Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Nanofibers with Different Crystal Forms
2.3. Preparation of Rutile–Anatase Core-Shell TiO2 Nanofibers
2.4. Preparation of Anatase–Rutile Core-Shell TiO2 Nanofibers
2.5. Characterization
2.6. Photocatalytic Activity Measurement
3. Results and Discussion
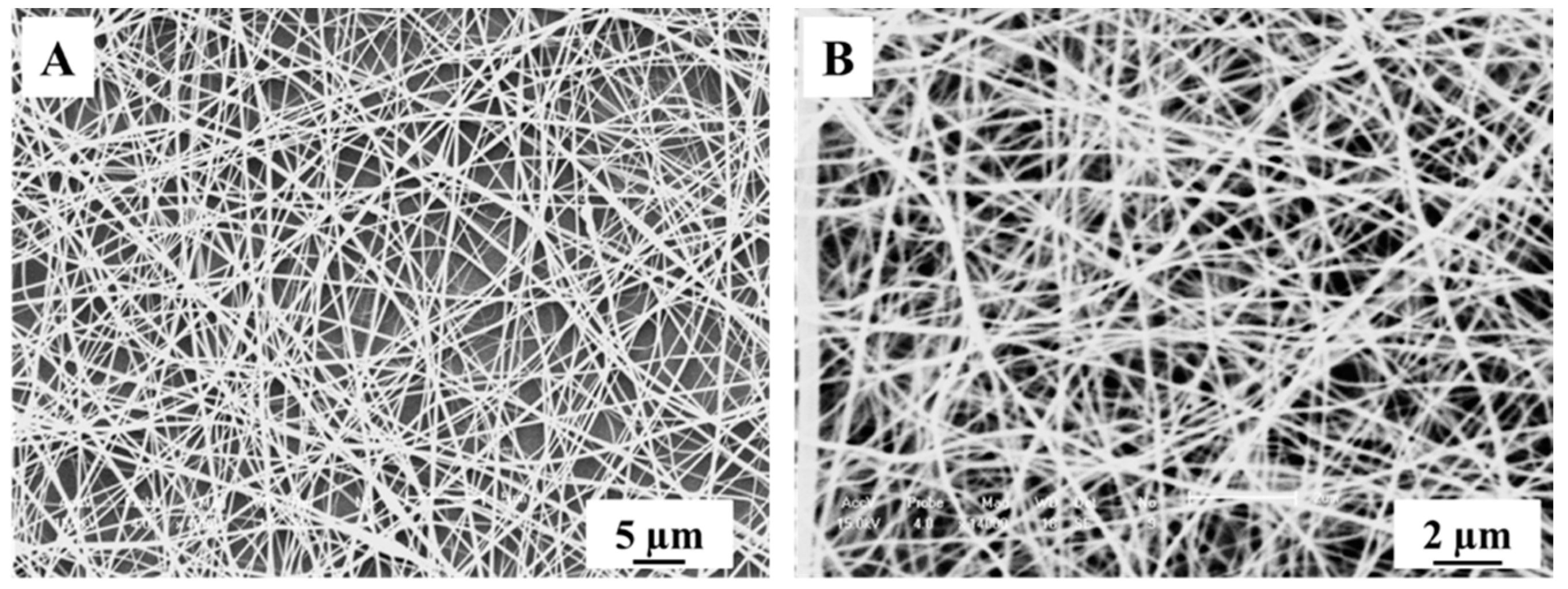
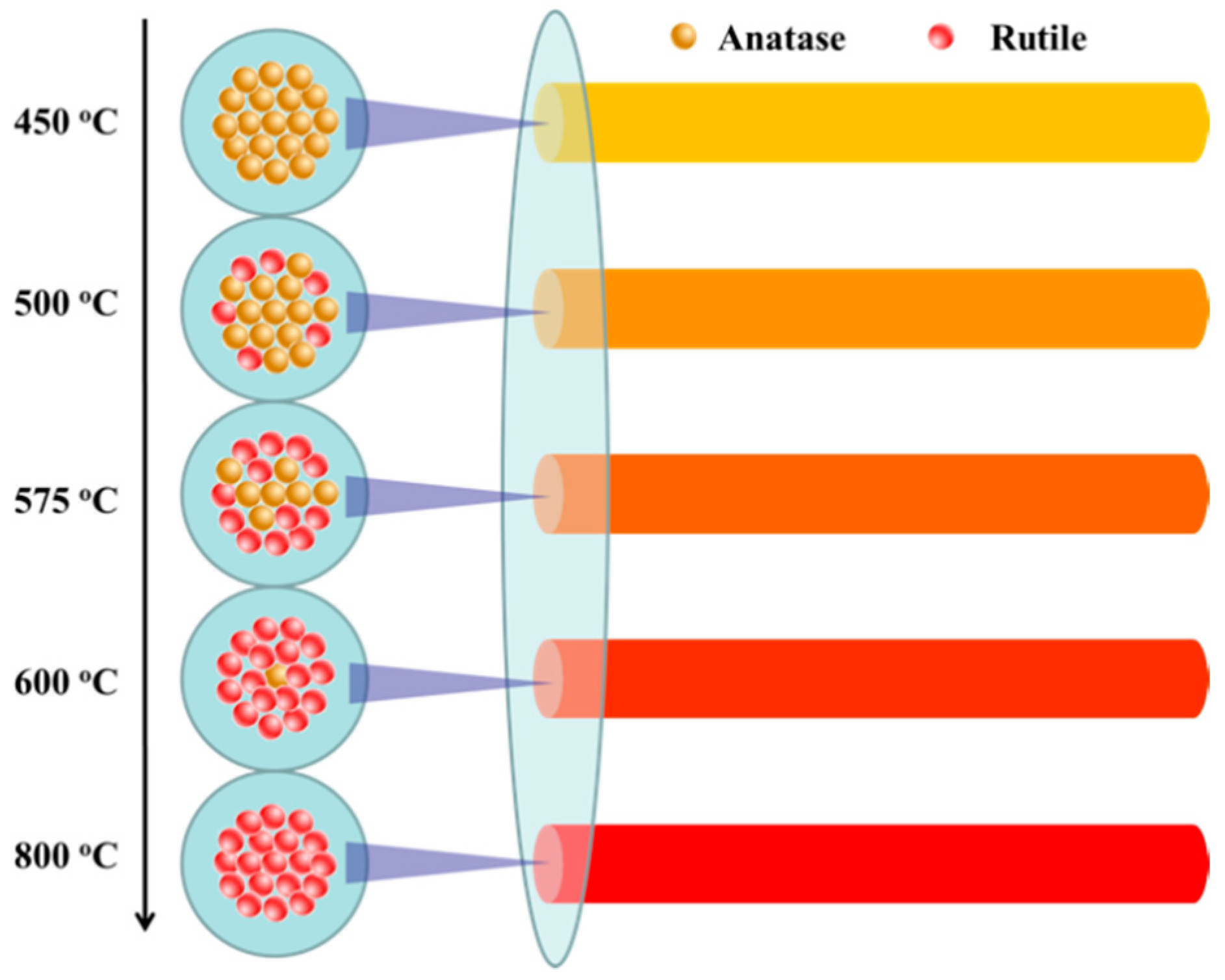
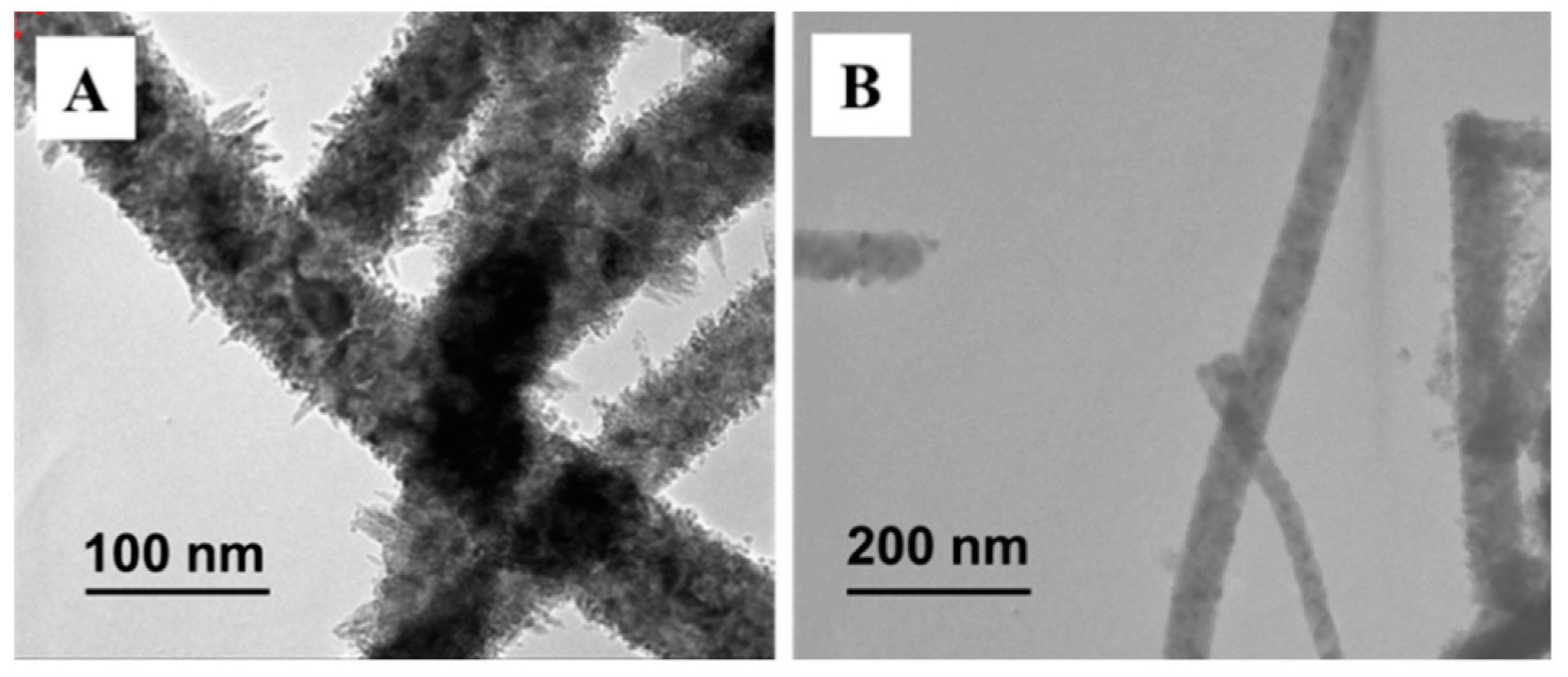
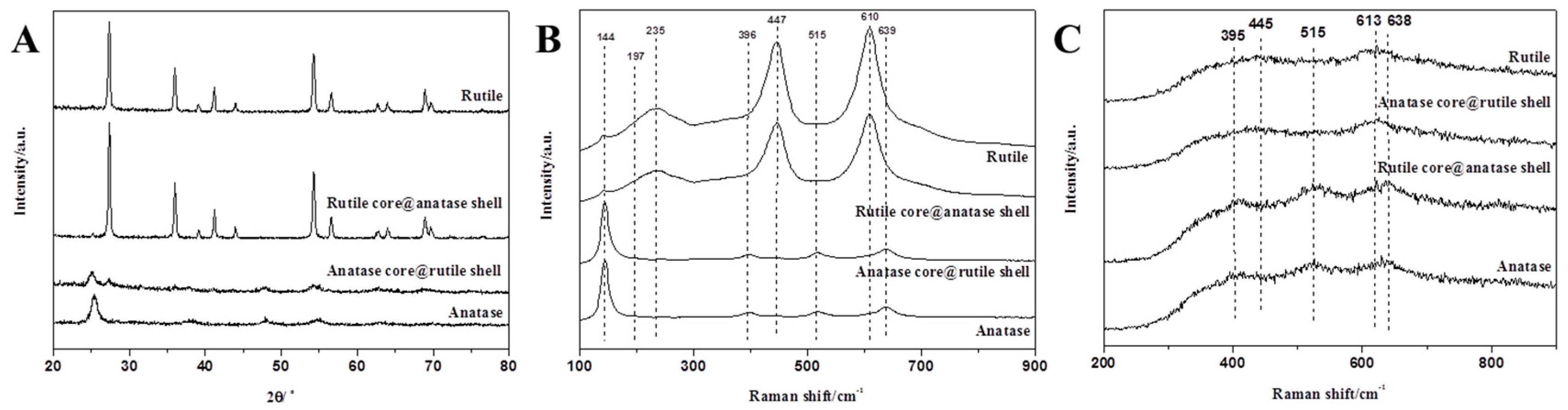
3.1. Characterization and Analysis of Mixed Crystalline TiO2 Nanofibers
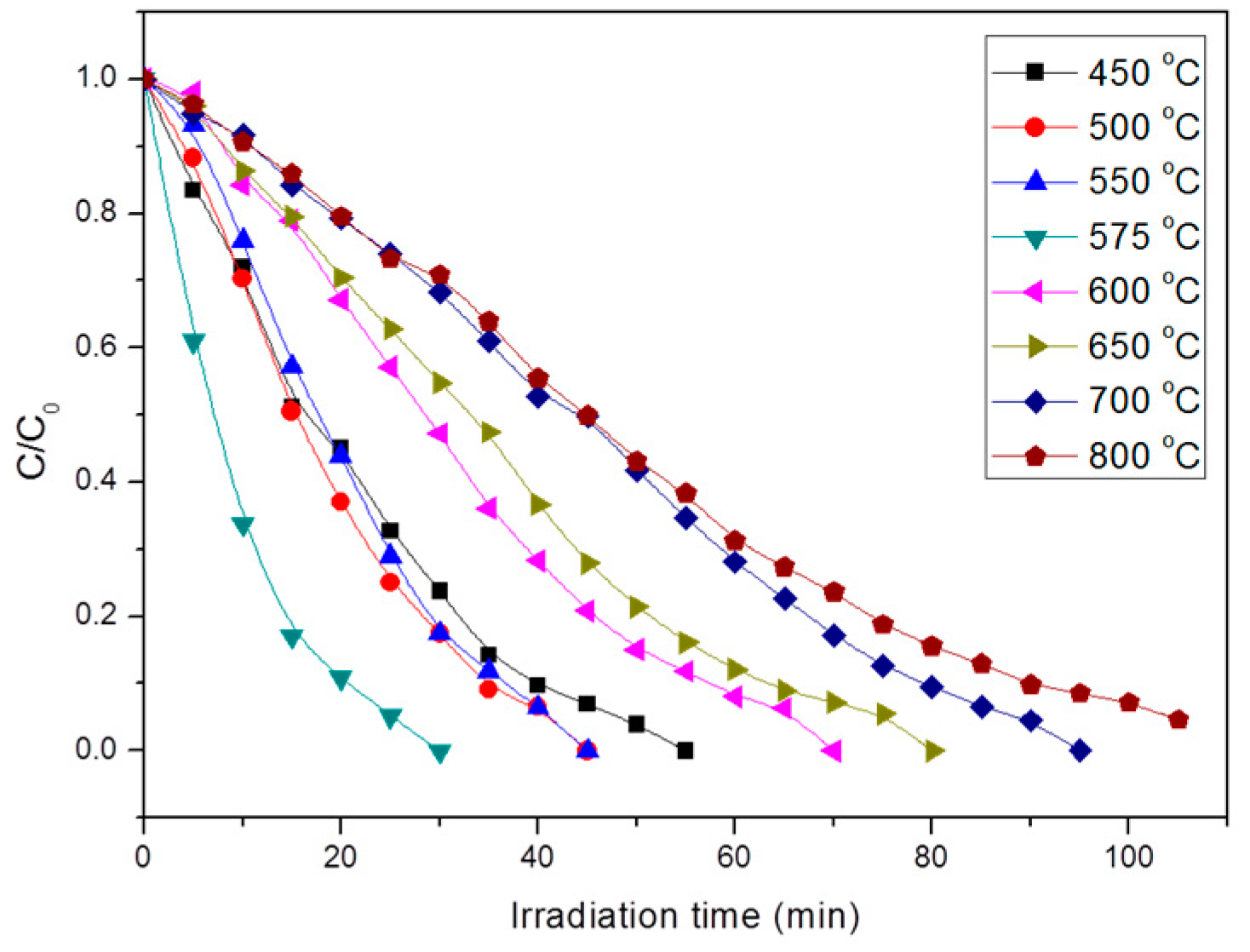
3.2. Photocatalytic Test of Mixed Crystalline TiO2 Nanofibers
3.3. Characterization and Analysis of Core-Shell Structure TiO2 Nanofibers
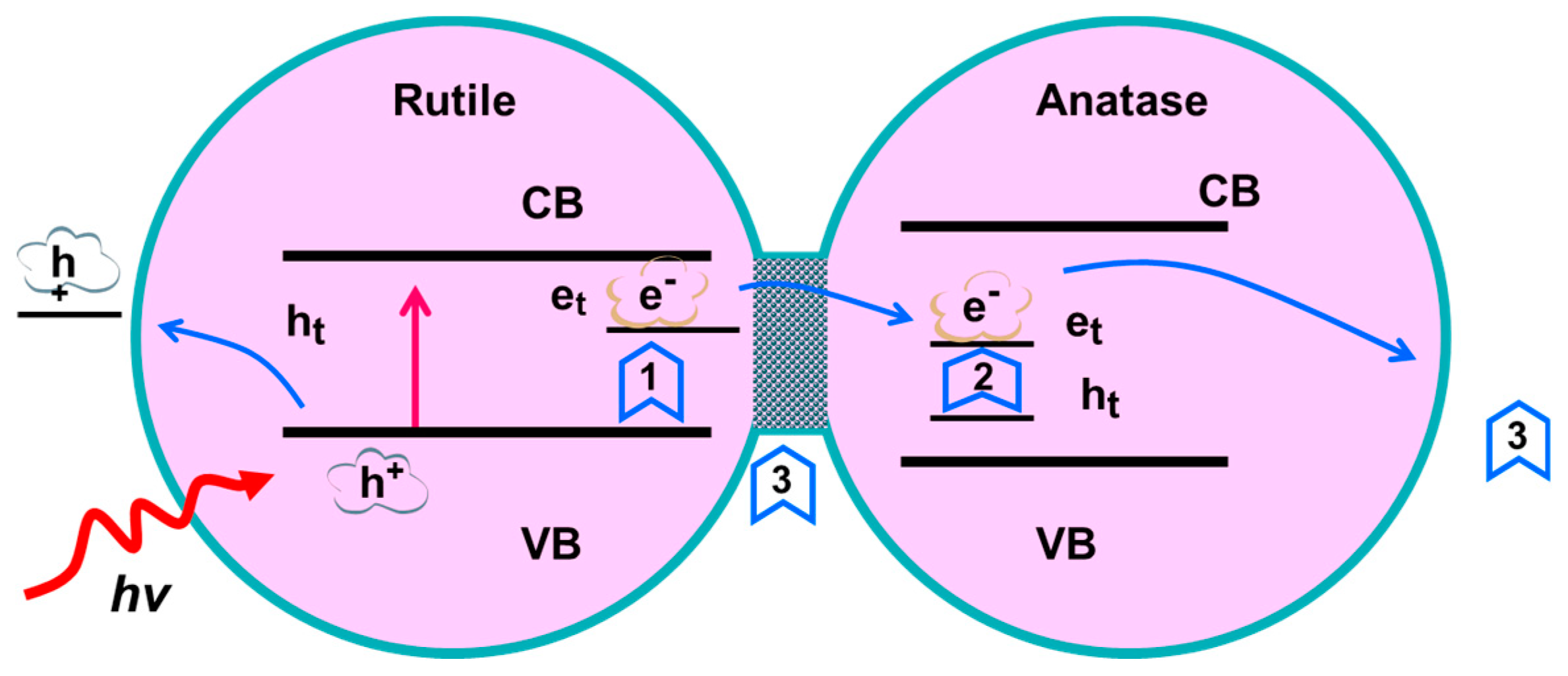
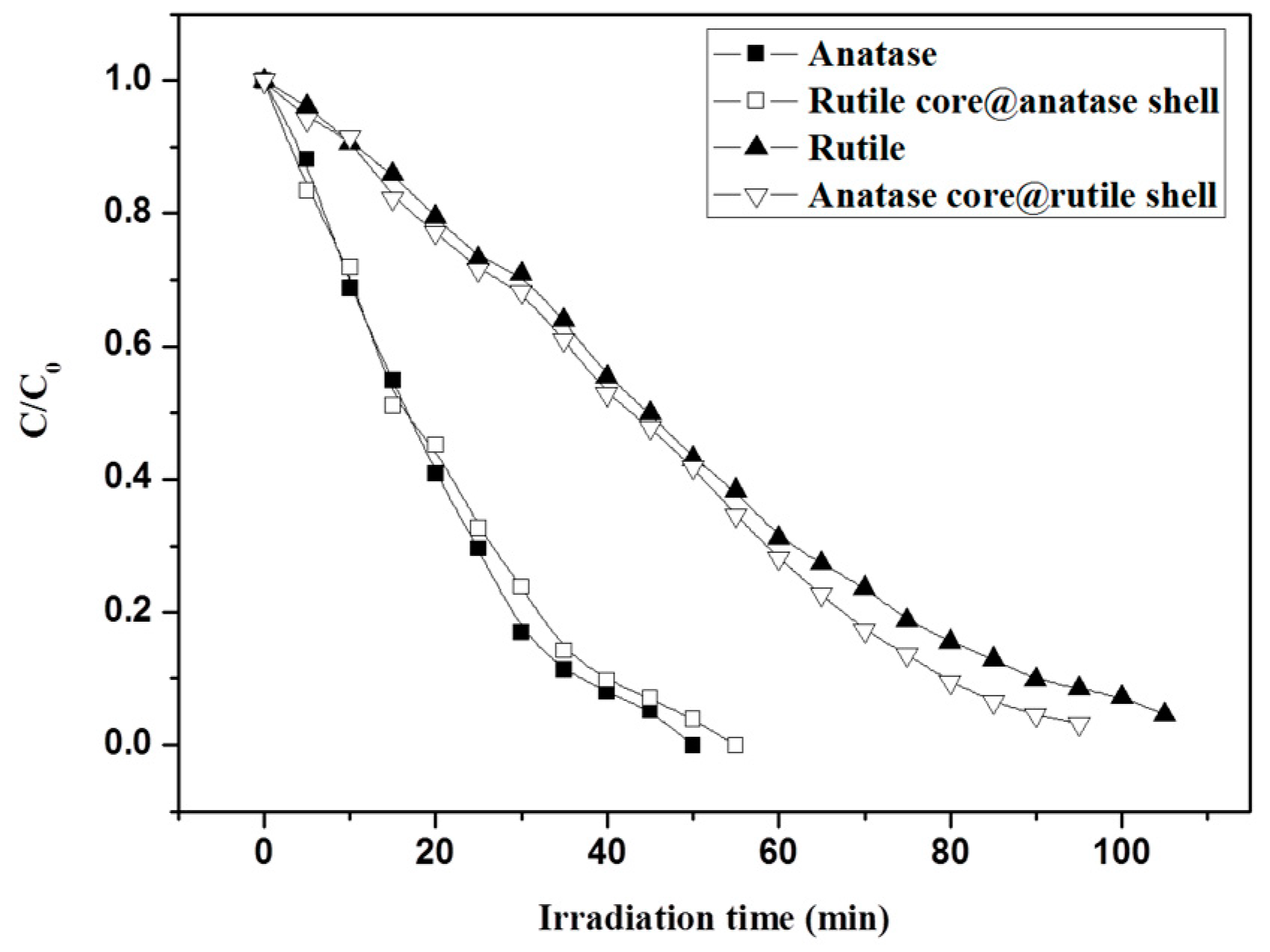
3.4. Photocatalytic Test of Core-Shell Structure TiO2 Nanofibers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasikhudin; Ismaya, E.P.; Diantoro, M.; Kusumaatmaja, A.; Triyana, K. Preparation of PVA/TiO2 composites nanofibers by using electrospinning method for photocatalytic degradation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012011. [Google Scholar] [CrossRef]
- Compagnoni, M.; Ramis, G.; Freyria, F.S.; Armandi, M.; Bonelli, B.; Rossetti, I. Photocatalytic processes for the abatement of N-containing pollutants from waste water. part 1: Inorganic pollutants. J. Nanosci. Nanotechnol. 2017, 17, 3632–3653. [Google Scholar] [CrossRef]
- Leudjo, T.A.; Pillay, K.; Yangkou, M.X. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Wei, Y.; Zhang, M.; Jiang, H.; Li, J.; Li, S.; Li, J. Electrospun TiO2 nanofibers surface-loaded with Ag nanoparticles as a sensitizer and their enhanced effect in photocatalytic applications. Eur. J. Inorg. Chem. 2015, 2015, 5039–5044. [Google Scholar] [CrossRef]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Su, C.; Yang, X.; Hu, J.; Lin, X. Ag-AgBr nanoparticles loaded on TiO2 nanofibers as an efficient heterostructured photocatalyst driven by visible light. J. Mol. Catal. A Chem. 2015, 410, 226–234. [Google Scholar] [CrossRef]
- Gong, C.; Du, J.; Li, X.; Yu, Z.; Ma, J.; Qi, W.; Zhang, K.; Yang, J.; Luo, M.; Peng, H. One-step acidic hydrothermal preparation of dendritic rutile TiO2 nanorods for photocatalytic performance. Nanomaterials 2018, 8, 683. [Google Scholar] [CrossRef]
- Wu, X.; Si, Y.; Yu, J.; Ding, B. Titania-based electrospun nanofibrous materials: A new model for organic pollutants degradation. MRS Commun. 2018, 8, 765–781. [Google Scholar] [CrossRef]
- Siah, W.R.; Lintang, H.O.; Shamsuddin, M.; Yuliati, L. High photocatalytic activity of mixed anatase-rutile phases on commercial TiO2 nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012005. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, M.; Zhang, X.; Wang, H.; Wei, Z.; Du, Q. Multi-type carbon doping of TiO2 photocatalyst. Chem. Phys. Lett. 2007, 444, 292–296. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Zhang, H.; Zhao, H.; Yu, H.; Xu, J.; Fu, Y.; Yang, D.; Liu, Y. Hydrothermal fabrication of rutile TiO2 submicrospheres on wood surface: An efficient method to prepare UV-protective wood. Mater. Chem. Phys. 2012, 133, 253–258. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernandez-Rodriguez, C.; Dona-Rodriguez, J.M.; Dionysiou, D.D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO2 photocatalysts. Appl. Catal. B Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Woll, C. Photocatalytic activity of bulk TiO2 anatase and rutile single crystals using infrared absorption spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Wu, S.; Peng, C.; Ji, H. Comparison of TiO2 degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem. Eng. J. 2014, 243, 254–264. [Google Scholar] [CrossRef]
- Liu, G.; Yan, X.; Chen, Z.; Wang, X.; Wang, L.; Lu, G.Q.; Cheng, H.M. Synthesis of rutile–anatase core–shell structured TiO2 for photocatalysis. J. Mater. Chem. 2009, 19, 6590–6596. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, W.; Li, Z.; Xiong, Y. Surface and interface engineering in photocatalysis. ChemNanoMat 2015, 1, 223–239. [Google Scholar] [CrossRef]
- Su, C.; Shao, C.; Liu, Y. Electrospun nanofibers of TiO2/CdS heteroarchitectures with enhanced photocatalytic activity by visible light. J. Colloid Interf. Sci. 2011, 359, 220–227. [Google Scholar] [CrossRef]
- Lee, J.A.; Krogman, K.C.; Ma, M.; Hill, R.M.; Hammond, P.T.; Rutledge, G.C. Highly reactive multilayer-assembled TiO2 coating on electrospun polymer nanofibers. Adv. Mater. 2009, 21, 1252–1256. [Google Scholar] [CrossRef]
- Vu, D.; Li, X.; Li, Z.; Wang, C. Phase-structure effects of electrospun TiO2 nanofiber membranes on as(III) adsorption. J. Chem. Eng. Data 2013, 58, 71–77. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Barakat, N.A.M.; Sheikh, F.A.; Kim, H.Y. Electronic characterization and photocatalytic properties of TiO2/CdO electrospun nanofibers. J. Mater. Sci. 2010, 45, 1272–1279. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Electrospun TiO2/SnO2 nanofibers with innovative structure and chemical properties for highly efficient photocatalytic H2 generation. Int. J. Hydrogen. Energy 2012, 37, 10575–10584. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Doh, S.J.; Kim, C.; Lee, S.G.; Lee, S.J.; Kim, H. Development of photocatalytic TiO2 nanofibers by electrospinning and its application to degradation of dye pollutants. J. Hazard. Mater. 2008, 154, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Chen, D.; Jiao, X.; Tao, C. Long TiO2 hollow fibers with mesoporous walls: Sol−gel combined electrospun fabrication and photocatalytic properties. J. Phys. Chem. B 2006, 110, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.C.; Leung, W.F. Enhanced photocatalytic activity of electrospun TiO2/ZnO nanofibers with optimal anatase/rutile ratio. Catal. Commun. 2013, 37, 100–104. [Google Scholar] [CrossRef]
- Riaz, A.; Qi, H.; Fang, Y.; Xu, J.; Zhou, C.; Jin, Z.; Hong, Z.; Zhi, M.; Liu, Y. Enhanced intrinsic photocatalytic activity of TiO2 electrospun nanofibers based on temperature assisted manipulation of crystal phase ratios. J. Mater. Res. 2006, 31, 3036–3043. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, M.; Feng, Z.; Li, C. UV Raman spectroscopic study on TiO2. II. effect of nanoparticle size on the outer/inner phase transformations. J. Phys. Chem. C 2009, 113, 1698–1704. [Google Scholar] [CrossRef]
- Watson, S.; Beydoun, D.; Scott, J.; Amal, R. Preparation of nanosized crystalline TiO2 particles at low temperature for photocatalysis. J. Nanopart. Res. 2004, 6, 193–207. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Crist, S.E.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Probing reaction mechanisms in mixed phase TiO2 by EPR. J. Electron Spectrosc. Relat. Phenom. 2006, 150, 155–163. [Google Scholar] [CrossRef]
- Miyagi, T.; Kamei, M.; Mitsuhashi, T.; Ishigaki, T.; Yamazaki, A. Charge separation at the rutile/anatase interface: A dominant factor of photocatalytic activity. Chem. Phys. Lett. 2004, 390, 399–402. [Google Scholar] [CrossRef]
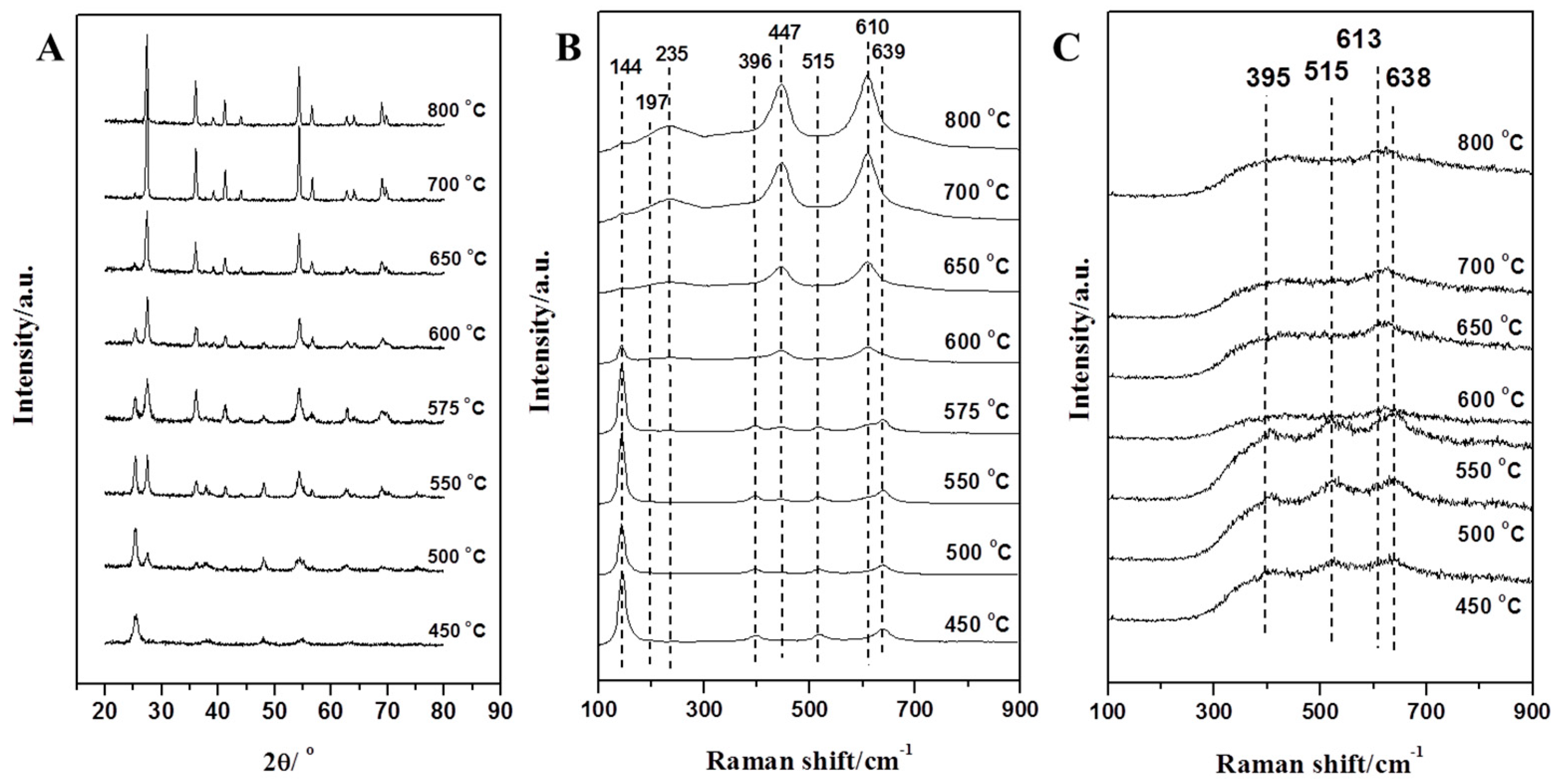
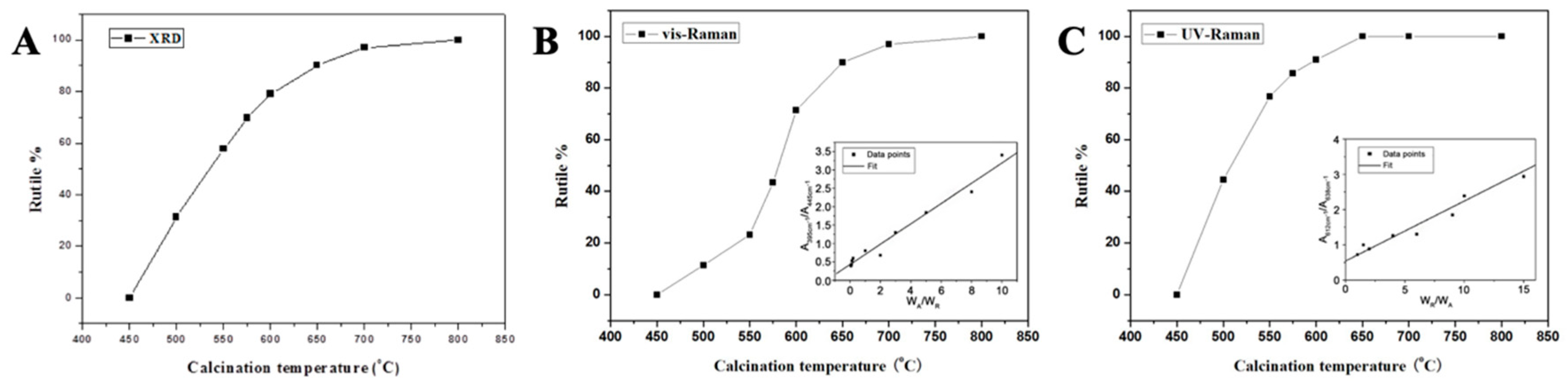
| Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|
| Phase | 450 °C | 500 °C | 550 °C | 575 °C | 600 °C | 650 °C | 700 °C | 800 °C |
| Anatase | 100 | 68.71 | 42.33 | 30.22 | 20.99 | 9.84 | 2.94 | 0 |
| Rutile | 0 | 31.29 | 57.67 | 69.78 | 79.01 | 90.16 | 97.06 | 100 |
| Anatase core@rutile Shell | Rutile core@anatase Shell | |||
|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | |
| XRD | 65.2 | 34.8 | 8.8 | 91.2 |
| vis-Raman | 91.1 | 8.9 | 0 | 100 |
| UV-Raman | 0 | 100 | 92.7 | 7.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yu, M.; Qin, X. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials 2019, 9, 535. https://doi.org/10.3390/nano9040535
Zhang H, Yu M, Qin X. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials. 2019; 9(4):535. https://doi.org/10.3390/nano9040535
Chicago/Turabian StyleZhang, Hongnan, Ming Yu, and Xiaohong Qin. 2019. "Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters" Nanomaterials 9, no. 4: 535. https://doi.org/10.3390/nano9040535
APA StyleZhang, H., Yu, M., & Qin, X. (2019). Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials, 9(4), 535. https://doi.org/10.3390/nano9040535






