Measurement of Radial Elasticity and Original Height of DNA Duplex Using Tapping-Mode Atomic Force Microscopy
Abstract
1. Introduction
2. Materials and Methods
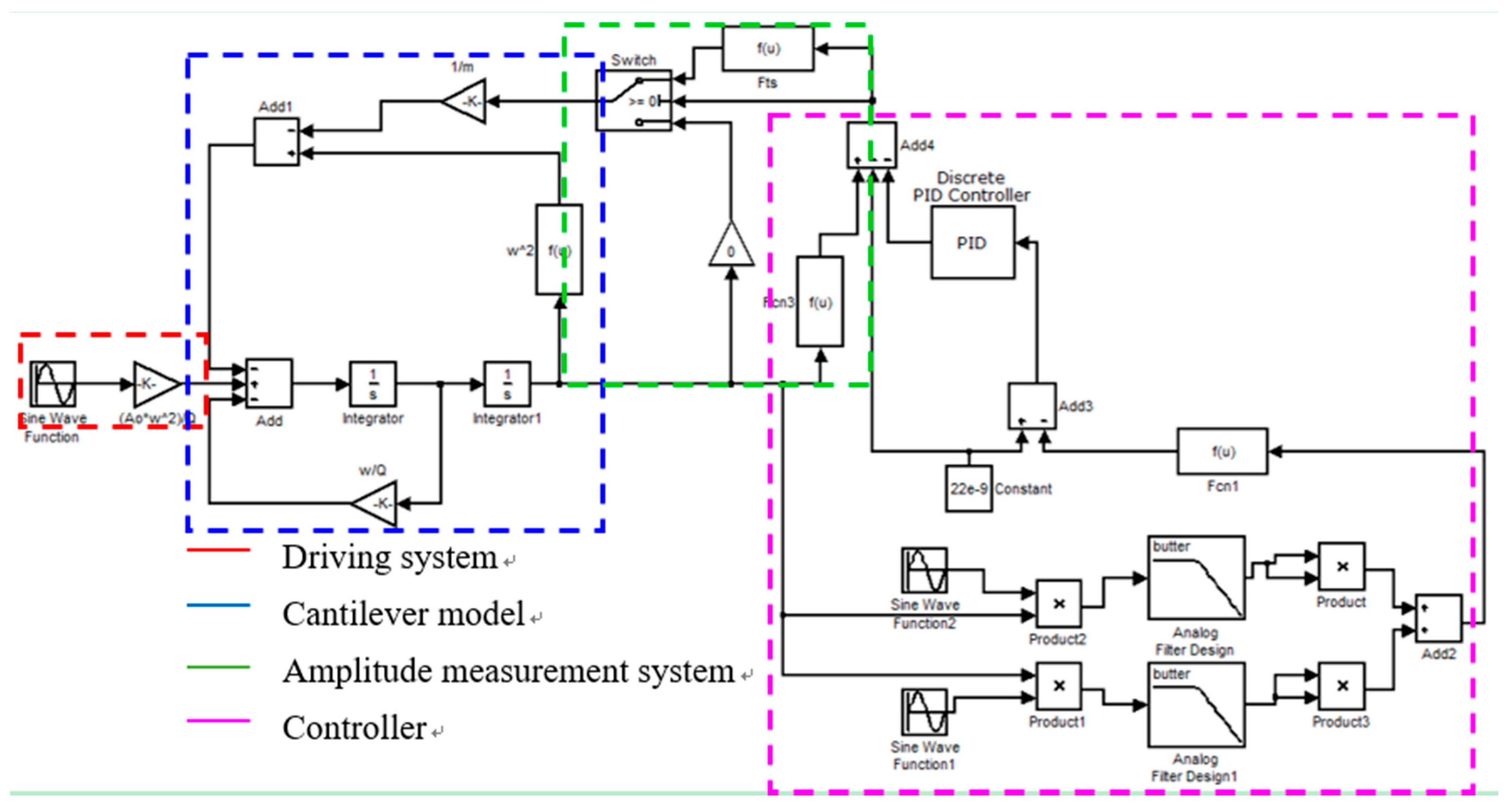
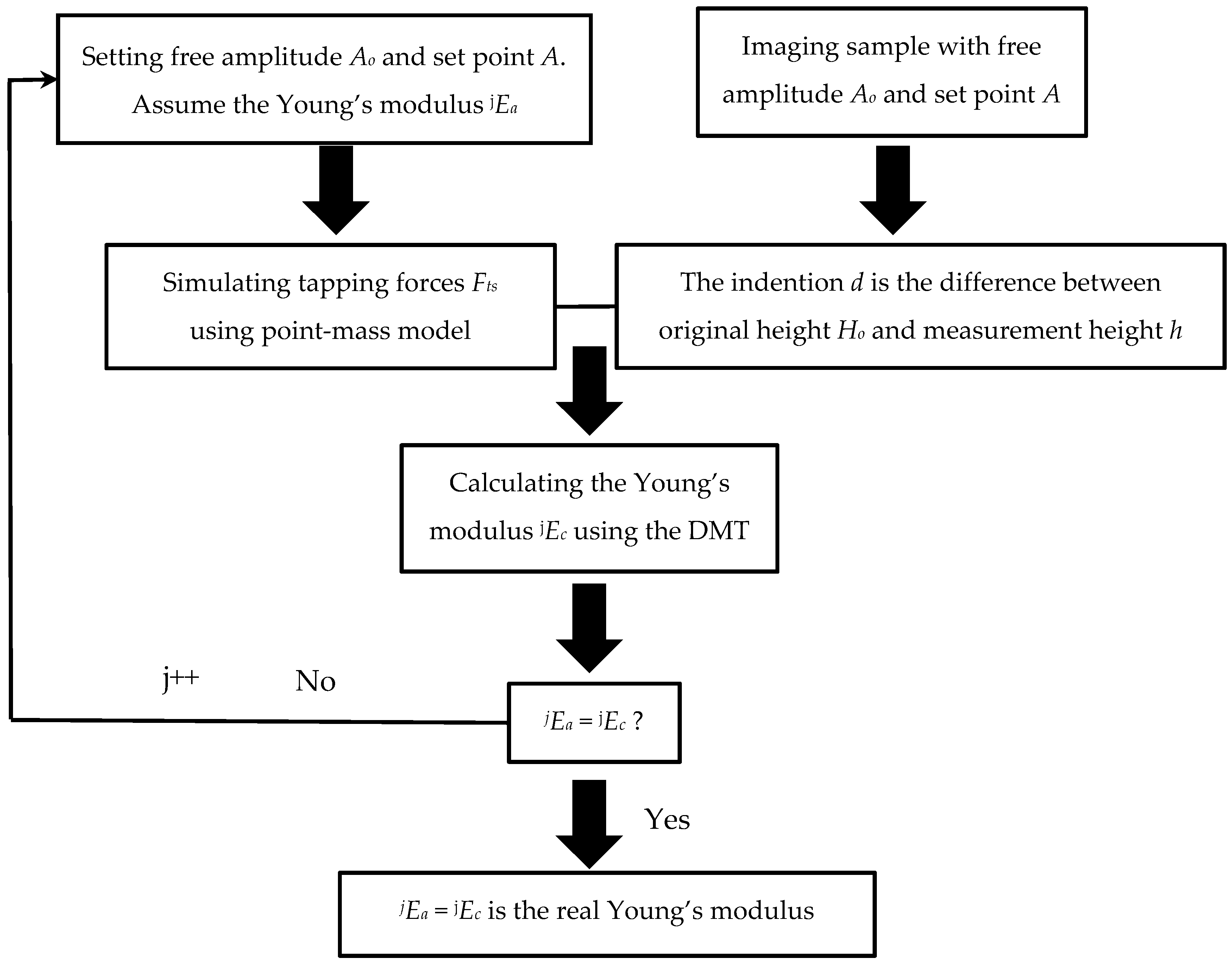
2.1. Assumption–Calculation Method (ACM)
2.2. AFM Experiments
2.2.1. Sample Preparation
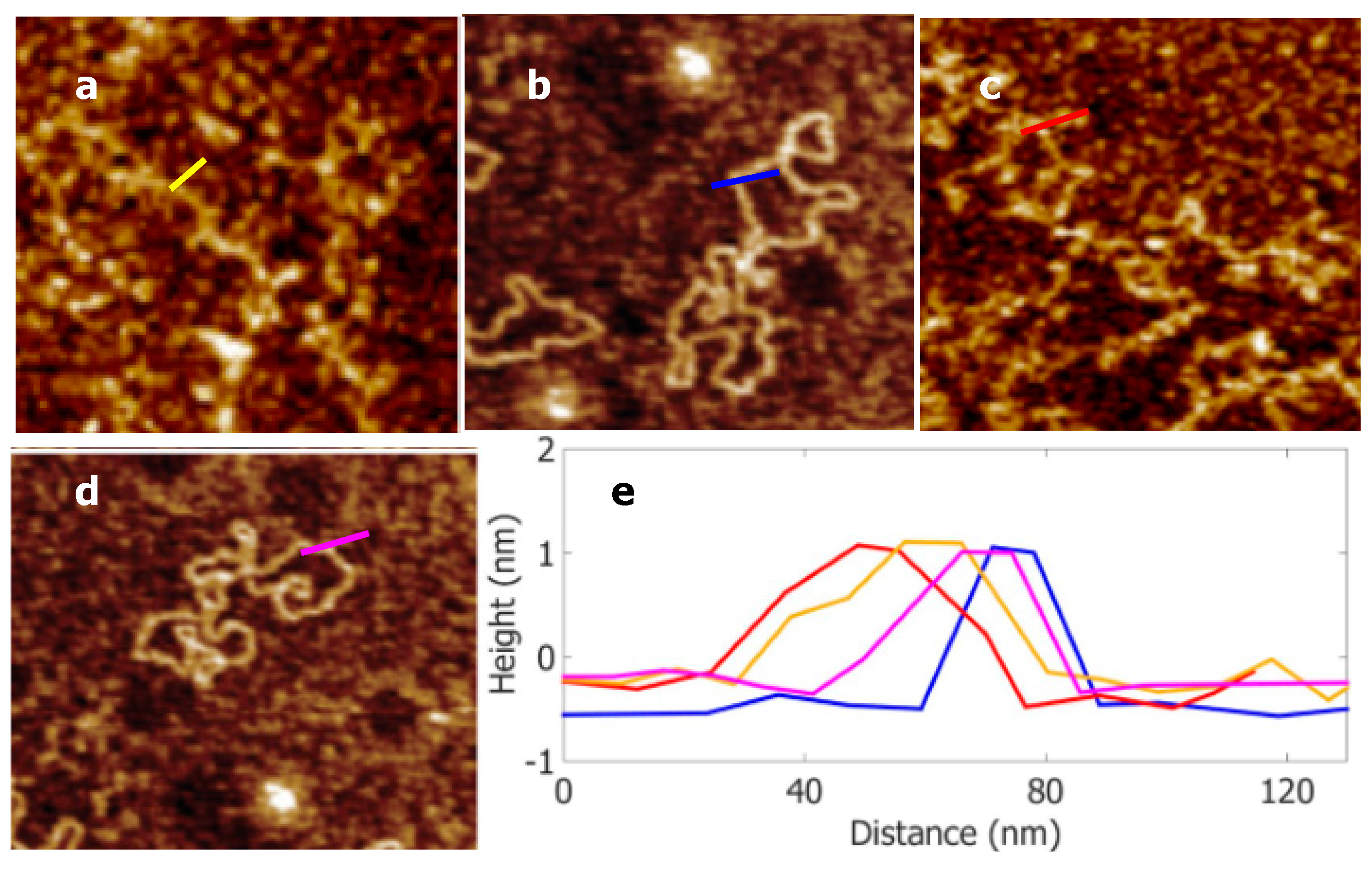
2.2.2. AFM Images
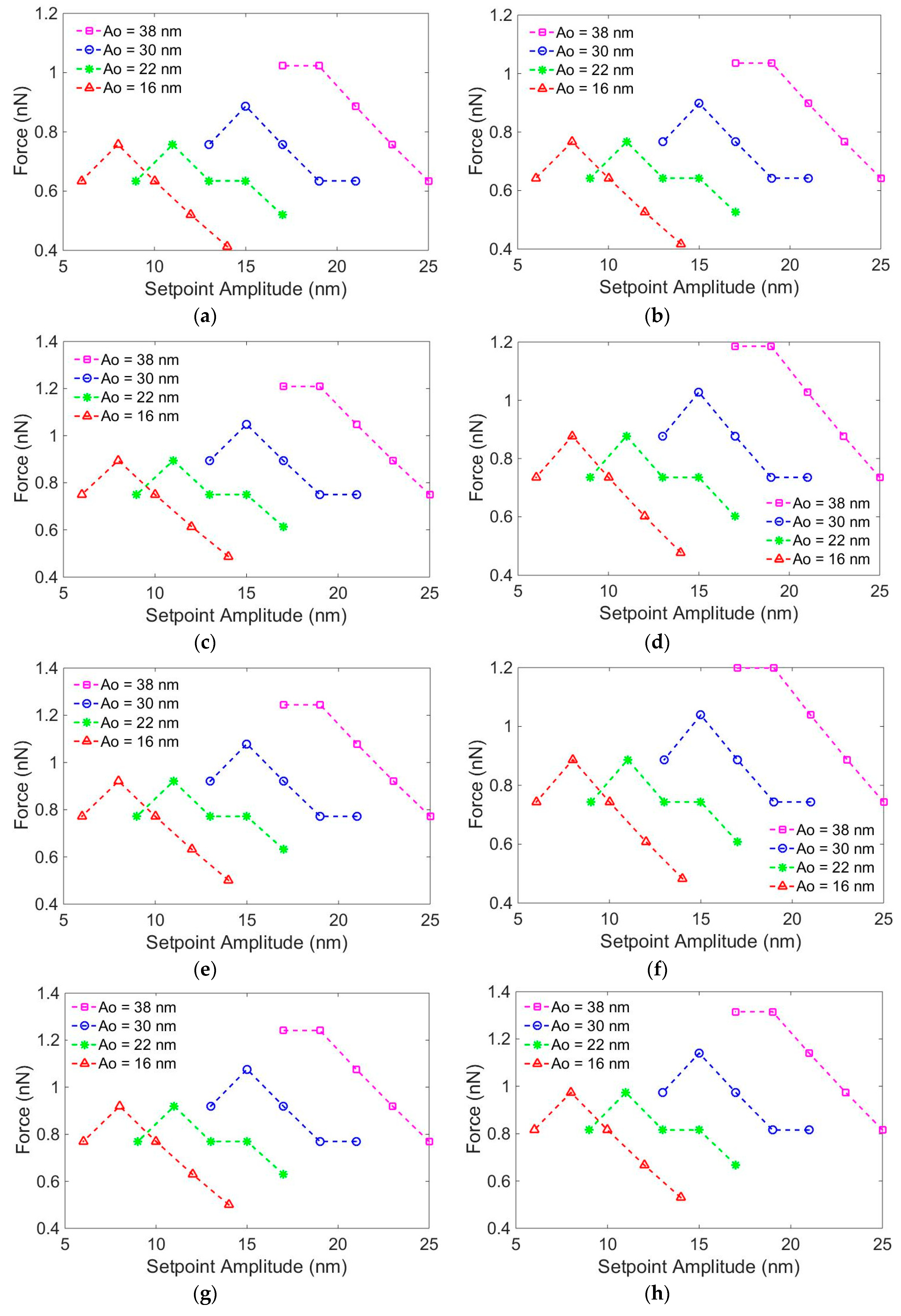
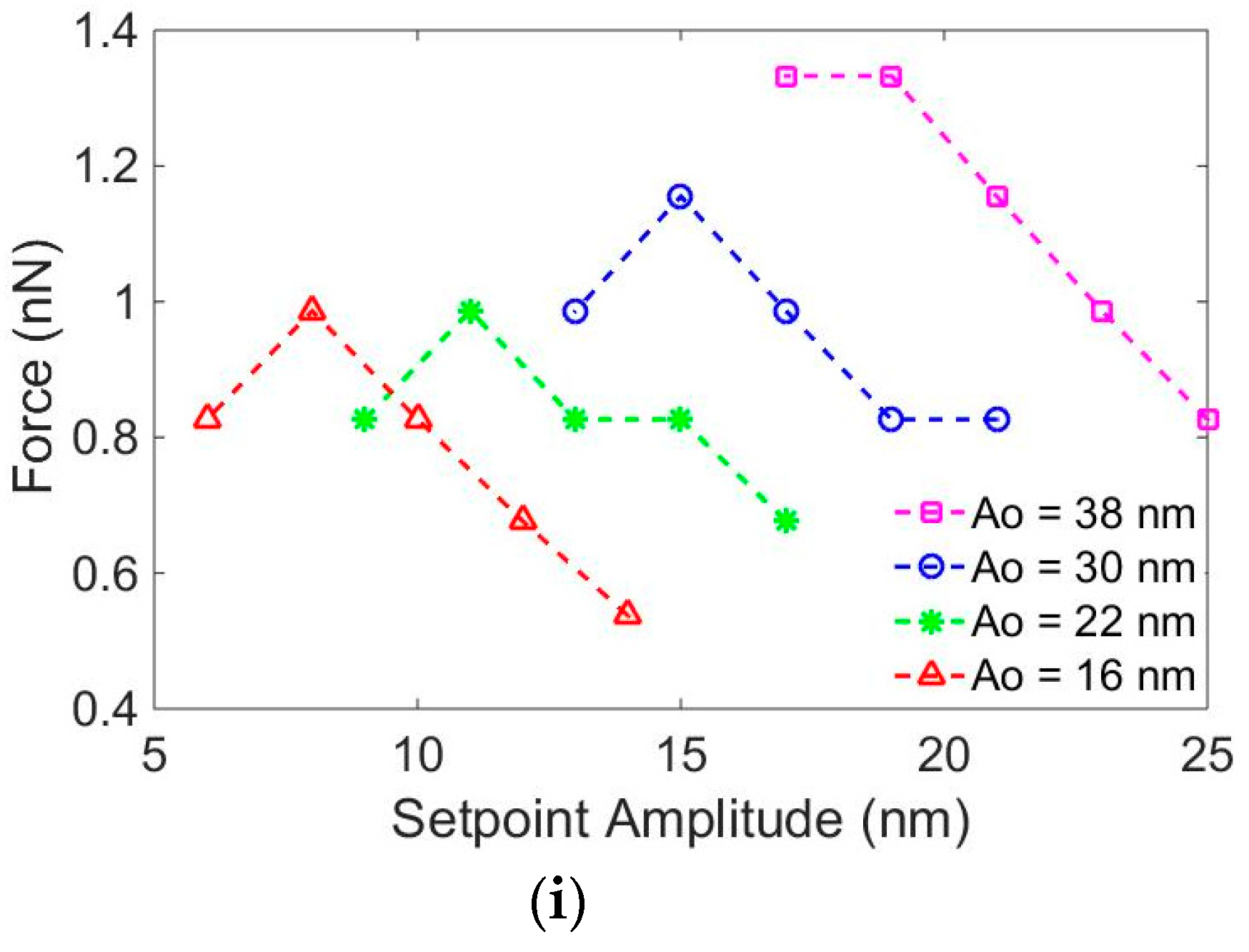
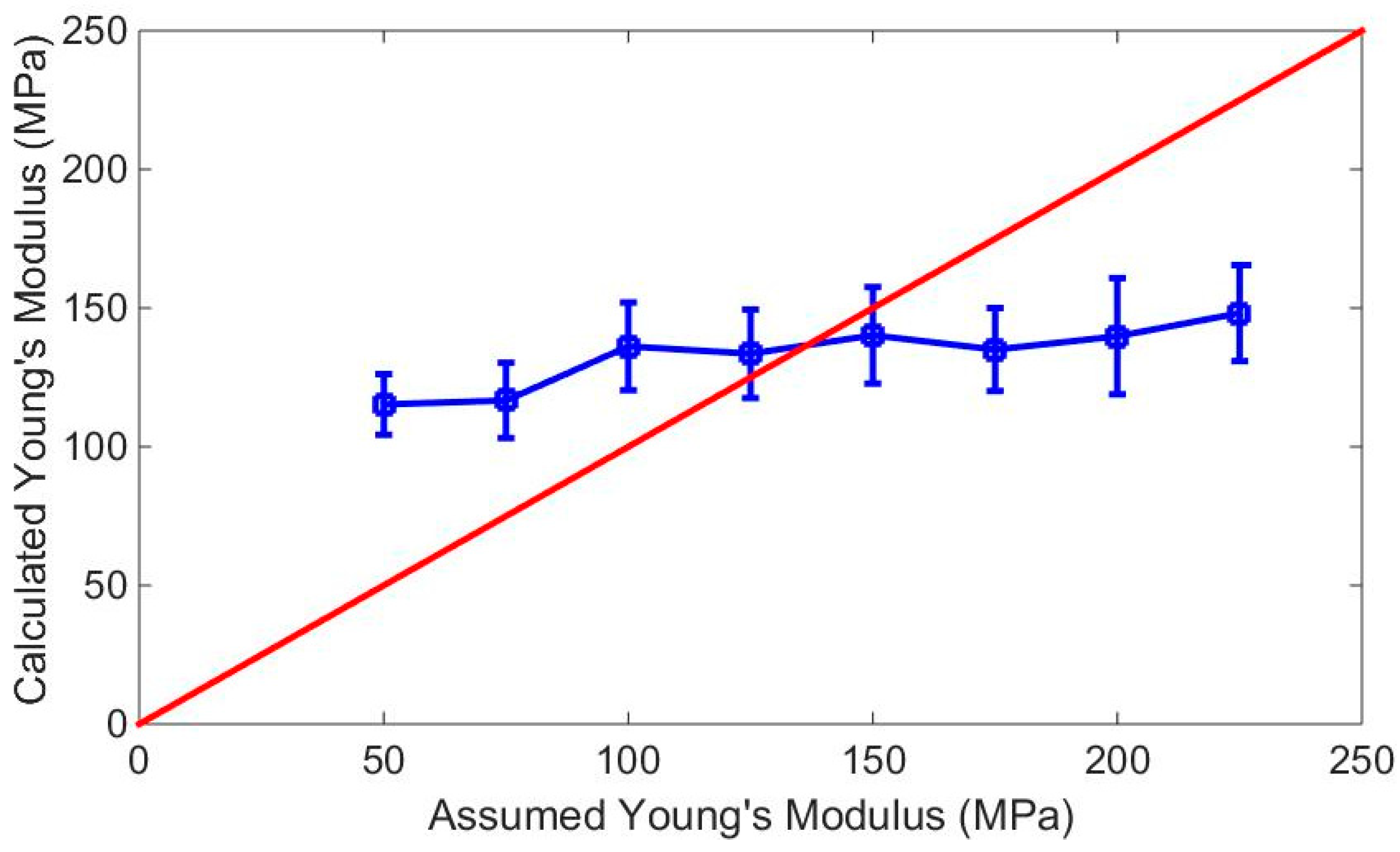
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414. [Google Scholar] [CrossRef] [PubMed]
- Maune, H.T.; Han, S.P.; Barish, R.D.; Bockrath, M.; Goddard, W.A., III; Rothemund, P.W.; Winfree, E. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nat. Nanotechnol. 2010, 5, 61. [Google Scholar] [CrossRef]
- Tasciotti, E. Smart cancer therapy with DNA origami. Nat. Biotechnol. 2018, 36, 234. [Google Scholar] [CrossRef]
- Bui, H.; Shah, S.; Mokhtar, R.; Song, T.; Garg, S.; Reif, J. Localized DNA hybridization chain reactions on DNA origami. ACS Nano 2018, 12, 1146–1155. [Google Scholar] [CrossRef]
- Ding, B.; Deng, Z.; Yan, H.; Cabrini, S.; Zuckermann, R.N.; Bokor, J. Gold nanoparticle self-similar chain structure organized by DNA origami. J. Am. Chem. Soc. 2010, 132, 3248–3249. [Google Scholar] [CrossRef]
- Yamashita, N.; Ma, Z.; Park, S.; Kawai, K.; Hirai, Y.; Tsuchiya, T.; Tabata, O. Formation of gold nanoparticle dimers on silicon by sacrificial DNA origami technique. Micro Nano Lett. 2017, 12, 854–859. [Google Scholar] [CrossRef]
- Saccà, B.; Meyer, R.; Erkelenz, M.; Kiko, K.; Arndt, A.; Schroeder, H.; Rabe, K.S.; Niemeyer, C.M. Orthogonal protein decoration of DNA origami. Angew. Chem. Int. Ed. Engl. 2010, 49, 9378–9383. [Google Scholar] [CrossRef]
- Baclayon, M.; Roos, W.H.; Wuite, G.J. Sampling protein form and function with the atomic force microscope. Mol. Cell Proteom. 2010, 9, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef]
- Li, M.; Tang, H.X.; Roukes, M.L. Ultra-sensitive NEMS-based cantilevers for sensing, scanned probe and very high-frequency applications. Nat. Nanotechnol. 2007, 2, 114. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Rong, G.; Cao, Y.; Liu, J. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Plunkett, M.A.; Feiler, A.; Rutland, M.W. Atomic force microscopy measurements of adsorbed polyelectrolyte layers. 2. Effect of composition and substrate on structure, forces, and friction. Langmuir 2003, 19, 4180–4187. [Google Scholar] [CrossRef]
- Butt, H.J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- McConney, M.E.; Singamaneni, S.; Tsukruk, V.V. Probing soft matter with the atomic force microscopies: Imaging and force spectroscopy. Polym. Rev. 2010, 50, 235–286. [Google Scholar] [CrossRef]
- Tombler, T.W.; Zhou, C.; Alexseyev, L.; Kong, J.; Dai, H.; Liu, L.; Jayanthi, C.S.; Tang, M.; Wu, S.Y. Reversible electromechanical characteristics of carbon nanotubes underlocal-probe manipulation. Nature 2000, 405, 769. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, N.; Xu, K.; Wang, Z.; Dong, Z.; Liu, L. Nanodot deposition and its application with atomic force microscope. J. Nanopart. Res. 2013, 15, 1687. [Google Scholar] [CrossRef]
- Weisenhorn, A.L.; Hansma, P.K.; Albrecht, T.R.; Quate, C.F. Forces in atomic force microscopy in air and water. Appl. Phys. Lett. 1989, 54, 2651–2653. [Google Scholar] [CrossRef]
- Dessinges, M.N.; Maier, B.; Zhang, Y.; Peliti, M.; Bensimon, D.; Croquette, V. Stretching single stranded DNA, a model polyelectrolyte. Phys. Rev. Lett. 2002, 89, 248102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Lee, S.M.; Na, K.; Yang, S.; Kim, J.; Yoon, E.S. An improved measurement of dsDNA elasticity using AFM. Nanotechnology 2010, 21, 075101. [Google Scholar] [CrossRef]
- Zhang, N.H.; Chen, J.Z. Mechanical properties of double-stranded DNA biolayers immobilized on microcantilever under axial compression. J. Biomech. 2009, 42, 1483–1487. [Google Scholar] [CrossRef]
- Zhou, X.F.; Sun, J.L.; An, H.J.; Guo, Y.C.; Fang, H.P.; Su, C.; Xiao, X.D.; Huang, W.H.; Li, M.Q.; Shen, W.Q.; Hu, J. Radial compression elasticity of single DNA molecules studied by vibrating scanning polarization force microscopy. Phys. Rev. E 2005, 71, 062901. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, X.; Wang, J.; Bao, L.; Zhang, Z.; Pang, D. Measuring radial Young’s modulus of DNA by tapping mode AFM. Chin. Sci. Bull. 2007, 52, 3189–3192. [Google Scholar] [CrossRef]
- Payton, O.D.; Picco, L.; Scott, T.B. High-speed atomic force microscopy for materials science. Int. Mater. Rev. 2016, 61, 473–494. [Google Scholar] [CrossRef]
- Legleiter, J. The effect of drive frequency and set point amplitude on tapping forces in atomic force microscopy: Simulation and experiment. Nanotechnology 2009, 20, 245703. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Ref. 1999, 34, 1–104. [Google Scholar] [CrossRef]
- Leite, F.L.; Bueno, C.C.; Da Róz, A.L.; Ziemath, E.C.; Oliveira, O.N. Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy. Int. J. Mol. Sci. 2012, 13, 12773–12856. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Li, J.; Wang, Y.; Wei, Y.; Hu, J.; Zhou, X.F.; Xu, B.; Li, B. Measurement of Nanomechanical Properties of DNA Molecules by PeakForce Atomic Force Microscopy Base on DNA Origamis. Nanoscale 2019, 11, 4707–4711. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Trigueros, S.; Cartagena, A.; Stevenson, A.P.Z.; Susilo, M.; Nauman, E.; Contera, S.A. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy. Nat. Nanotechnol. 2011, 6, 809. [Google Scholar] [CrossRef] [PubMed]
- Cartagena-Rivera, A.X.; Wang, W.H.; Geahlen, R.L.; Raman, A. Fast, multi-frequency, and quantitative nanomechanical mapping of live cells using the atomic force microscope. Sci. Rep. 2015, 5, 11692. [Google Scholar] [CrossRef]
- Cartagena, A.; Hernando-Pérez, M.; Carrascosa, J.L.; de Pablo, P.J.; Raman, A. Mapping in vitro local material properties of intact and disrupted virions at high resolution using multi-harmonic atomic force microscopy. Nanoscale 2013, 5, 4729–4736. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, X.; Wang, H.; Lang, Q.; Chen, H.; Liu, L.Q. Measurement of Radial Elasticity and Original Height of DNA Duplex Using Tapping-Mode Atomic Force Microscopy. Nanomaterials 2019, 9, 561. https://doi.org/10.3390/nano9040561
Li L, Zhang X, Wang H, Lang Q, Chen H, Liu LQ. Measurement of Radial Elasticity and Original Height of DNA Duplex Using Tapping-Mode Atomic Force Microscopy. Nanomaterials. 2019; 9(4):561. https://doi.org/10.3390/nano9040561
Chicago/Turabian StyleLi, Longhai, Xu Zhang, Hongfei Wang, Qian Lang, Haitao Chen, and Lian Qing Liu. 2019. "Measurement of Radial Elasticity and Original Height of DNA Duplex Using Tapping-Mode Atomic Force Microscopy" Nanomaterials 9, no. 4: 561. https://doi.org/10.3390/nano9040561
APA StyleLi, L., Zhang, X., Wang, H., Lang, Q., Chen, H., & Liu, L. Q. (2019). Measurement of Radial Elasticity and Original Height of DNA Duplex Using Tapping-Mode Atomic Force Microscopy. Nanomaterials, 9(4), 561. https://doi.org/10.3390/nano9040561






