Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of WO3 H2O Nanosheets, SnO2 Nanoparticles and Their Composite
2.2. Material Characterization
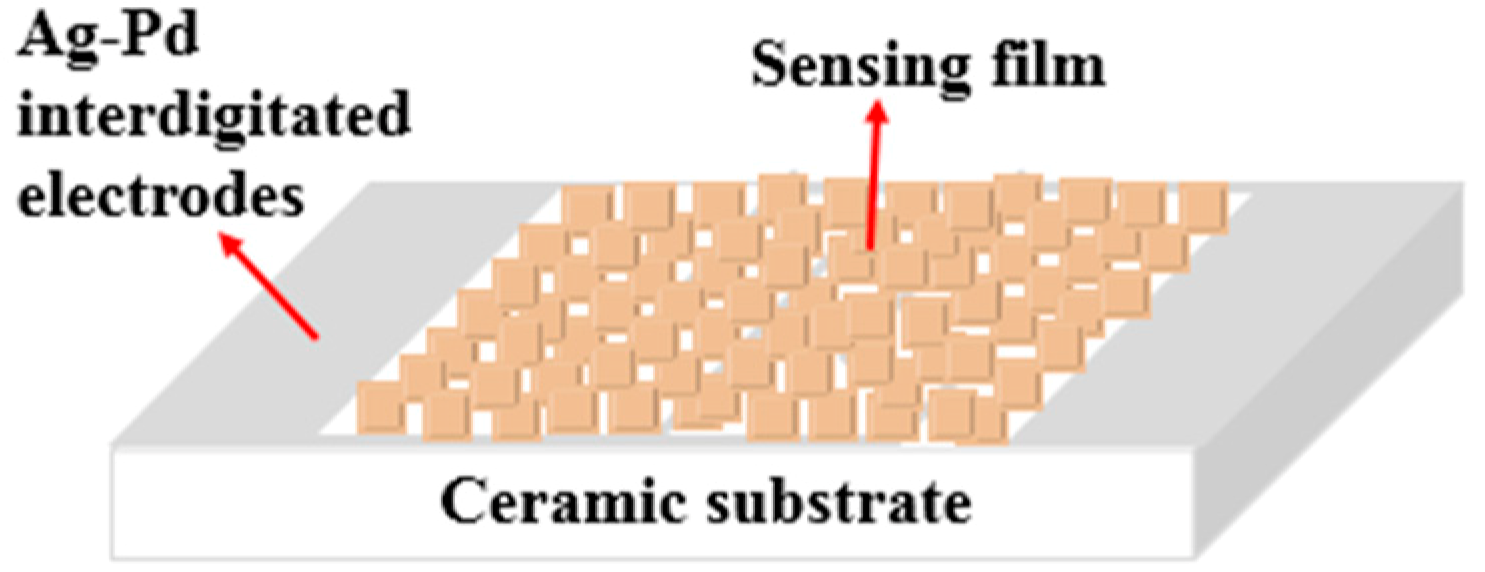
2.3. Gas-Sensing Measurement
3. Results and Discussion
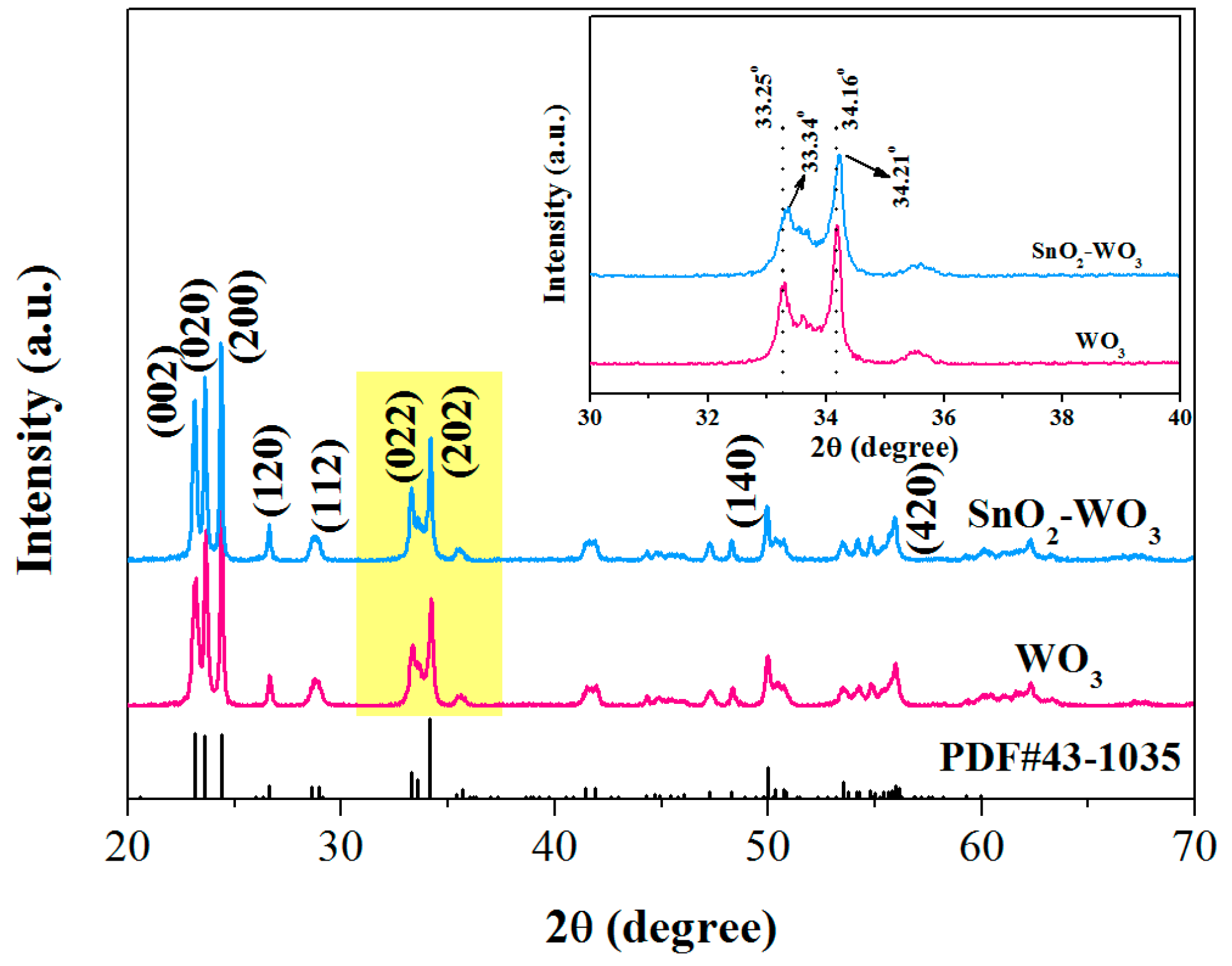
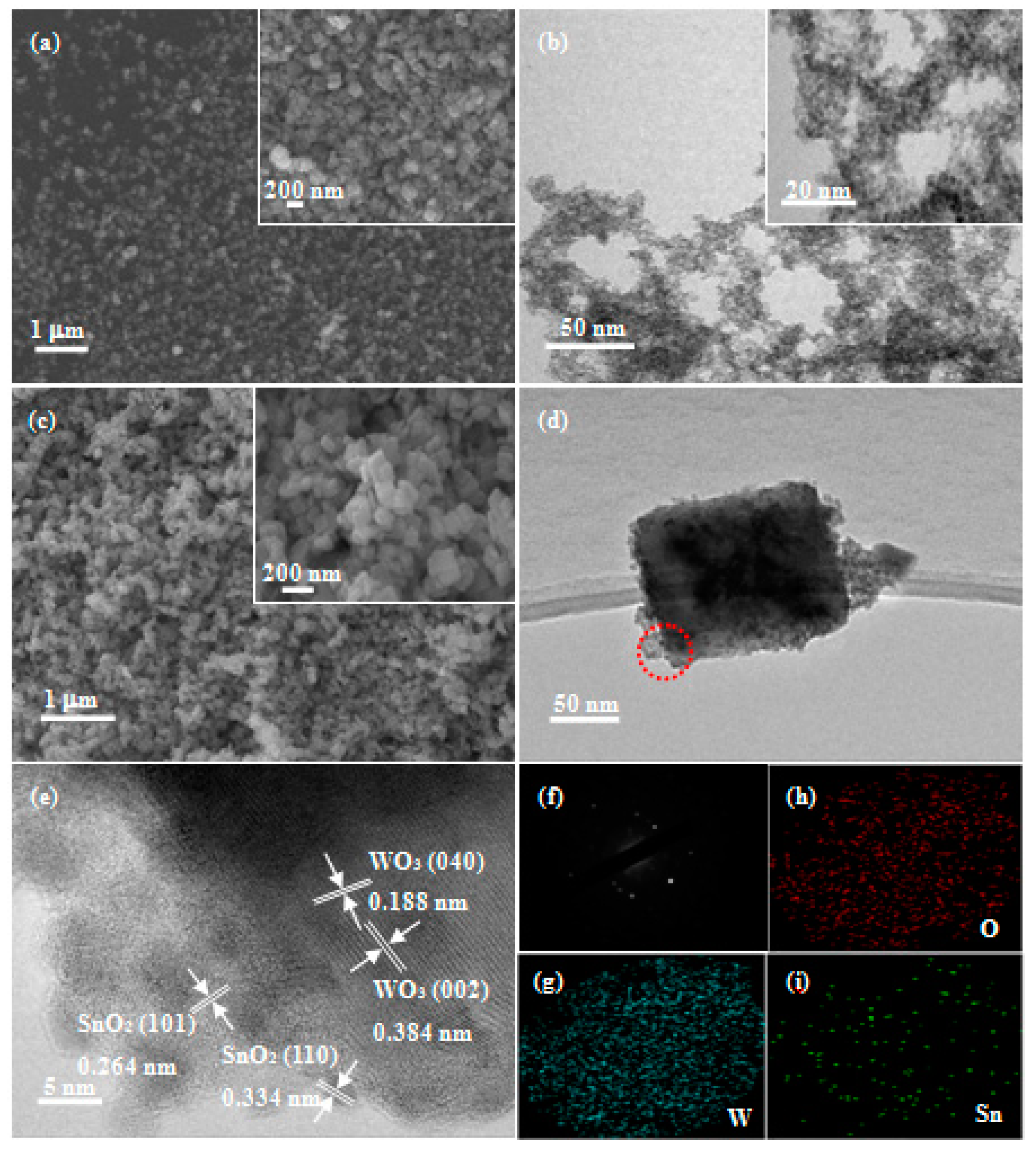
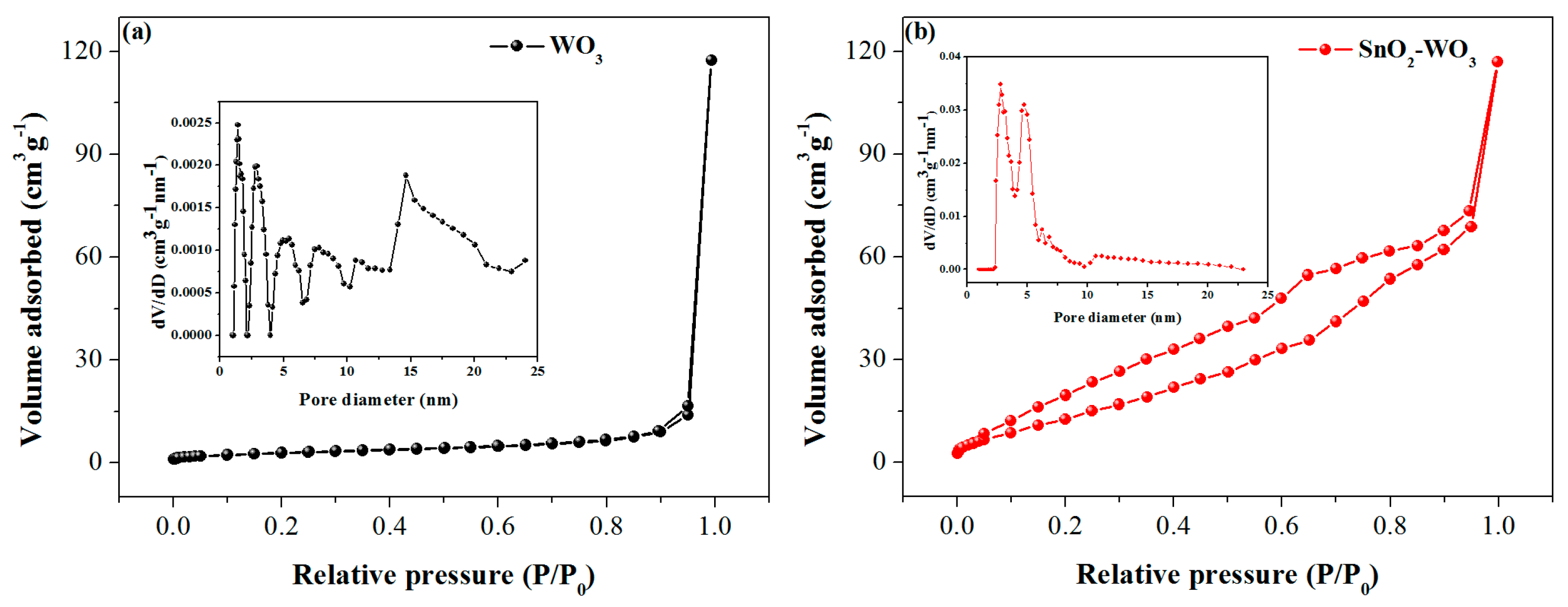
3.1. Characterizations of the As-Prepared Samples
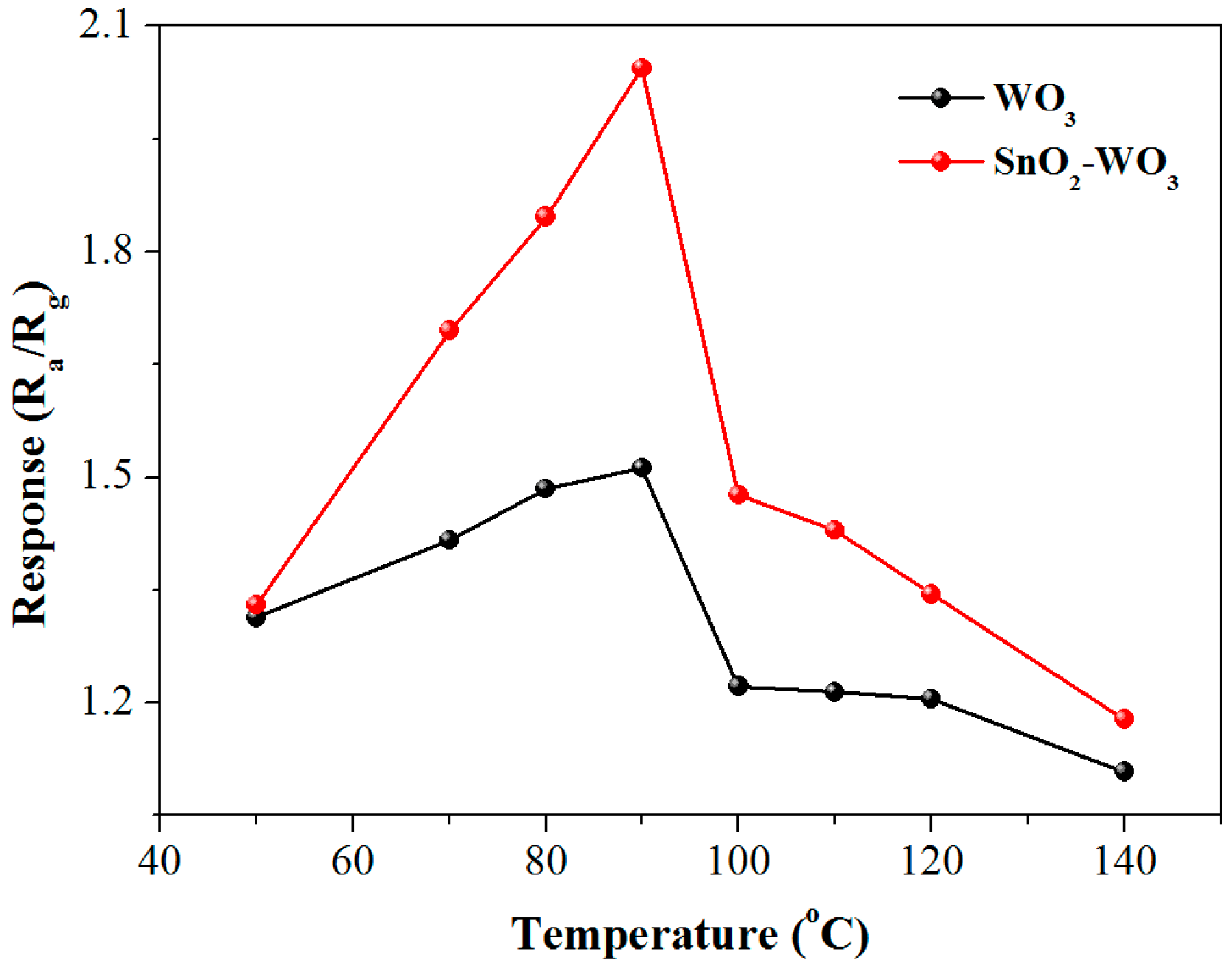
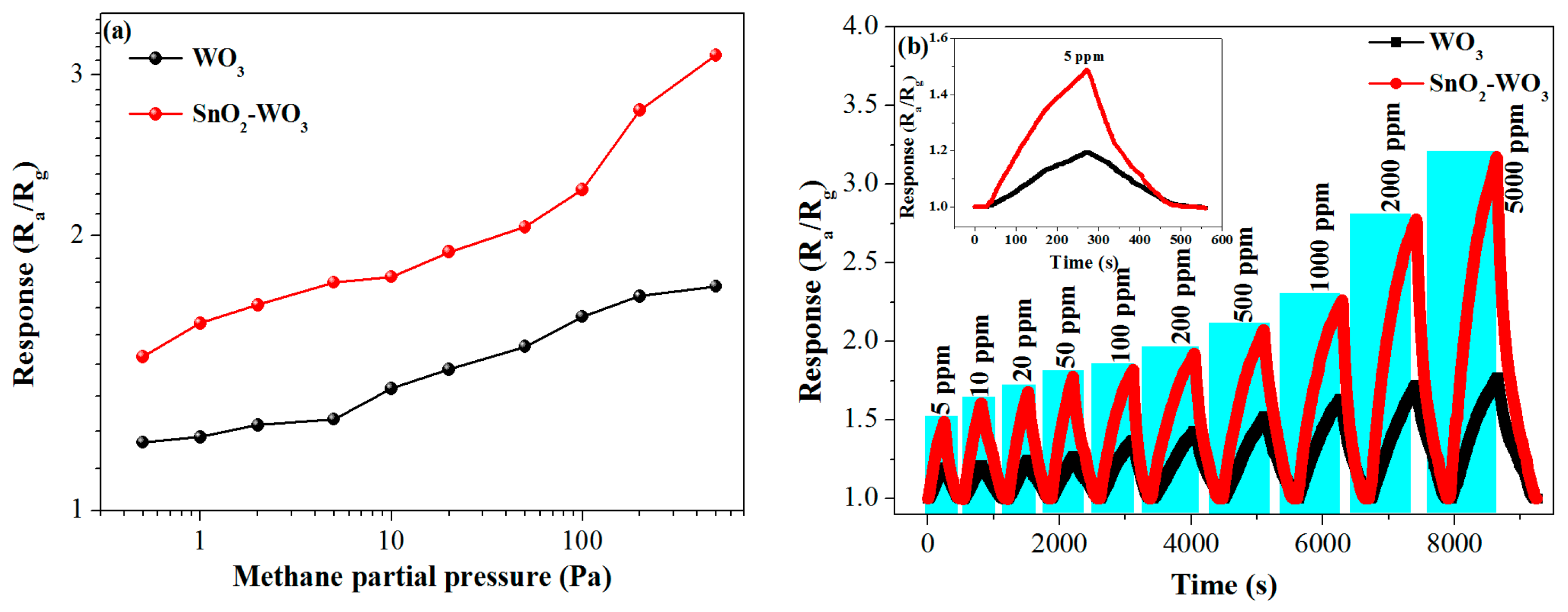
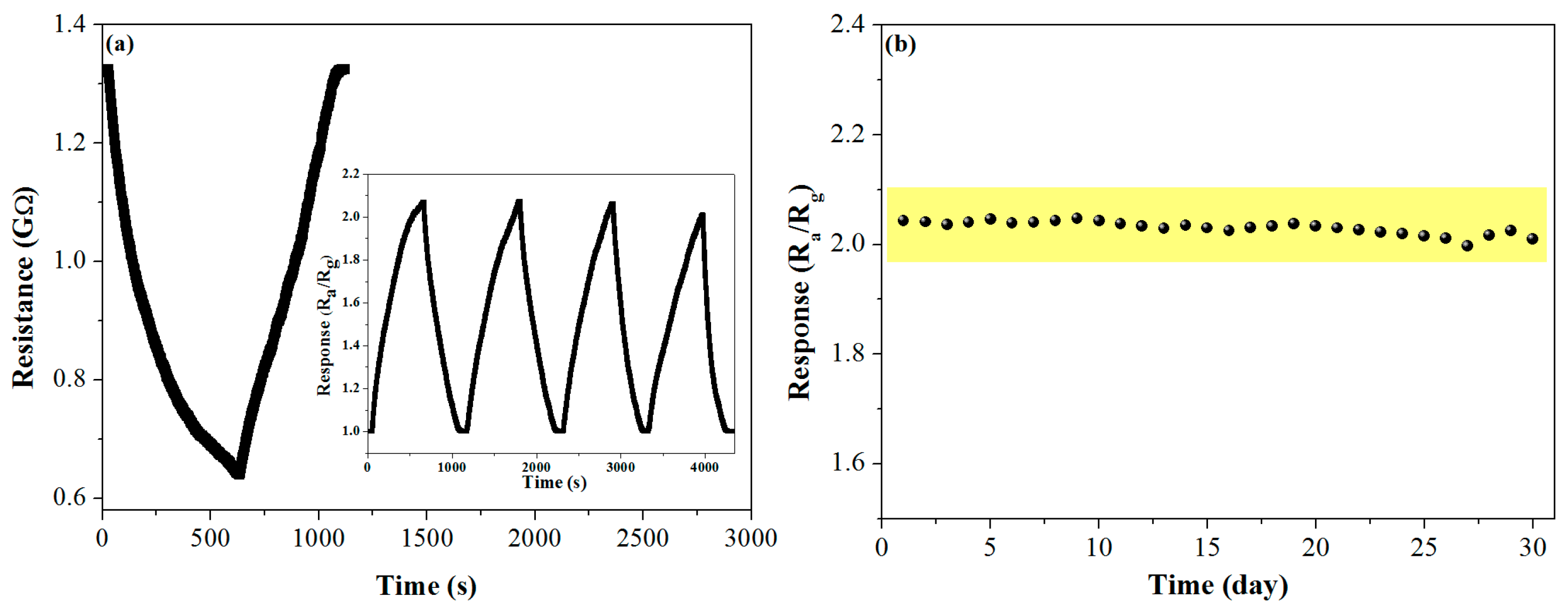
3.2. Gas-Sensing Properties
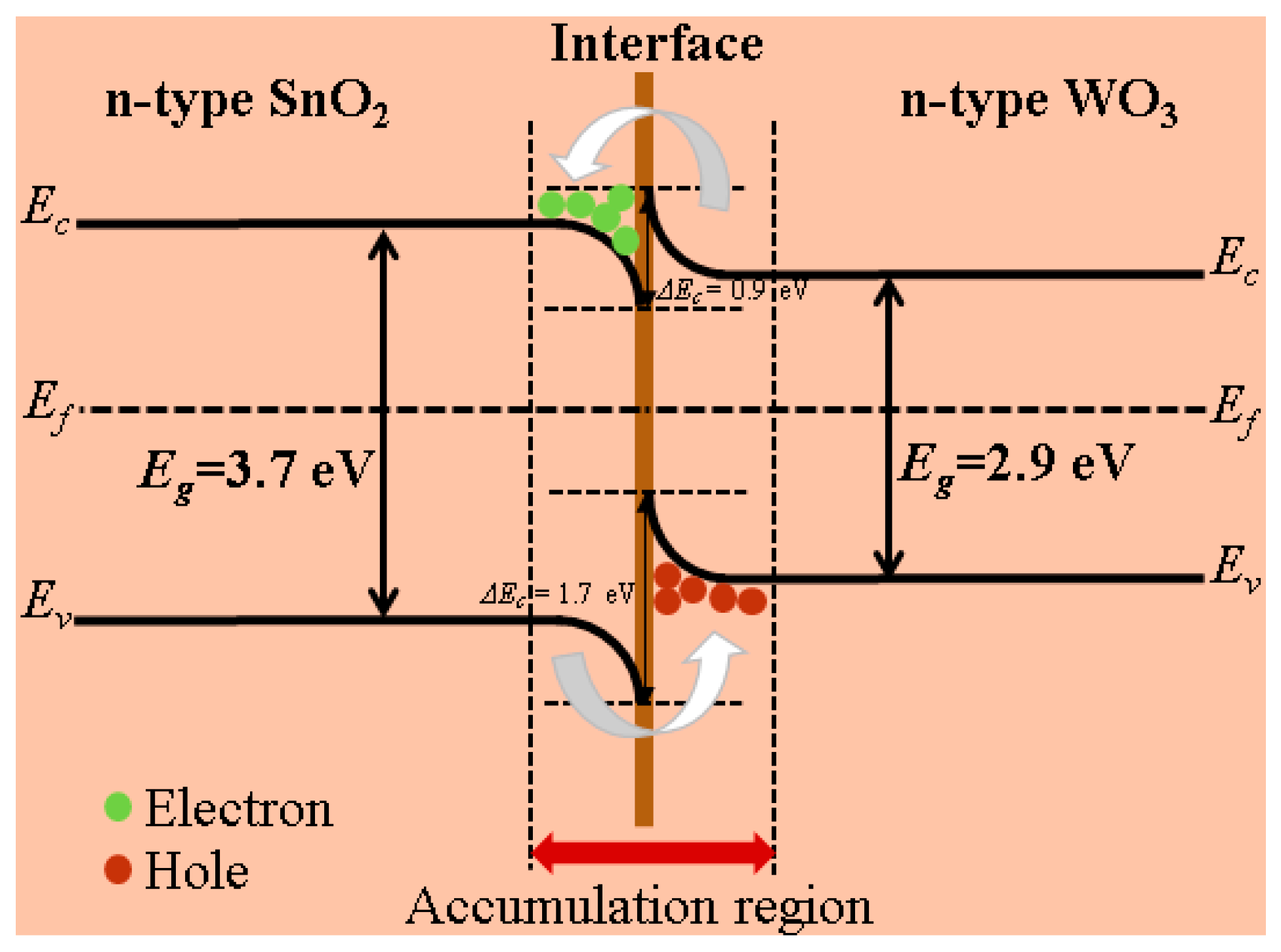
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, J.L.; Qin, C.; Wang, Y.; Zhang, H.L.; Zhang, B.; Gong, Y.X.; Wang, X.D.; Sun, G.; Bala, H.; Zhang, Z.Y. Synthesis of g-C3N4 nanosheet modified SnO2 composites with improved performance for ethanol gas sensing. RSC Adv. 2007, 7, 25504–25511. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.-S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Amutha, A.; Amirthapandian, S.; Prasad, A.K.; Panigrahi, B.K.; Thangadurai, P. Methane gas sensing at relatively low operating temperature by hydrothermally prepared SnO2 nanorods. J. Nanopart. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.H.; Tohidi, M.; Zarifkar, A. Methane gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Gao, T.; Su, X.; Wan, F. Pd-doped SnO2-based sensor detecting characteristic fault hydrocarbon gases in transformer oil. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Kim, K.; Choi, K.; Jeong, H.; Kim, H.J.; Kim, H.P.; Lee, J. Highly sensitive and selective trimethylamine sensors using Ru-doped SnO2 hollow spheres. Sens. Actuators B Chem. 2012, 166–167, 733–738. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.; Gong, M. Gas-sensing properties of sensors based on single crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B Chem. 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Tomer, V.K.; Devi, S.; Malik, R.; Nehra, S.P.; Duhan, S. Fast response with high performance humidity sensing of Ag-SnO2 /SBA-15 nanohybrid sensors. Microporous Mesoporous Mater. 2016, 219, 240–248. [Google Scholar] [CrossRef]
- Lai, X.; Shen, G.; Xue, P.; Yan, B.; Wang, H.; Li, P.; Xia, W.; Fang, J. Ordered mesoporous NiO with thin pore walls and its enhanced sensing performance for formaldehyde. Nanoscale 2015, 7, 4005–4012. [Google Scholar] [CrossRef] [PubMed]
- Lepoutre, S.; López, B.J.; Sanchez, C.; Amenitsch, H.; Linden, M.; Grosso, D. Nanocasted mesoporous nanocrystalline ZnO thin films. J. Mater. Chem. 2010, 20, 537–542. [Google Scholar] [CrossRef]
- Duhan, S.; Tomer, V.K. Mesoporous silica: Making “sense” of sensors. In Advanced Sensor and Detection Materials; Tiwari, A., Demir, M.M., Eds.; Scrivener Publishing: Linkoping, Sweden, 2014; pp. 149–192. [Google Scholar]
- Zhao, J.; Wang, W.; Liu, Y.; Ma, J.; Li, X.; Du, Y.; Lu, G. Ordered mesoporous Pd/SnO2 synthesized by a nanocasting route for high hydrogen sensing performance. Sens. Actuators B 2011, 160, 604–608. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Feng, D.; Zhuo, M.; Zhang, M.; Zhang, E.; Xu, Z.; Li, Q.; Wang, T. Superior ethanol-sensing properties based on Ni-doped SnO2 p-heterojunction hollow spheres. Sens. Actuators B 2012, 166, 61–67. [Google Scholar] [CrossRef]
- Akiyama, M.; Tamaki, J.; Miura, N.; Yamazoe, N. Tungsten oxide-based semiconductor sensor highly sensitive to NO and NO2. Chem. Lett. 1991, 1991, 1611–1614. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Tamaki, J.; Hashishin, T.; Uno, Y.; Dao, D.V.; Sugiyama, S. Ultrahigh-sensitive WO3 nanosensor with interdigitated Au nanoelectrode for NO2 detection. Sens. Actuators B 2008, 132, 234–238. [Google Scholar] [CrossRef]
- Choi, Y.G.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Wet process- based fabrication of WO3 thin film for NO2 detection. Sens. Actuators B 2004, 101, 107–111. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, J.; Yu, M.; Lu, P.; Wei, J.; Qian, Y.; Wang, Y.; Yu, C. Green synthesis of hexagonal-shaped WO3 0.33 H2O nanodiscs composed of nanosheets. Cryst. Growth Des. 2008, 8, 3993–3998. [Google Scholar] [CrossRef]
- Liu, Z.; Miyauchi, M.; Yamazaki, T.; Shen, Y. Facile synthesis and NO2 gas sensing of tungsten oxide nanorods assembled microspheres. Sens. Actuators B 2009, 140, 514–519. [Google Scholar] [CrossRef]
- Cao, B.; Chen, J.; Tang, X.; Zhou, W. Growth of monoclinic WO3 nanowire array for highly sensitive NO2 detection. J. Mater. Chem. 2009, 19, 2323–2327. [Google Scholar] [CrossRef]
- Zhu, L.F.; She, J.C.; Luo, J.Y.; Deng, S.Z.; Chen, J.; Xu, N.S. Study of physical and chemical processes of H2 sensing of Pt-coated WO3 nanowire films. J. Phys. Chem. C 2010, 114, 15504–15509. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kang, Y.C.; Lee, J.H. Highly selective and sensitive detection of trimethylamine using WO3 hollow spheres prepared by ultrasonic spray pyrolysis. Sens. Actuators B 2013, 176, 971–977. [Google Scholar] [CrossRef]
- Li, X.L.; Lou, T.J.; Sun, X.M.; Li, Y.D. Highly sensitive WO3 hollow-sphere gas sensors. Inorg. Chem. 2004, 43, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Zhen, L.; Shao, W.Z.; Chen, Z.L. Sodium chloride induced formation of square-shaped cadmium molybdate nanoplates. Mater. Lett. 2014, 131, 292–294. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ding, E.J.; Xu, S.C.; Li, Z.H.; Wang, X.X.; Song, F. Sensitization of an optical fiber methane sensor with graphene. Opt. Fiber Technol. 2017, 37, 26–29. [Google Scholar] [CrossRef]
- Alaya, M.N.; Rabah, M.A. Surface acidity and catalytic activity of aged SO42− /SnO2 catalyst supported with WO3. J. Alloys Compd. 2013, 575, 285–291. [Google Scholar] [CrossRef]
- Liu, L.; Song, P.; Wei, Q.; Zhong, X.; Yang, Z.; Wang, Q. Synthesis of porous SnO2 hexagon nanosheets loaded with Au nanoparticles for high performance gas sensors. Mater. Lett. 2017, 201, 211–215. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.; Ju, D.; Cao, B. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuators B Chem. 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Koinkar, P.M.; Maiti, N.; Sonawane, K.M. Synthesis of Ag doped SnO2 thin films for the evaluation of H2S gas sensing properties. Phys. B Condens. Matter 2017, 524, 90–96. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Yang, J.; Wang, S. Pt nanoparticles functionalized 3D SnO2 nanoflowers for gas sensor application. Solid. State. Electron. 2017, 130, 20–27. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Chen, N.; Xing, X.; Liu, X.; Xiao, X.; Wang, Y. Pd nanoparticles composited SnO2 microspheres as sensing materials for gas sensors with enhanced hydrogen response performances. J. Alloys Compd. 2017, 710, 216–224. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2017, 204, 250–272. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Sun, G.; Jia, T.K.; Jia, L.; Zhang, F.M.; Lin, L.; Zhang, B.; Cao, J.L.; Zhang, Z. Carbon nitride decorated ball-flower like Co3O4 hybrid composite: Hydrothermal synthesis and ethanol gas sensing application. Nanomaterials 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.L.; Gong, Y.X.; Wang, Y.; Zhang, B.; Zhang, H.L.; Sun, G.; Bala, H.; Zhang, Z.Y. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. [Google Scholar] [CrossRef]
- Xue, D.P.; Wang, Y.; Cao, J.L.; Zhang, Z.Y. Hydrothermal synthesis of CeO2-SnO2 nano-flowers for improving triethylamine gas sensing property. Nanomaterials 2018, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chen, H.; Li, Y.; Chen, Z.; Zhang, S.; Ma, G.; Jia, T.; Cao, J.; Bala, H.; Wang, X.; et al. Synthesis and improved gas sensing properties of NiO-decorated SnO2 microflowers assembled with porous nanorods. Sens. Actuators B Chem. 2016, 233, 180–192. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Q.S.; Zhan, X.Y.; Wang, F.M.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, Q.; Wang, Z.; Zhang, R.; Gao, Y.; Sun, P.; Sun, Y.; Lu, G. Improvement of NO2 gas sensing performance based on discoid tin oxide modified by reduced graphene oxide. Sens. Actuators B 2016, 227, 419–426. [Google Scholar] [CrossRef]
- Mizsei, J. Semiconducting gas sensor incorporating a sparking decomposer. Sens. Actuators B 1990, 2, 199–203. [Google Scholar] [CrossRef]
- Mizsei, J. How can sensitive and selective semiconductor gas sensors be made? Sens. Actuators B 1995, 23, 173–176. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Askarieh, M.; Carlo, A.D.; Agresti, A. Facile synthesis of a SnO2@rGO nanohybrid and optimization of its methane-sensing parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Faglia, G.; Sberveglieri, G. Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC Adv. 2016, 6, 34225–34232. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.; Wang, J.; Gang, D.D.; Islam, F.; Lian, Q.; Zappi, M.E. Neodymium embedded ordered mesoporous carbon (OMC) for enhanced adsorption of sunset yellow: Characterizations, adsorption study and adsorption mechanism. Chem. Eng. J. 2019, 359, 814–826. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Kida, T.; Nishiyama, A.; Hua, Z.; Suematsu, K.; Yuasa, M.; Shimanoe, K. WO3 nanolamella gas sensor: Porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir 2014, 30, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, H. Nanorods assembled hierarchical urchin-like WO3 nanostructures: Hydrothermal synthesis, characterization, and their gas sensing properties. J. Alloys Compd. 2017, 702, 644–648. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 nanosheet composites: Hydrothermal synthesis and gas sensing mechanism. J. Alloys Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Jia, Q.-Q.; Ji, H.-M.; Wang, D.-H.; Bai, X.; Sun, X.-H.; Jin, Z.-G. Exposed facets induced enhanced acetone selective sensing property of nanostructured tungsten oxide. J. Mater. Chem. A 2014, 2, 13602–13611. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Xie, S.; Li, H. Hexagonal single crystal growth of WO3 nanorods along a [110] axis with enhanced adsorption capacity. Chem. Commun. 2011, 47, 4403–4405. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Han, X.; Li, L.; Wang, C. Controlling the morphologies of WO3 particles and tuning the gas sensing properties. New J. Chem. 2012, 36, 2205–2208. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, D.; Wang, F.; Zhao, Q.; Zhai, C.; Zhang, M. Preparation of hierarchical WO3 dendrites and their applications in NO2 sensing. J. Alloys Compd. 2017, 43, 8183–8189. [Google Scholar]
- Li, H.; Liu, B.; Cai, D.P.; Wang, Y.; Liu, Y.; Mei, L.; Wang, L.; Wang, D.; Li, Q.; Wang, T. High-temperature humidity sensors based on WO3-SnO2 composite hollow nanospheres. J. Mater. Chem. A 2014, 2, 6854–6862. [Google Scholar] [CrossRef]
- Wang, F.; di Valentin, C.; Pacchioni, G. Electronic and structural properties of WO3: A systematic hybrid DFT study. J. Phys. Chem. C 2011, 115, 8345–8353. [Google Scholar] [CrossRef]
- Floriano, E.A.; Scalvi, L.V.A.; Saeki, M.J.; Sambrano, J.R. Preparation of TiO2/SnO2 thin films by sol-gel method and periodic B3LYP simulations. J. Phys. Chem. A 2014, 118, 5857–5865. [Google Scholar] [CrossRef] [PubMed]
- Sukunta, J.K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.K.; Liewhiran, C.K. WO3 nanotubes-SnO2 nanoparticles heterointerfaces for ultrasensitive and selective NO2 detections. Appl. Surf. Sci. 2018, 458, 319–332. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, D.; Wang, J.; Wang, Y.; Sun, G.; Cao, J.; Bala, H.; Zhang, Z. Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials 2019, 9, 351. https://doi.org/10.3390/nano9030351
Xue D, Wang J, Wang Y, Sun G, Cao J, Bala H, Zhang Z. Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials. 2019; 9(3):351. https://doi.org/10.3390/nano9030351
Chicago/Turabian StyleXue, Dongping, Junjun Wang, Yan Wang, Guang Sun, Jianliang Cao, Hari Bala, and Zhanying Zhang. 2019. "Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles" Nanomaterials 9, no. 3: 351. https://doi.org/10.3390/nano9030351
APA StyleXue, D., Wang, J., Wang, Y., Sun, G., Cao, J., Bala, H., & Zhang, Z. (2019). Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials, 9(3), 351. https://doi.org/10.3390/nano9030351






