Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis
3.2. Preparation of Hydrogels
3.3. TEM Studies
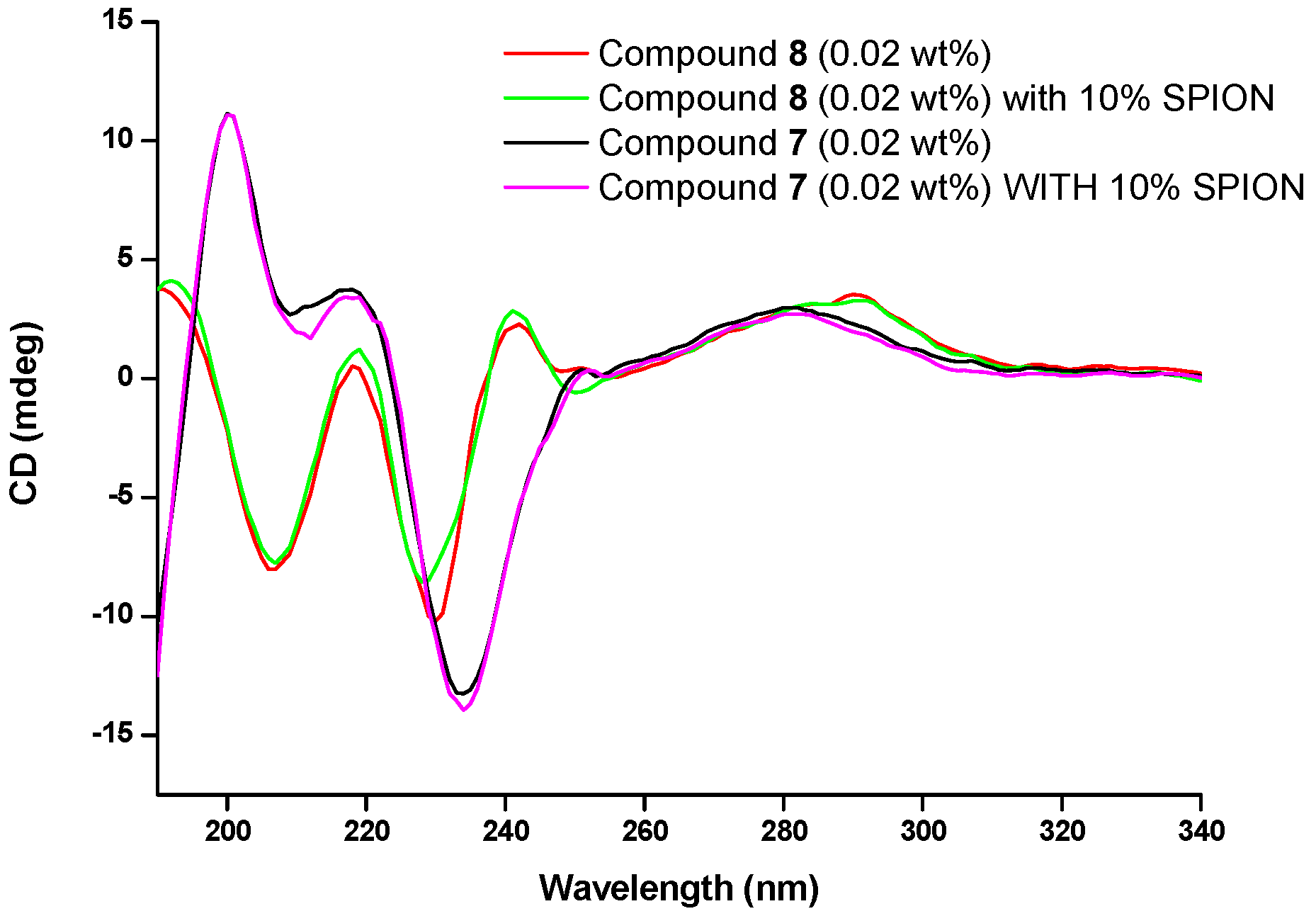
3.4. Circular Dichroism
3.5. Rheology
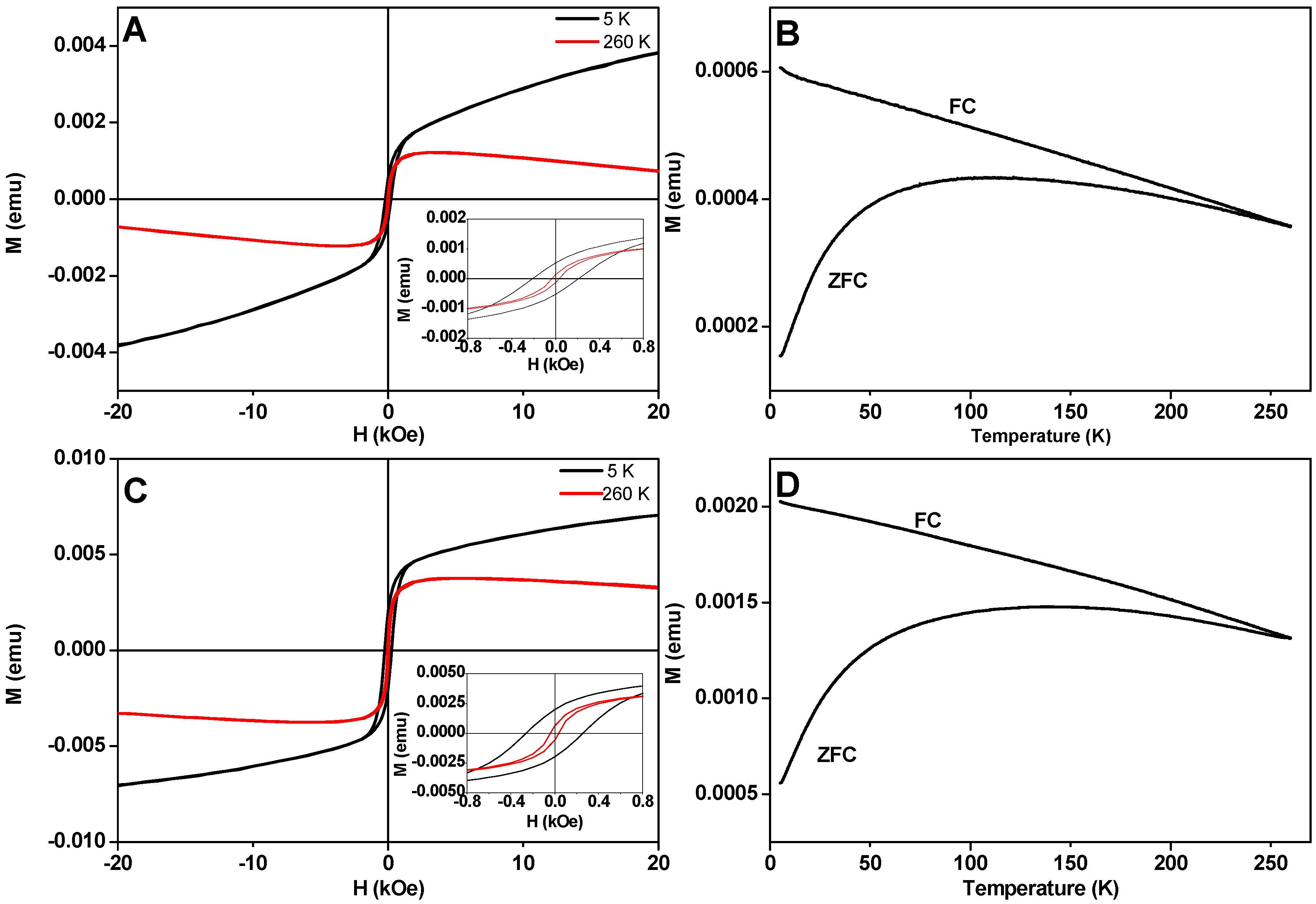
3.6. Magnetic Properties
3.7. Magnetic Resonance Imaging
3.8. Hyperthermia Studies
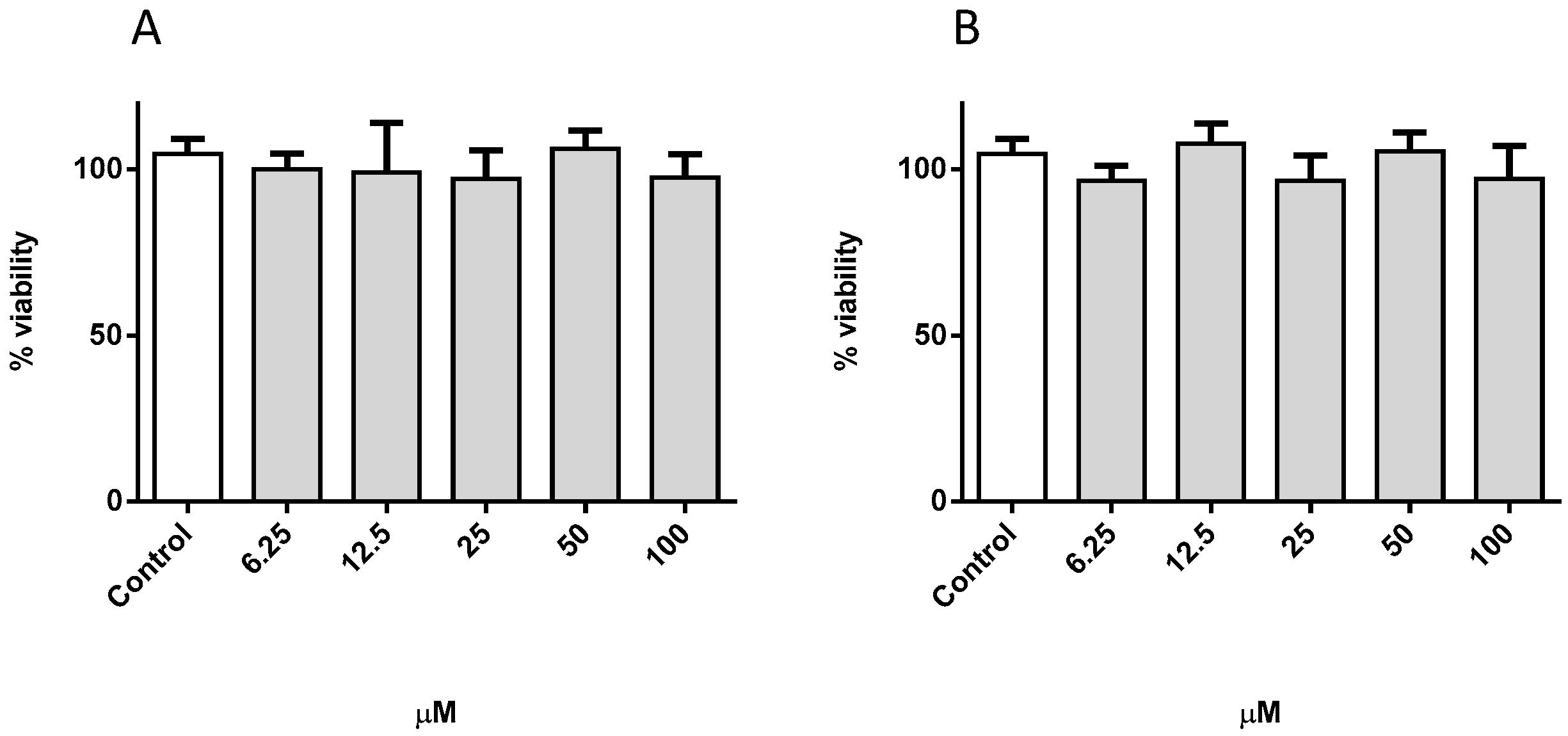
3.9. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolen’Ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I.; et al. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Piñeiro-Redondo, Y.; Bañobre-López, M.; Pardiñas-Blanco, I.; Goya, G.; López-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; Mcmurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Laurent, S.; Bhujwalla, Z.M.; Muller, R.N.; Qin, J.; Jo, Y.S.; Mikhaylova, M.; Muhammed, M.; Roch, A. A High-Performance Magnetic Resonance ImagingT2 Contrast Agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar]
- Muller, R.N.; Port, M.; Vander Elst, L.; Forge, D.; Robic, C.; Laurent, S.; Roch, A. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar]
- Park, J.W.; Bae, K.H.; Kim, C.; Park, T.G. Clustered magnetite nanocrystals cross-linked with PEI for efficient siRNA delivery. Biomacromolecules 2011, 12, 457–465. [Google Scholar] [CrossRef]
- Pineiro, Y.; Vargas-Osorio, Z.; Banobre-Lopez, M.; Kolen’Ko, Y.V.; Lopez-Quintela, M.A.; Rivas, J. Relevant parameters for magnetic hyperthermia in biological applications: Agglomeration, concentration, and viscosity. IEEE Trans. Magn. 2016, 52, 18–21. [Google Scholar] [CrossRef]
- Ribeiro, C.; Cardoso, V.F.; Francesko, A.; Martins, P.; Bañobre-López, M.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2017, 7, 1700845. [Google Scholar]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüß, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH®300F for clinical magnetic fluid hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Kim, J.; Kim, I.-S.; Choi, J.; Jang, J.; Park, K.I.; Cheon, J.; Kim, J.-G.; Lee, J.-H.; Moon, S.H.; Noh, S. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli Responsive Self-Assembled Hydrogel of a Low Molecular Weight Free Dipeptide with Potential for Tunable Drug Delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic hydrogelation of small molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef]
- Ehrbar, M.; Rizzi, S.C.; Schoenmakers, R.G.; San Miguel, B.; Hubbell, J.A.; Weber, F.E.; Lutoff, M.P. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules 2007, 8, 3000–3007. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, H.; Pereira, G.; Castro, T.G.; Hermenegildo, B.F.; Shi, J.; Faria, T.Q.; Micaêlo, N.; Brito, R.M.M.; Xu, B.; Castanheira, E.M.S.; et al. New self-assembled supramolecular hydrogels based on dehydropeptides. J. Mater. Chem. B 2015, 3, 6355–6367. [Google Scholar] [CrossRef]
- Vilaça, H.; Castro, T.; Costa, F.M.G.; Melle-Franco, M.; Hilliou, L.; Hamley, I.W.; Castanheira, E.M.S.; Martins, J.A.; Ferreira, P.M.T. Self-assembled RGD dehydropeptide hydrogels for drug delivery applications. J. Mater. Chem. B 2017, 5, 8607–8617. [Google Scholar] [CrossRef]
- Vilaça, H.; Hortelão, A.C.L.; Castanheira, E.M.S.; Queiroz, M.J.R.P.; Hilliou, L.; Hamley, I.W.; Martins, J.A.; Ferreira, P.M.T. Dehydrodipeptide Hydrogelators Containing Naproxen N-Capped Tryptophan: Self-Assembly, Hydrogel Characterization, and Evaluation as Potential Drug Nanocarriers. Biomacromolecules 2015, 16, 3562–3573. [Google Scholar] [CrossRef]
- Dionigi, C.; Lungaro, L.; Goranov, V.; Riminucci, A.; Piñeiro-Redondo, Y.; Bañobre-López, M.; Rivas, J.; Dediu, V. Smart magnetic poly(N-isopropylacrylamide) to control the release of bio-active molecules. J. Mater. Sci. Mater. Med. 2014, 25, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; De, M.; Chou, S.S.; Vasavada, S.; Bleher, R.; Prasad, P.V.; Bahadur, D.; Dravid, V.P. Thermoresponsive magnetic hydrogels as theranostic nanoconstructs. ACS Appl. Mater. Interfaces 2014, 6, 6237–6247. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Du, J.; Gao, J.; Zhang, B.; Zhang, X.; Xu, B. Self-assembled hybrid nanofibers confer a magnetorheological supramolecular hydrogel. Tetrahedron 2007, 63, 7349–7357. [Google Scholar] [CrossRef]
- Contreras-Montoya, R.; Bonhome-Espinosa, A.B.; Orte, A.; Miguel, D.; Delgado-López, J.M.; Duran, J.D.G.; Cuerva, J.M.; Lopez-Lopez, M.T.; Álvarez de Cienfuegos, L. Iron nanoparticles-based supramolecular hydrogels to originate anisotropic hybrid materials with enhanced mechanical strength. Mater. Chem. Front. 2018, 2, 686–699. [Google Scholar] [CrossRef]
- Pereira, D.M.; Correia-da-Silva, G.; Valentão, P.; Teixeira, N.; Andrade, P.B. Anti-inflammatory effect of unsaturated fatty acids and ergosta-7,22-dien-3-ol from Marthasterias glacialis: Prevention of CHOP-mediated ER-stress and NF-κB activation. PLoS ONE 2014, 9, e88341. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pinto, D.C.G.A.; Pereira, D.M.; Gomes, N.G.M.; Silva, A.M.S.; Andrade, P.B.; Valentão, P. UHPLC-MS/MS profiling of Aplysia depilans and assessment of its potential therapeutic use: Interference on iNOS expression in LPS-stimulated RAW 264.7 macrophages and caspase-mediated pro-apoptotic effect on SH-SY5Y cells. J. Funct. Foods 2017, 37, 164–175. [Google Scholar] [CrossRef]
- Ramagopal, U.; Ramakumar, S.; Sahal, D.; Chauhan, V.S. De novo design and characterization of an apolar helical hairpin peptide at atomic resolution: Compaction mediated by weak interactions. Proc. Natl. Acad. Sci. USA 2001, 98, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Pritchard, R.H.; Shery Huang, Y.Y.; Terentjev, E.M. Mechanics of biological networks: From the cell cytoskeleton to connective tissue. Soft Matter 2014, 10, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, A.V.; Carrillo, J.M.Y. Universality in nonlinear elasticity of biological and polymeric networks and gels. Macromolecules 2011, 44, 140–146. [Google Scholar] [CrossRef]
- Gisler, T.; Ball, R.C.; Weitz, D.A. Strain hardening of fractal colloidal gels. Phys. Rev. Lett. 1999, 82, 1064–1067. [Google Scholar] [CrossRef]
- Hemar, Y.; Yang, Z.; Hilliou, L.; Williams, M.A.K.; Chaieb, S.; McGillivray, D.J.; Gilbert, E.P. Nonlinear Behavior of Gelatin Networks Reveals a Hierarchical Structure. Biomacromolecules 2015, 17, 590–600. [Google Scholar]
- Hilliou, L.; Wilhelm, M.; Yamanoi, M.; Gonçalves, M.P. Structural and mechanical characterization of κ/ι-hybrid carrageenan gels in potassium salt using Fourier Transform rheology. Food Hydrocoll. 2009, 23, 2322–2330. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Surface controlled synthesis of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic characteristics. CrystEngComm 2013, 15, 524–532. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Martínez-Junza, V.; Fibikar, S.; De Cola, L.; Luppi, G.; Clemente-León, M. Manipulation and Orientation of Zeolite L by Using a Magnetic Field. ChemPlusChem 2014, 80, 62–67. [Google Scholar]
- Knobel, M.; Nunes, W.C.; Winnischofer, H.; Rocha, T.C.R.; Socolovsky, L.M.; Mayorga, C.L.; Zanchet, D. Effects of magnetic interparticle coupling on the blocking temperature of ferromagnetic nanoparticle arrays. J. Non. Cryst. Solids 2007, 353, 743–747. [Google Scholar] [CrossRef]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as: T 1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Guldris, N.; Argibay, B.; Kolen’ko, Y.V.; Carbó-Argibay, E.; Sobrino, T.; Campos, F.; Salonen, L.M.; Bañobre-López, M.; Castillo, J.; Rivas, J. Influence of the separation procedure on the properties of magnetic nanoparticles: Gaining in vitro stability and T1-T2 magnetic resonance imaging performance. J. Colloid Interface Sci. 2016, 472, 229–236. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kallumadil, M.; Abe, M.; Pankhurst, Q.A.; Southern, P.; Nakagawa, T. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar]
- Duggan, K.C.; Walters, M.J.; Harp, J.M.; Kiefer, J.R.; Oates, A.; Marnett, L.J.; Duggan, K.C.; Walters, M.J.; Musee, J.; Harp, J.M.; et al. Molecular Basis for Cyclooxygenase Inhibition by the Non-steroidal Anti-inflammatory Drug Naproxen. J. Biol. Chem. 2010, 285, 34950–34959. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Shi, J.; Gao, Y.; Zhou, J.; Xu, B. The conjugation of nonsteroidal anti-inflammatory drugs (NSAID) to small peptides for generating multifunctional supramolecular nanofibers/hydrogels. Beilstein J. Org. Chem. 2013, 9, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflamatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Vane, S.J.R.; Botting, J. Selective COX-2 Inhibitors: Pharmacology, Clinical Effects and Therapeutic Potential; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]






| Hydrogel 7 | Hydrogel 8 | |||
|---|---|---|---|---|
| SPIONs % | G′ (Pa) | G″ (Pa) | G′ (Pa) | G″ (Pa) |
| 0 | 39,300 | 3530 | 119,000 | 5520 |
| 10 | 7390 | 593 | 80,000 | 3400 |
| 20 | 1310 | 30 | 76,600 | 3300 |
| 30 | 122 | 13 | 43,500 | 1400 |
| SPIONs in Water | SPIONs Incorporated into Hydrogel 7 | SPIONs Incorporated into Hydrogel 8 | |
|---|---|---|---|
| r2 (mM−1 s−1) (25 °C; 3 T) | 122 | 30 | 44 |
| SPIONs (%) | SAR (w/g) | ||
|---|---|---|---|
| SPIONs in Water | SPIONs in Hydr. 7 | SPIONs in Hydr. 8 | |
| 25 | 357 | 161 | 347 |
| 30 | 325 | 100 | 305 |
| 35 | 227 | 65 | 240 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. https://doi.org/10.3390/nano9040541
Carvalho A, Gallo J, Pereira DM, Valentão P, Andrade PB, Hilliou L, Ferreira PMT, Bañobre-López M, Martins JA. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials. 2019; 9(4):541. https://doi.org/10.3390/nano9040541
Chicago/Turabian StyleCarvalho, André, Juan Gallo, David M. Pereira, Patrícia Valentão, Paula B. Andrade, Loic Hilliou, Paula M.T. Ferreira, Manuel Bañobre-López, and José A. Martins. 2019. "Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications" Nanomaterials 9, no. 4: 541. https://doi.org/10.3390/nano9040541
APA StyleCarvalho, A., Gallo, J., Pereira, D. M., Valentão, P., Andrade, P. B., Hilliou, L., Ferreira, P. M. T., Bañobre-López, M., & Martins, J. A. (2019). Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials, 9(4), 541. https://doi.org/10.3390/nano9040541









