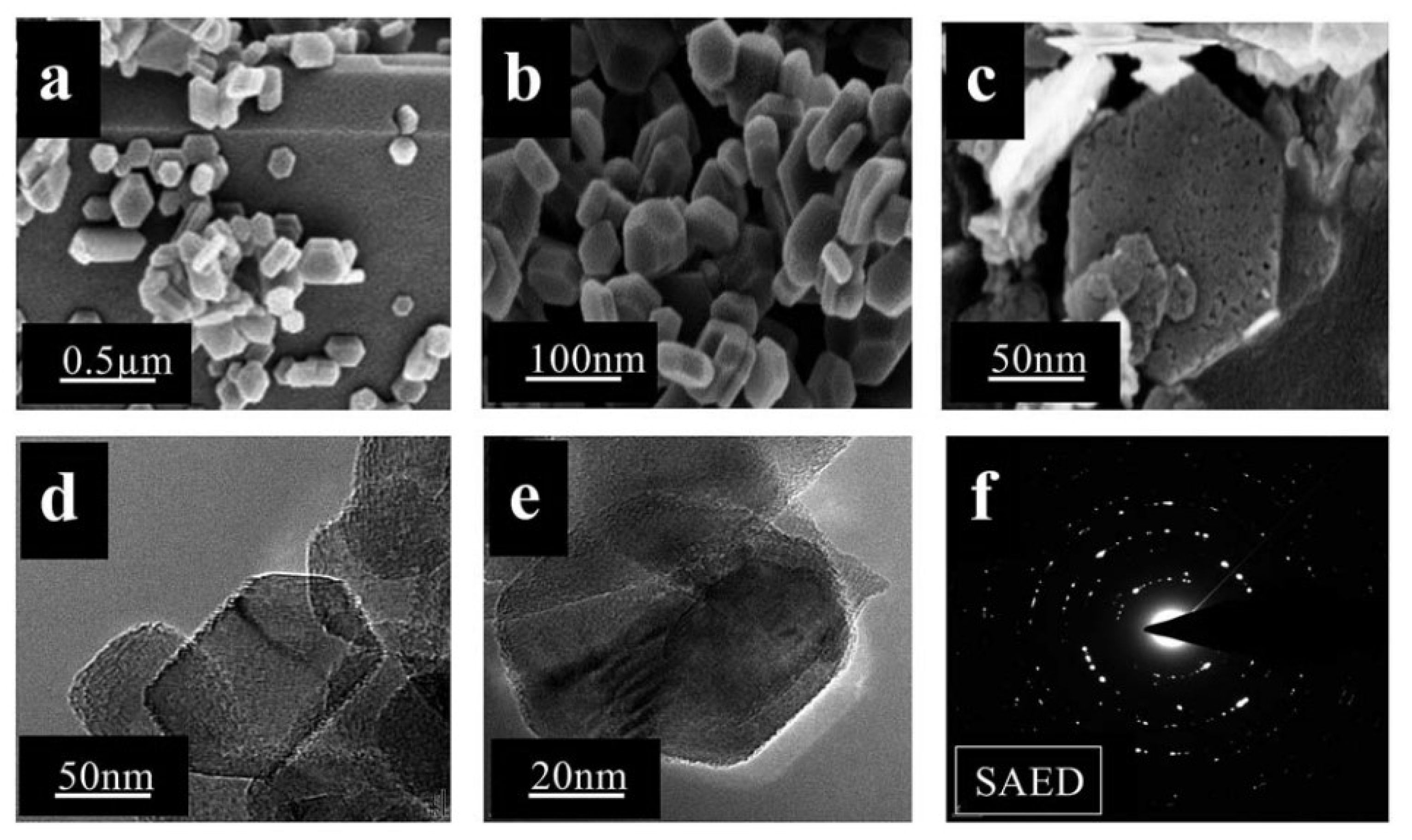
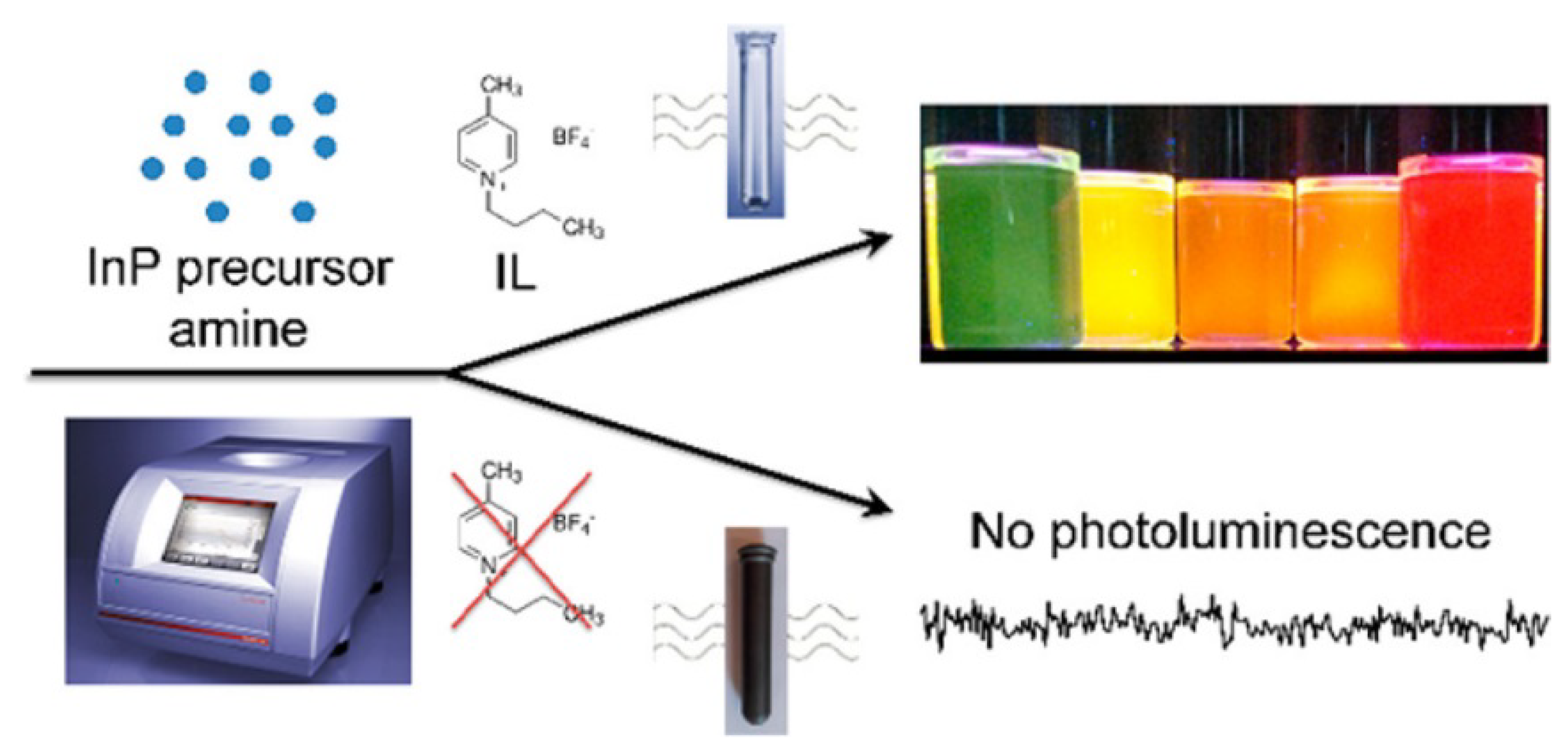

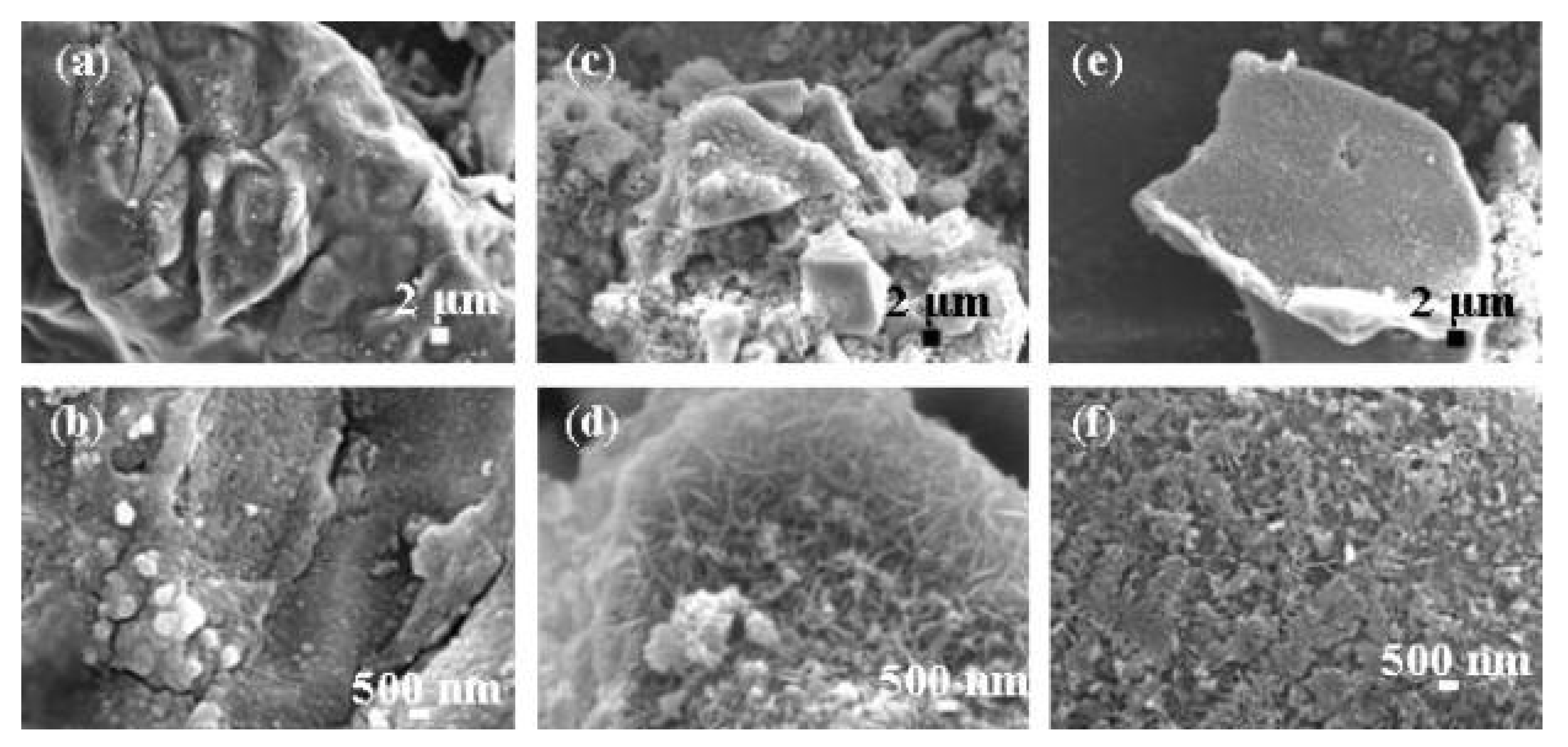
| Nanomaterial Properties. | ILs | Microwave Condition | Applications | Ref. |
|---|---|---|---|---|
| One-dimensional (1D) and 2D mesoporous nickel cobaltite (NiCo2O4) rods and sheets | [Bmim][BF4] | 100 °C for 10 min | Efficient electrode material for supercapacitors | [80] |
| Metal-fluoride NPs, (MFx-NPs) with M = Fe, Co, Pr, Eu | [Bmim][BF4] | Irradiated for 10 min (Co) or 15 min (Fe, Pr, Eu) at a power of 50 W to a temperature of 220 °C | Cathode material for lithium-ion batteries | [81] |
| Nanoparticle morphology of Sb2Te3 | 1-alkyl-3-methylimidazolium and 1,3-dialkylimidazolium-based ILs | 30 s at 100 °C, subsequently for 5 s at 150 °C and finally for 5 min at 170 °C | Thermoelectrics | [82] |
| Hierarchical microcube-like BiOBr | 1-hexadecyl-3-methylimidazolium-bromide | 160 °C | / | [83] |
| Sr1−xBaxSnO3 Perovskite | [C4mim][Tf2N] | 10 min at 85 °C | Photocatalytic applications for the hydroxylation of terephthalic acid | [84] |
| Co2P/CNTs | Tetrabutylphosphonium chloride ([P4,4,4,4]Cl), trihexyl(tetradecyl)phosphonium chloride ([P6,6,6,14]Cl) | Microwave oven for 6 min | Hydrogen evolution | [85] |
| SnSex NDs@rGO | [Bmmim]Cl | 120 °C for 5 min followed by 180 °C for 55 min | Electrochemical application | [86] |
| MoS2/BiOBr | [C16mim]Br | 140 °C for 10 min | Photocatalyst | [87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hou, Q.; Ju, M.; Li, W. New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation. Nanomaterials 2019, 9, 647. https://doi.org/10.3390/nano9040647
Wang Y, Hou Q, Ju M, Li W. New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation. Nanomaterials. 2019; 9(4):647. https://doi.org/10.3390/nano9040647
Chicago/Turabian StyleWang, Yannan, Qidong Hou, Meiting Ju, and Weizun Li. 2019. "New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation" Nanomaterials 9, no. 4: 647. https://doi.org/10.3390/nano9040647
APA StyleWang, Y., Hou, Q., Ju, M., & Li, W. (2019). New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation. Nanomaterials, 9(4), 647. https://doi.org/10.3390/nano9040647