Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CdTe-SA Spinning Dope (SD)
Measurements of SDs
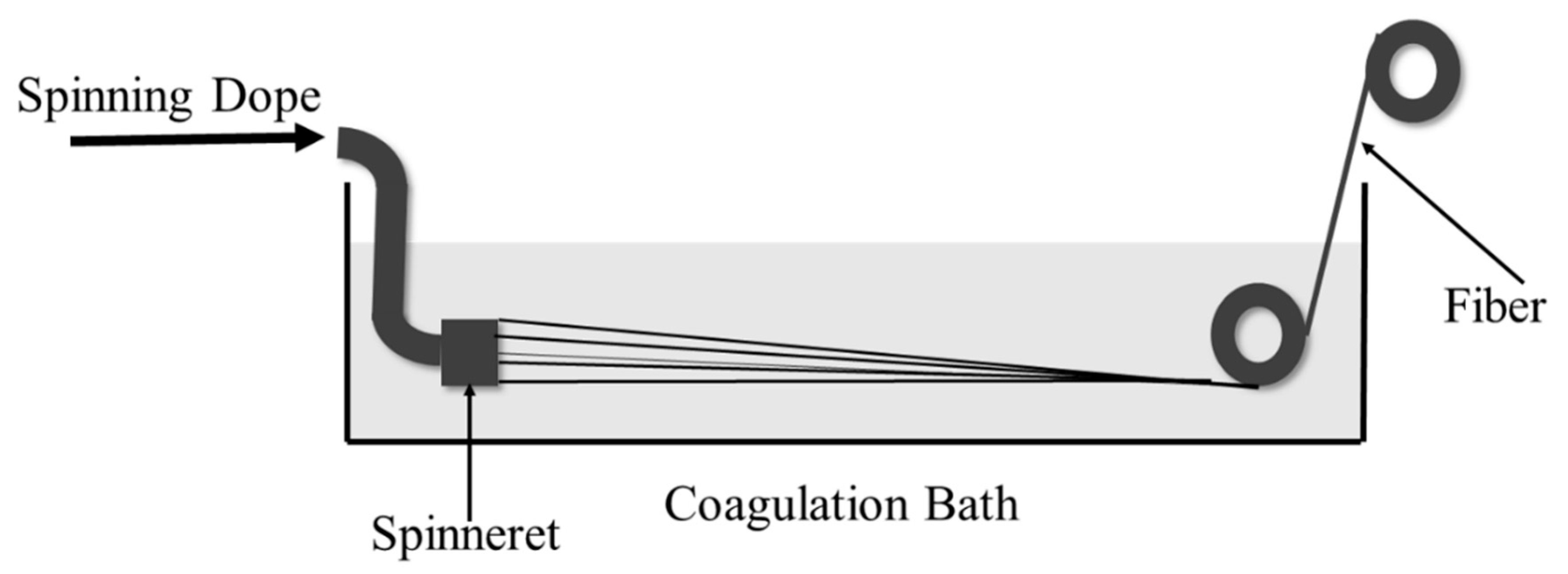
2.3. Preparation of CdTe/Calcium Alginate (CA) FTFs via Wet Spinning
Measurement of FTFs
2.4. Detection of Hg2+ Ions
3. Results and Discussion
3.1. Selection of CdTe NCs with the Most Appropriate Surface Ligands to Prepare SD
3.2. Preparation, Morphology and Structure of FTFs
3.3. Photoluminescence (PL) Properties and Possible Composition of FTFs
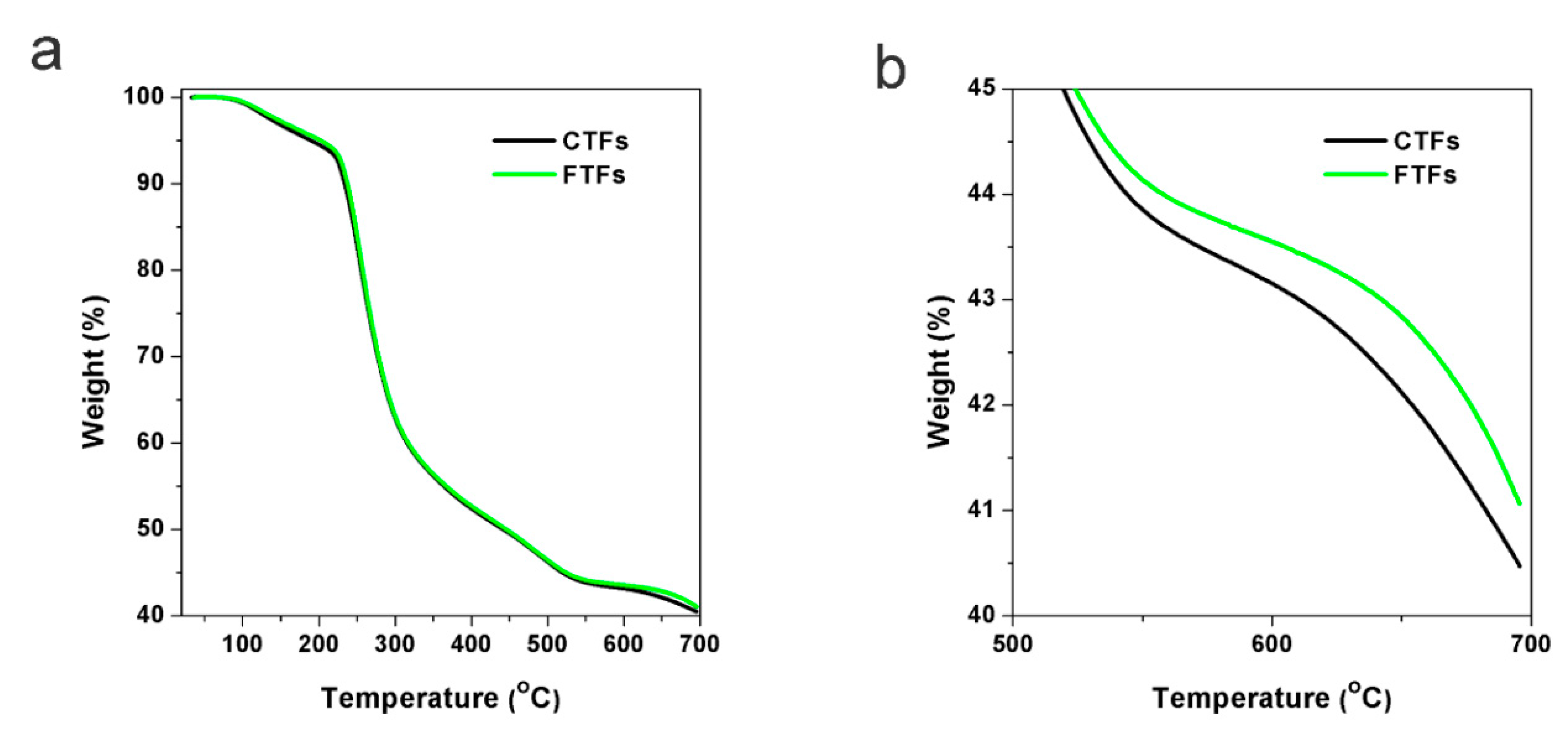
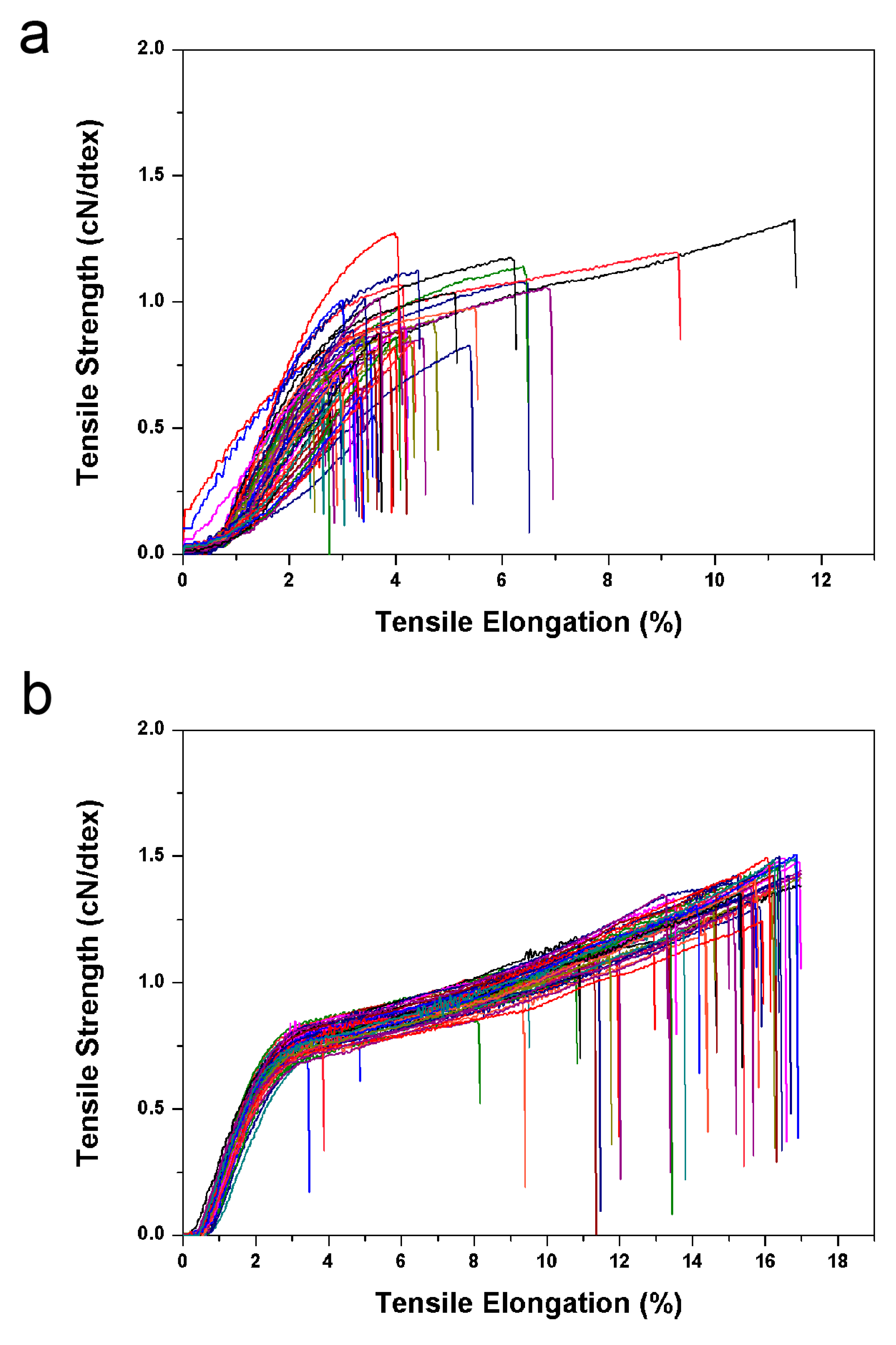
3.4. Thermal Stability, LOI and Mechanical Strength of FTFs
3.5. Detection of Hg2+ Using FTFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haug, A.L.B.; Smidsrod, O. Studies on the Sequence of Uronic Acid Composition of Alginates. Acta Chem. Scand. 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xia, Y.; Wang, Y.; Zhao, X.; Xue, Z.; Quan, F.; Geng, C.; Zhao, Z. Preparation and property investigation of crosslinked alginate/silicon dioxide nanocomposite films. J. Appl. Polym. Sci. 2016, 133, 43489. [Google Scholar] [CrossRef]
- Sui, K.; Li, Y.; Liu, R.; Zhang, Y.; Zhao, X.; Liang, H.; Xia, Y. Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydr. Polym. 2012, 90, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Perez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar]
- Scherr, G.H. Alginate Fibrous Dressing and Method of Making the Same. U.S. Patent 5674524, 7 October 1997. [Google Scholar]
- Wang, C.; Zhang, H.; Zhang, J.; Lv, N.; Li, M.; Sun, H.; Yang, B. Ligand Dynamics of Aqueous CdTe Nanocrystals at Room Temperature. J. Phys. Chem. C 2008, 112, 6330–6336. [Google Scholar] [CrossRef]
- Yoon, D.H.; Tanaka, D.; Sekiguchi, T.; Shoji, S. Mechanical Reinforcement of Low-Concentration Alginate Fibers by Microfluidic Embedding of Multiple Cores. Macromol. Mater. Eng. 2018, 303, 1700516. [Google Scholar] [CrossRef]
- Ge, M.; Guo, X.; Yan, Y. Preparation and Study on the Structure and Properties of Rare-Earth Luminescent Fiber. Text. Res. J. 2012, 82, 677–684. [Google Scholar]
- Tansil, N.C.; Koh, L.D.; Han, M.-Y. Functional Silk: Colored and Luminescent. Adv. Mater. 2012, 24, 1388–1397. [Google Scholar] [CrossRef]
- Murase, N.; Yang, P.; Ando, M. Fluorescent Fiber Containing Semiconductor Nanoparticles. U.S. Patent 20100295016A1, 25 November 2010. [Google Scholar]
- Chu, M.; Liu, G. Fluorescent Silkworm Silk Prepared via Incorporation of Green, Yellow, Red, and Near-Infrared Fluorescent Quantum Dots. IEEE Trans. Nanotechnol. 2008, 7, 308–315. [Google Scholar]
- Zhang, J.; Ge, M. Effecting factors of the emission spectral characteristics of rare-earth strontium aluminate for anti-counterfeiting application. J. Lumin. 2011, 131, 1765–1769. [Google Scholar] [CrossRef]
- Guo, X.; Ge, M. The afterglow characteristics and trap level distribution of chromatic rare-earth luminous fiber. Text. Res. J. 2013, 83, 1263–1272. [Google Scholar] [CrossRef]
- Chang, S.-Q.; Kang, B.; Dai, Y.-D.; Chen, D. A Novel Route to Synthesize CdS Quantum Dots on the Surface of Silk Fibers via g-radiation. Mater. Lett. 2008, 62, 3447–3449. [Google Scholar] [CrossRef]
- Smyder, J.A.; Krauss, T.D. Coming attractions for semiconductor quantum dots. Mater. Today 2011, 14, 382–387. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Zeng, Q.; Cheng, Z.; Du, X.; Jin, G.; Zhang, H.; Yang, B. Efficient aqueous-processed hybrid solar cells from a polymer with a wide bandgap. J. Mater. Chem. A 2015, 3, 10969–10975. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Xiong, H.; Hu, L.; Yang, B.; Li, W. Hydrothermal Synthesis for High-Quality CdTe Nanocrystals. Adv. Mater. 2003, 15, 1712–1715. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Sun, H.; Yang, B. Preparation of polymer-nanocrystals hybrid solar cells through aqueous approaches. Nano Today 2012, 7, 316–326. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Z.; Wang, Y.; Zhang, K.; Ji, X.; Lü, C.; Yang, B.; Gao, M. From Water-Soluble CdTe Nanocrystals to Fluorescent Nanocrystal–Polymer Transparent Composites Using Polymerizable Surfactants. Adv. Mater. 2003, 15, 777–780. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Chemometric prediction of alginate monomer composition: A comparative spectroscopic study using IR, Raman, NIR and NMR. Carbohydr. Polym. 2008, 72, 730–739. [Google Scholar] [CrossRef]
- Joshi, A.; Keerthiprasad, R.; Jayant, R.D.; Srivastava, R. Nano-in-micro alginate based hybrid particles. Carbohydr. Polym. 2010, 81, 790–798. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Y.; Guo, X.; Ge, M. The effects of inorganic pigments on the luminescent properties of colored luminous fiber. Text. Res. J. 2014, 84, 785–792. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Ji, Q.; Xia, Y.; Guo, Z.; Yu, J. Thermal Degradation and Flame Retardancy of Calcium Alginate Fibers. Chin. J. Polym. Sci. 2009, 27, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis Products and Thermal Degradation Mechanism of Intrinsically Flame-Retardant Calcium Alginate Fibre. Polym. Degrad. Stabil. 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Sun, N.; Swatloski, R.P.; Maxim, M.L.; Rahman, M.; Harland, A.G.; Haque, A.; Spear, S.K.; Daly, D.T.; Rogers, R.D. Magnetite-Embedded Cellulose Fibers Prepared from Ionic Liquid. J. Mater. Chem. 2008, 18, 283–290. [Google Scholar] [CrossRef]
- Ding, L.; Yang, J. Research on Acid and Alkali Resistance of Outlast Viscose Fiber. J. Text. Res. 2009, 30, 17–20. [Google Scholar]
- Labeb, M.; Sakr, A.-H.; Soliman, M.; Abdel-Fattah, T.M.; Ebrahim, S. Effect of capping agent on selectivity and sensitivity of CdTe quantum dots optical sensor for detection of mercury ions. Opt. Mater. 2018, 79, 331–335. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Z.-J.; Li, J.-J.; Zhao, J.-W. CdTe quantum dot-based fluorescent probes for selective detection of Hg (II): The effect of particle size. Spectrochim. Acta A 2017, 177, 140–146. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R. Switchable electrochemiluminescence aptasensor coupled with resonance energy transfer for selective attomolar detection of Hg2+ via CdTe@CdS/dendrimer probe and Au nanoparticle quencher. Biosens. Bioelectron. 2018, 102, 328–335. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Geng, C.; Zhao, X.; Xue, Z.; Quan, F.; Xia, Y. Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions. Nanomaterials 2019, 9, 570. https://doi.org/10.3390/nano9040570
Zhao Z, Geng C, Zhao X, Xue Z, Quan F, Xia Y. Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions. Nanomaterials. 2019; 9(4):570. https://doi.org/10.3390/nano9040570
Chicago/Turabian StyleZhao, Zhihui, Cunzhen Geng, Xihui Zhao, Zhixin Xue, Fengyu Quan, and Yanzhi Xia. 2019. "Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions" Nanomaterials 9, no. 4: 570. https://doi.org/10.3390/nano9040570
APA StyleZhao, Z., Geng, C., Zhao, X., Xue, Z., Quan, F., & Xia, Y. (2019). Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions. Nanomaterials, 9(4), 570. https://doi.org/10.3390/nano9040570



