Metal-Support Cooperative Effects in Au/VPO for the Aerobic Oxidation of Benzyl Alcohol to Benzyl Benzoate
Abstract
1. Introduction
2. Materials and Methods
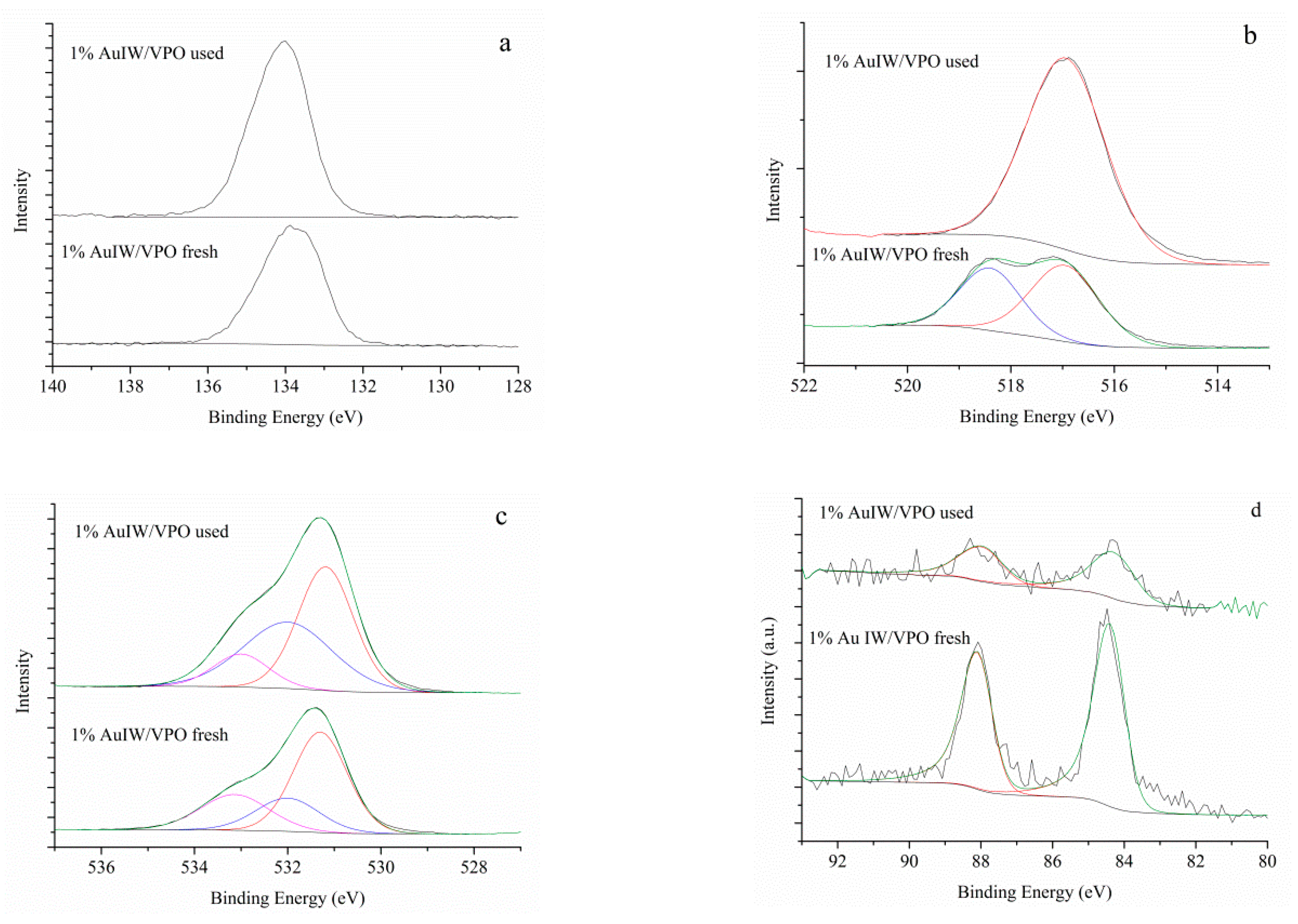
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Hutchings, G.J. Selective liquid phase oxidation with supported metal nanoparticles. Chem. Sci. 2012, 3, 20–44. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, B.; Wang, X.; Zhao, J.; Wang, X.; Cai, Q. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by biphasic catalysis. Chem. Eng. J. 2010, 162, 738–742. [Google Scholar] [CrossRef]
- Luo, J.; Yu, H.; Wang, H.; Wang, H.; Peng, F. Aerobic oxidation of benzyl alcohol to benzaldehyde catalyzed by carbon nanotubes without any promoter. Chem. Eng. J. 2014, 240, 434–442. [Google Scholar] [CrossRef]
- Alberici, F.; Pagani, L.; Ratti, G.; Viale, P. Ivermectin alone or in combination with benzyl benzoate in the treatment of human immunodeficiency virus-associated scabies. Br. J. Dermatol. 2000, 142, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Zheng, Q.K.; Zheng, J.Q. Effect of Carrier on the Structure and Performance of Polyphenylene Sulfide Fiber. Adv. Mater. Res. 2012, 554–556, 147–152. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Rossetti, I.; Villa, A.; Prati, L. Benzyl alcohol oxidation on carbon-supported Pd nanoparticles: Elucidating the reaction mechanism. ChemCatChem 2015, 6, 3464–3473. [Google Scholar] [CrossRef]
- Enache, D.I.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using supported gold catalysts. Catal. Lett. 2005, 103, 43–52. [Google Scholar] [CrossRef]
- Cao, E.; Sankar, M.; Firth, S.; Lam, K.F.; Bethell, D.; Knight, D.K.; Hutchings, G.J.; McMillan, P.F.; Gavriilidis, A. Reaction and Raman spectroscopic studies of alcohol oxidation on gold-palladium catalysts in microstructured reactors. Chem. Eng. J. 2011, 167, 734–743. [Google Scholar] [CrossRef]
- Ferri, D.; Mondelli, C.; Krumeich, F.; Baiker, A. Discrimination of active palladium sites in catalytic liquid-phase oxidation of benzyl alcohol. J. Phys. Chem. B 2006, 110, 22982–22986. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Enache, D.I.; Edwards, J.; Carley, A.F.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of benzyl alcohol with oxygen using zeolite-supported Au and Au-Pd catalysts. Catal. Lett. 2006, 110, 7–13. [Google Scholar] [CrossRef]
- Rogers, S.M.; Catlow, C.R.A.; Chan-Thaw, C.E.; Gianolio, D.; Gibson, E.K.; Gould, A.L.; Jian, N.; Logsdail, A.J.; Palmer, R.E.; Prati, L.; et al. Tailoring Gold Nanoparticle Characteristics and the Impact on Aqueous-Phase Oxidation of Glycerol. ACS Catal. 2015, 5. [Google Scholar] [CrossRef]
- Personick, M.L.; Madix, R.J.; Friend, C.M. Selective Oxygen-Assisted Reactions of Alcohols and Amines Catalyzed by Metallic Gold: Paradigms for the Design of Catalytic Processes. ACS Catal. 2017, 7, 965–985. [Google Scholar] [CrossRef]
- Lackmann, A.; Mahr, C.; Schowalter, M.; Fitzek, L.; Weissmüller, J.; Rosenauer, A.; Wittstock, A. A comparative study of alcohol oxidation over nanoporous gold in gas and liquid phase. J. Catal. 2017, 353, 99–106. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Zhou, Z.; Zhao, G. Highly selective aerobic oxidation of biomass alcohol to benzaldehyde by an: In situ doped Au/TiO2 nanotube photonic crystal photoanode for simultaneous hydrogen production promotion. J. Mater. Chem. A 2017, 5, 12407–12415. [Google Scholar] [CrossRef]
- Dias Ribeiro de Sousa Martins, L.M.; Carabineiro, S.A.C.; Wang, J.; Rocha, B.G.M.; Maldonado-Hódar, F.J.; Latourrette de Oliveira Pombeiro, A.J. Supported Gold Nanoparticles as Reusable Catalysts for Oxidation Reactions of Industrial Significance. ChemCatChem 2017, 9, 1211–1221. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Hutchings, G.J. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002, 7, 696–697. [Google Scholar] [CrossRef]
- Ketchie, W.C.; Murayama, M.; Davis, R.J. Selective oxidation of glycerol over carbon-supported AuPd catalysts. J. Catal. 2007, 250, 264–273. [Google Scholar] [CrossRef]
- Villa, A.; Campisi, S.; Mohammed, K.M.H.; Dimitratos, N.; Vindigni, F.; Manzoli, M.; Jones, W.; Bowker, M.; Hutchings, G.J.; Prati, L. Tailoring the selectivity of glycerol oxidation by tuning the acid–base properties of Au catalysts. Catal. Sci. Technol. 2015, 5, 1126–1132. [Google Scholar] [CrossRef]
- Villa, A.; Chan-Thaw, C.E.; Veith, G.M.; More, K.L.; Ferri, D.; Prati, L. Au on nanosized NiO: A cooperative effect between au and nanosized NiO in the base-free alcohol oxidation. ChemCatChem 2011, 3, 1612–1618. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Q.; Chen, J.; Deng, W.; Wang, Y. Gold nanoparticles on hydrotalcites as efficient catalysts for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2010, 46, 1547. [Google Scholar] [CrossRef] [PubMed]
- Patrícia, R.; Castro, K.; Aurélio, S.; Garcia, M.; de Abreu, W.C.; Anderson, A.; de Sousa, S.; Verônica, R.; de Moura, C.; Cláudio, S.; et al. Aerobic Oxidation of Benzyl Alcohol on a Strontium-Based Gold Material: Remarkable Intrinsic Basicity and Reusable Catalyst. Catalysts 2018, 8, 83. [Google Scholar] [CrossRef]
- Meng, Y.; Zou, S.; Zhou, Y.; Yi, W.; Yan, Y.; Ye, B.; Xiao, L.; Liu, J.; Kobayashi, H.; Fan, J. Activating molecular oxygen by Au/ZnO to selectively oxidize glycerol to dihydroxyacetone. Catal. Sci. Technol. 2018, 8, 2524–2528. [Google Scholar] [CrossRef]
- Villa, A.; Veith, G.M.; Ferri, D.; Weidenkaff, A.; Perry, K.A.; Campisi, S.; Prati, L. NiO as a peculiar support for metal nanoparticles in polyols oxidation. Catal. Sci. Technol. 2013, 3, 394–399. [Google Scholar] [CrossRef]
- Jian, J.; You, K.; Duan, X.; Gao, H.; Luo, Q.; Deng, R.; Liu, P.; Ai, Q.; Luo, H. Boosting one-step conversion of cyclohexane to adipic acid by NO2 and VPO composite catalysts. Chem. Commun. 2016, 52, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.L.; Katryniok, B.; Paul, S.; Nardello-Rataj, V.; Pera-Titus, M.; Clacens, J.-M.; De Campo, F.; Dumeignil, F. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over intercalated vanadium phosphate oxides. RSC Adv. 2013, 3, 9942. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Vanadium phosphorus oxide as an efficient catalyst for hydrocarbon oxidations using hydrogen peroxide. New J. Chem. 2003, 27, 525–528. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R. Vanadium phosphorus oxide catalyst promoted by cobalt doping for mild oxidation of benzyl alcohol to benzaldehyde in the liquid phase. Appl. Catal. A Gen. 2014, 482, 189–197. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R.; Abdollahi, S. Liquid Phase Selective Oxidation of Alcohols over VPO Catalysts Supported on Mesoporous Hexagonal Molecular Sieves (HMS). J. Chin. Chem. Soc. 2010, 57, 189–198. [Google Scholar] [CrossRef]
- Luciani, S.; Cavani, F.; Dal Santo, V.; Dimitratos, N.; Rossi, M.; Bianchi, C.L. The mechanism of surface doping in vanadyl pyrophosphate, catalyst for n-butane oxidation to maleic anhydride: The role of Au promoter. Catal. Today 2011, 169, 200–206. [Google Scholar] [CrossRef]
- Wang, D.; Villa, A.; Spontoni, P.; Su, D.S.; Prati, L. In situ formation of Au-Pd bimetallic active sites promoting the physically mixed monometallic catalysts in the liquid-phase oxidation of alcohols. Chem. A Eur. J. 2010, 16, 10007–10013. [Google Scholar] [CrossRef] [PubMed]
- Carniti, P.; Gervasini, A.; Marzo, M. Silica-niobia oxides as viable acid catalysts in water: Effective vs. intrinsic acidity. Catal. Today 2010, 152, 42–47. [Google Scholar] [CrossRef]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef]
- Dimitratos, N.; Villa, A.; Prati, L.; Hammond, C.; Chan-Thaw, C.E.; Cookson, J.; Bishop, P.T. Effect of the preparation method of supported Au nanoparticles in the liquid phase oxidation of glycerol. Appl. Catal. A Gen. 2016, 514, 267–275. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Intrinsic and effective acidity study of niobic acid and niobium phosphate by a multitechnique approach. Chem. Mater. 2005, 17, 6128–6136. [Google Scholar] [CrossRef]
- Védrine, J.C. Acid-base characterization of heterogeneous catalysts: An up-to-date overview. Res. Chem. Intermed. 2015, 41, 9387–9423. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Su, D.S.; Prati, L. Gold sols as catalysts for glycerol oxidation: The role of stabilizer. ChemCatChem 2009, 1, 510–514. [Google Scholar] [CrossRef]
- Wang, F.; Dubois, J.-L.; Ueda, W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J. Catal. 2009, 268, 260–267. [Google Scholar] [CrossRef]
- Dong, W.-S.; Bartley, J.K.; Girgsdies, F.; Schlögl, R.; Hutchings, G.J. The hydration and transformation of vanadyl pyrophosphate. J. Mater. Chem. 2005, 15, 4147. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Higgins, R. Effect of Promoters on the Selective Oxidation ofn-Butane with Vanadium-Phosphorus Oxide Catalysts. J. Catal. 1996, 162, 153–168. [Google Scholar] [CrossRef]
- Abon, M.; Bere, K.E.; Tuel, A.; Delichere, P. Evolution of a VPO catalyst in n-butane oxidation reaction during the activation time. J. Catal. 1995, 156, 28–36. [Google Scholar] [CrossRef]
- Sananés-Schulz, M.T.; Ben Abdelouahab, F.; Hutchings, G.J.; Volta, J.C. On the role of Fe and Co dopants during the activation of the VO(HPO4), 0.5 H2O precursor of the vanadium phosphorus catalyst as studied by in situ laser Raman spectroscopy: II. Study of VO(HPO4), 0.5 H2O precur. J. Catal. 1996, 163, 346–353. [Google Scholar] [CrossRef]
- Solsona, B.; Zazhigalov, V.A.; López Nieto, J.M.; Bacherikova, I.V.; Diyuk, E.A. Oxidative dehydrogenation of ethane on promoted VPO catalysts. Appl. Catal. A Gen. 2003, 249, 81–92. [Google Scholar] [CrossRef]
- Richter, F.; Papp, H.; Wolf, G.U.; Götze, T.; Kubias, B. Study of the surface composition of vanadyl pyrophosphate catalysts by XPS and ISS–Influence of Cs+ and water vapor on the surface P/V ratio of (VO)2P2O7 catalysts. Fresenius J. Anal. Chem. 1999, 365, 150–153. [Google Scholar] [CrossRef]
- Centi, G.; Golinelli, G.; Busca, G. Modification of the surface pathways in alkane oxidation by selective doping of Broensted acid sites of vanadyl pyrophosphate. J. Phys. Chem. 1990, 94, 6813–6819. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Ottone, M.; Santos, M.C.; Junior, G.C.; Lima, C.D.; Glososki, G.C.; Lopes, N.P.; Klein, S.I. Benzyl benzoate and dibenzyl ether from of benzoic acid and benzyl alcohol under microwave irradiation using a SiO2-SO3H catalyst. Catal. Commun. 2015, 68, 97–100. [Google Scholar] [CrossRef]
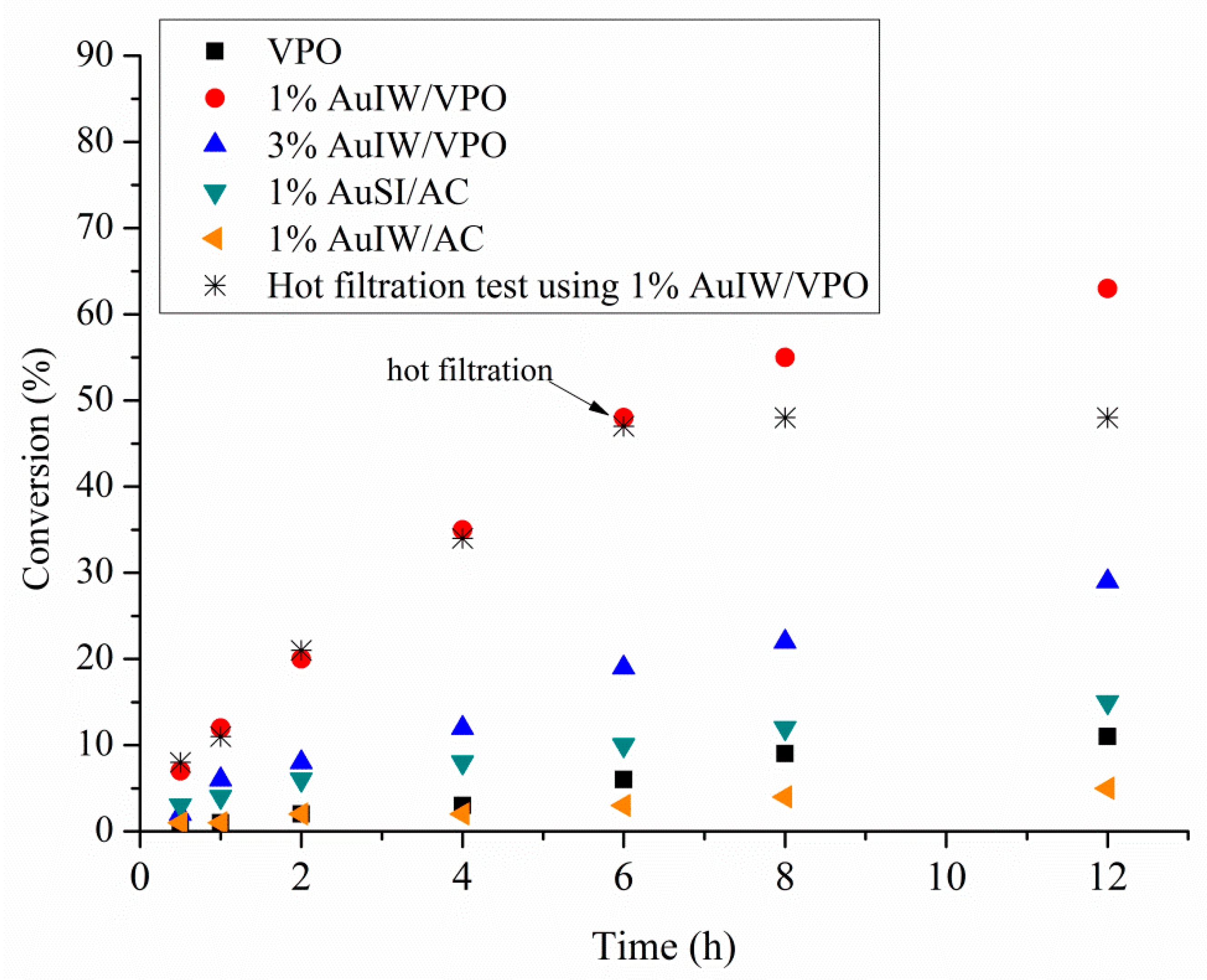
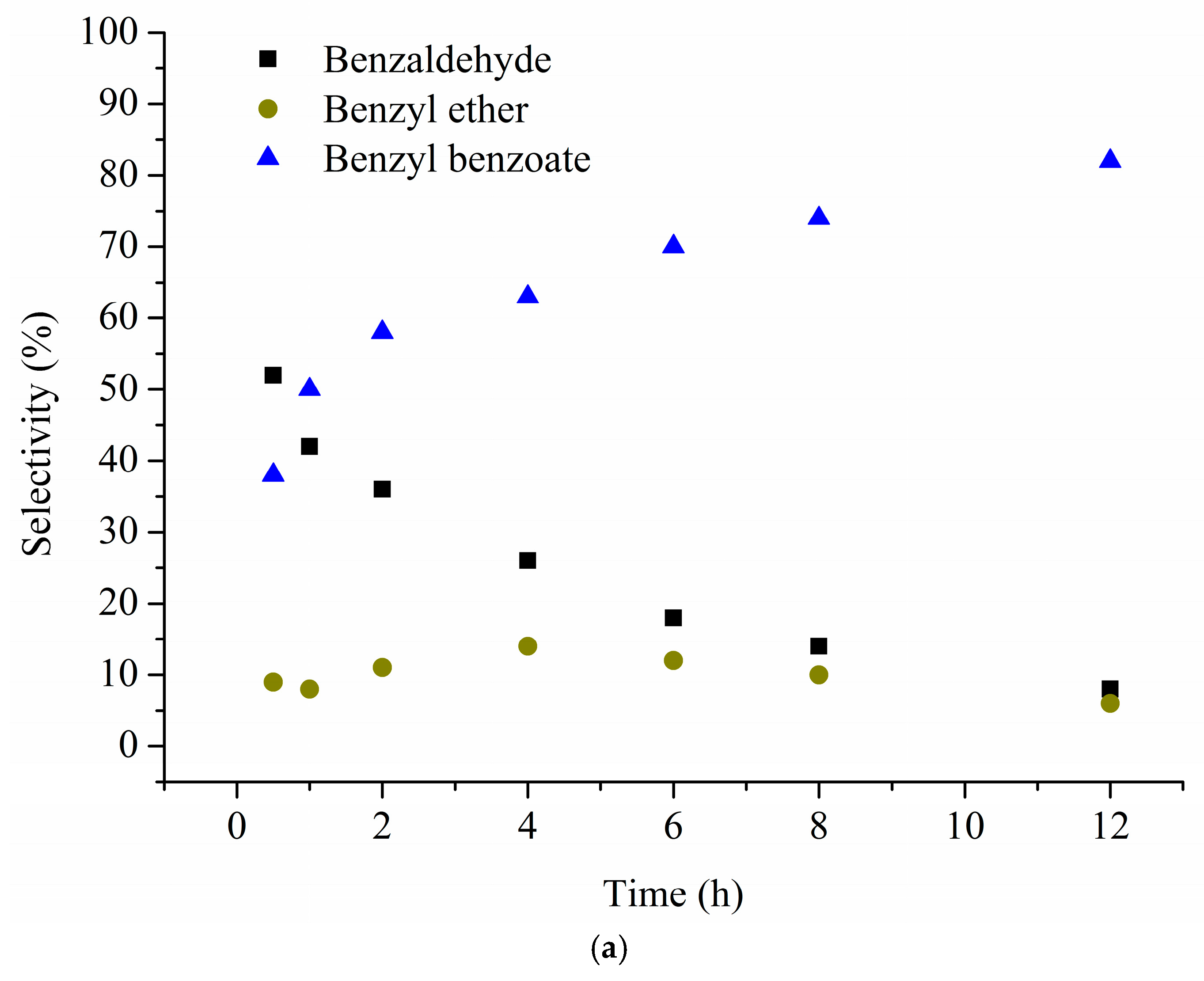
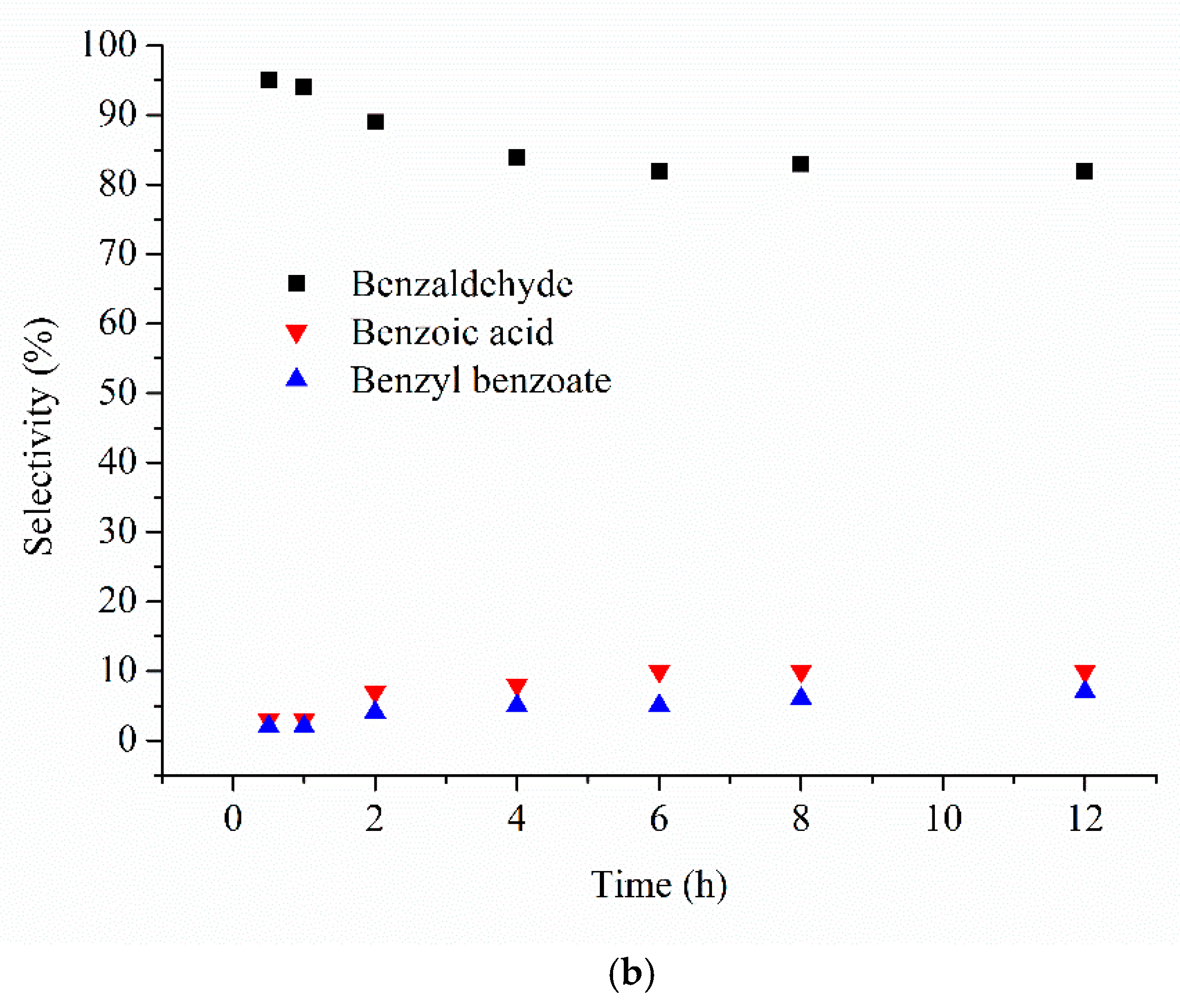
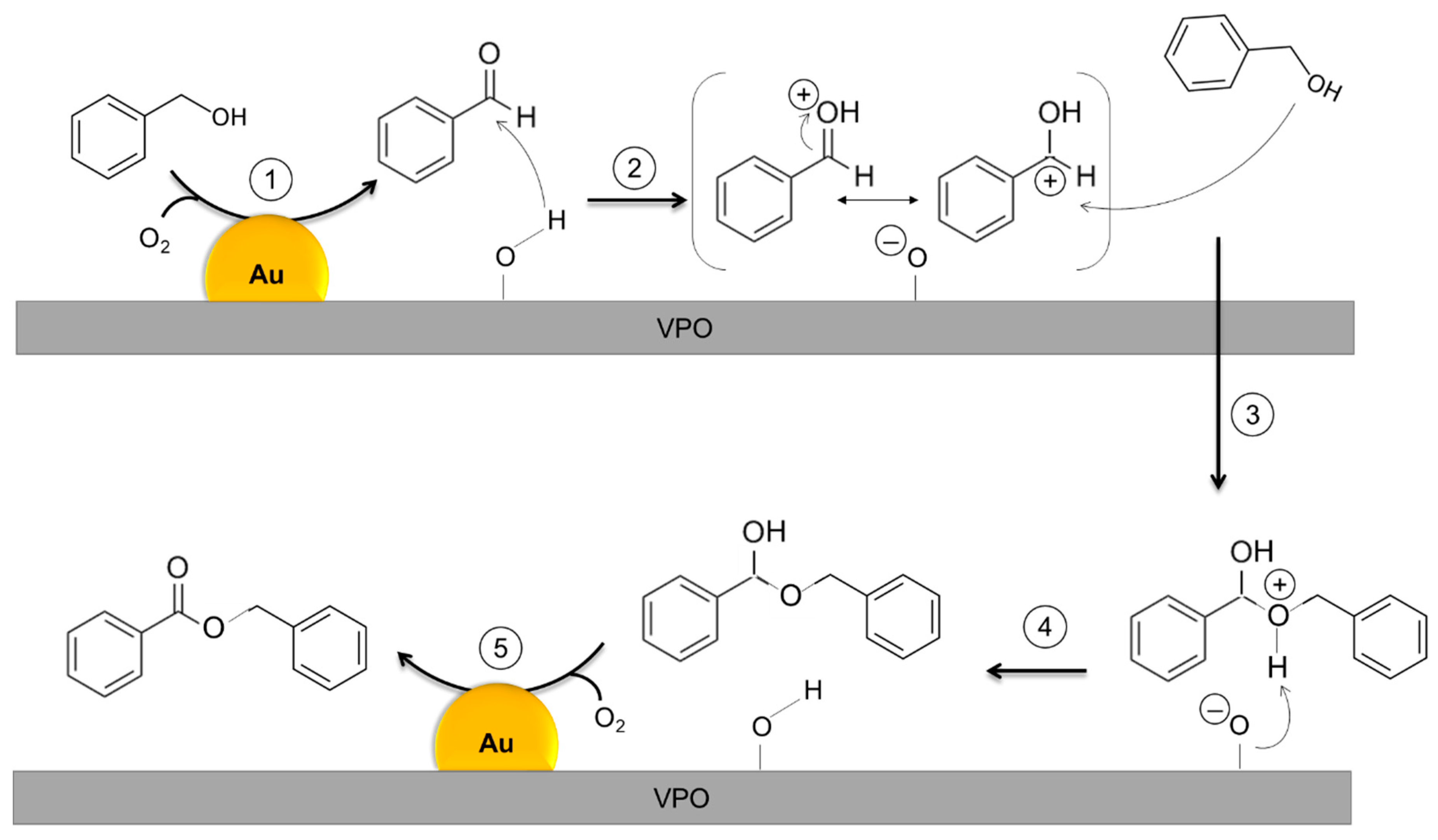
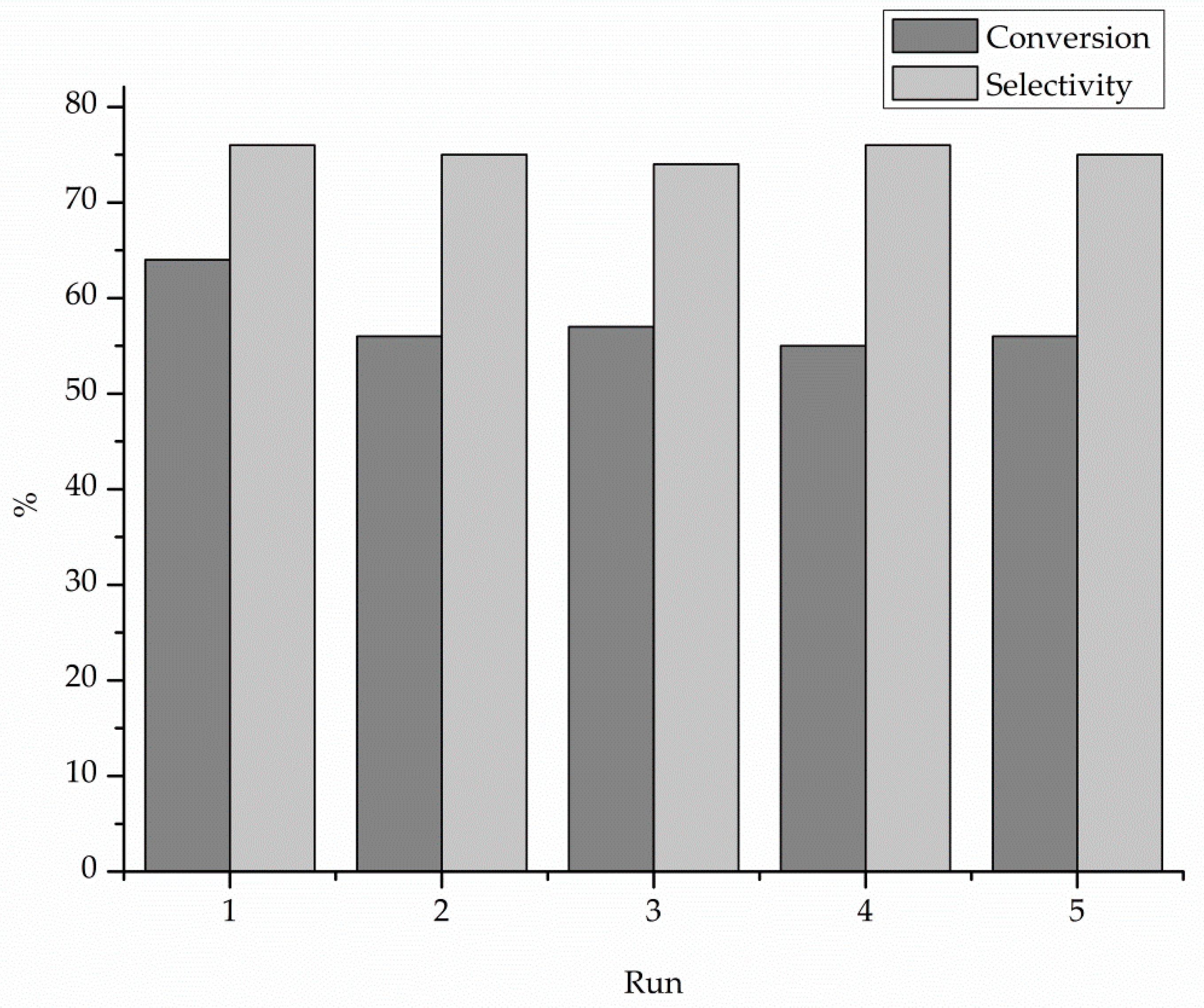
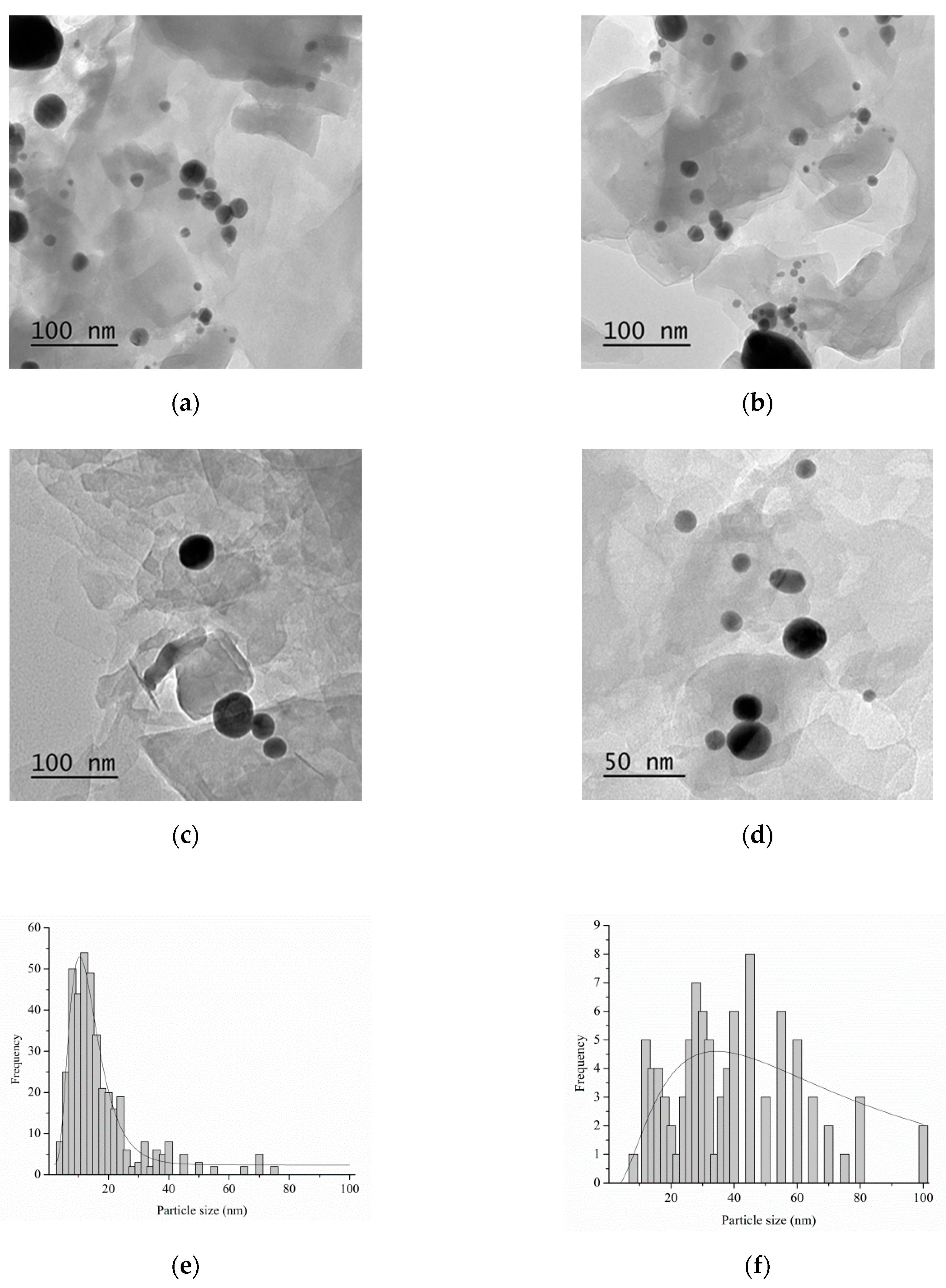
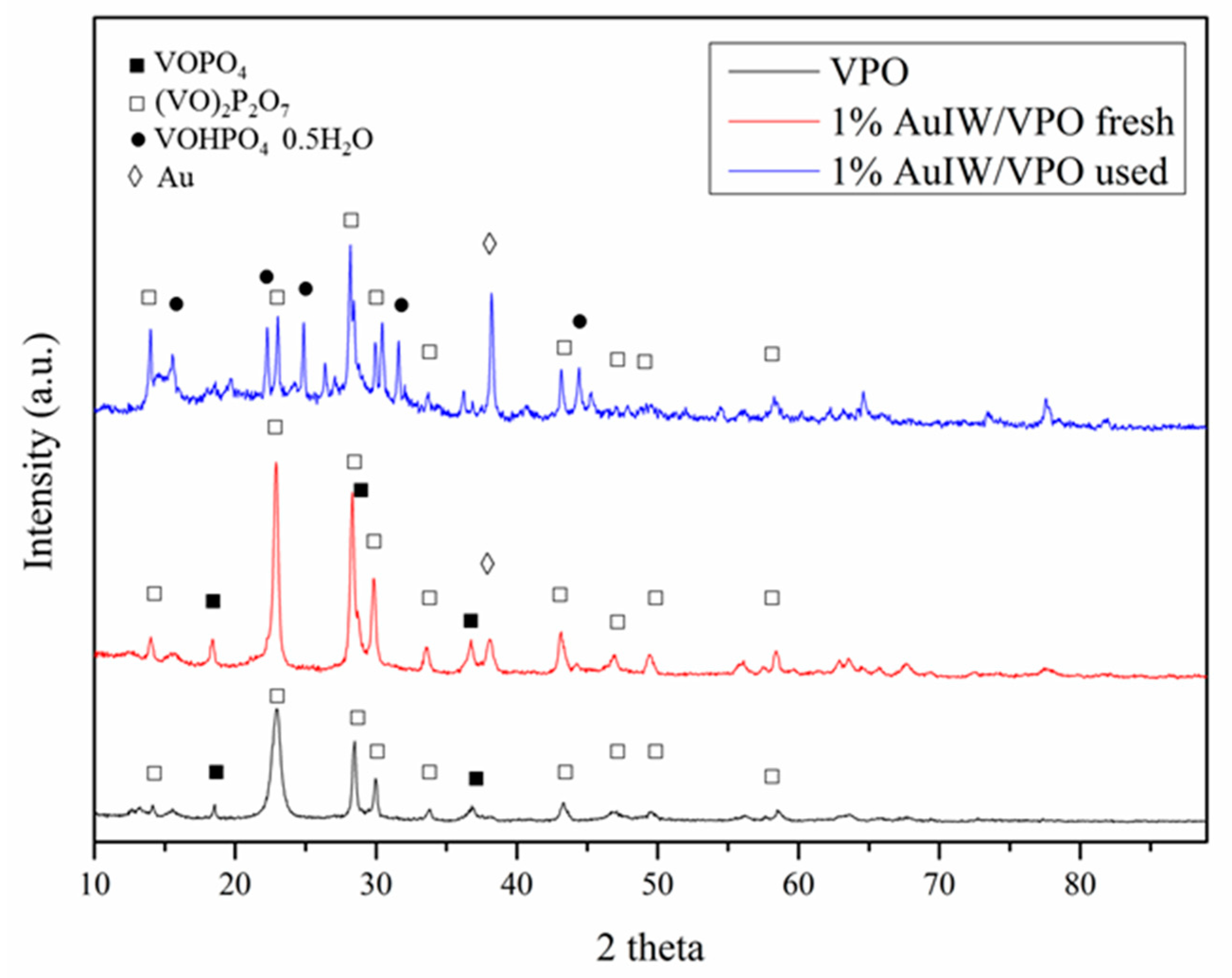
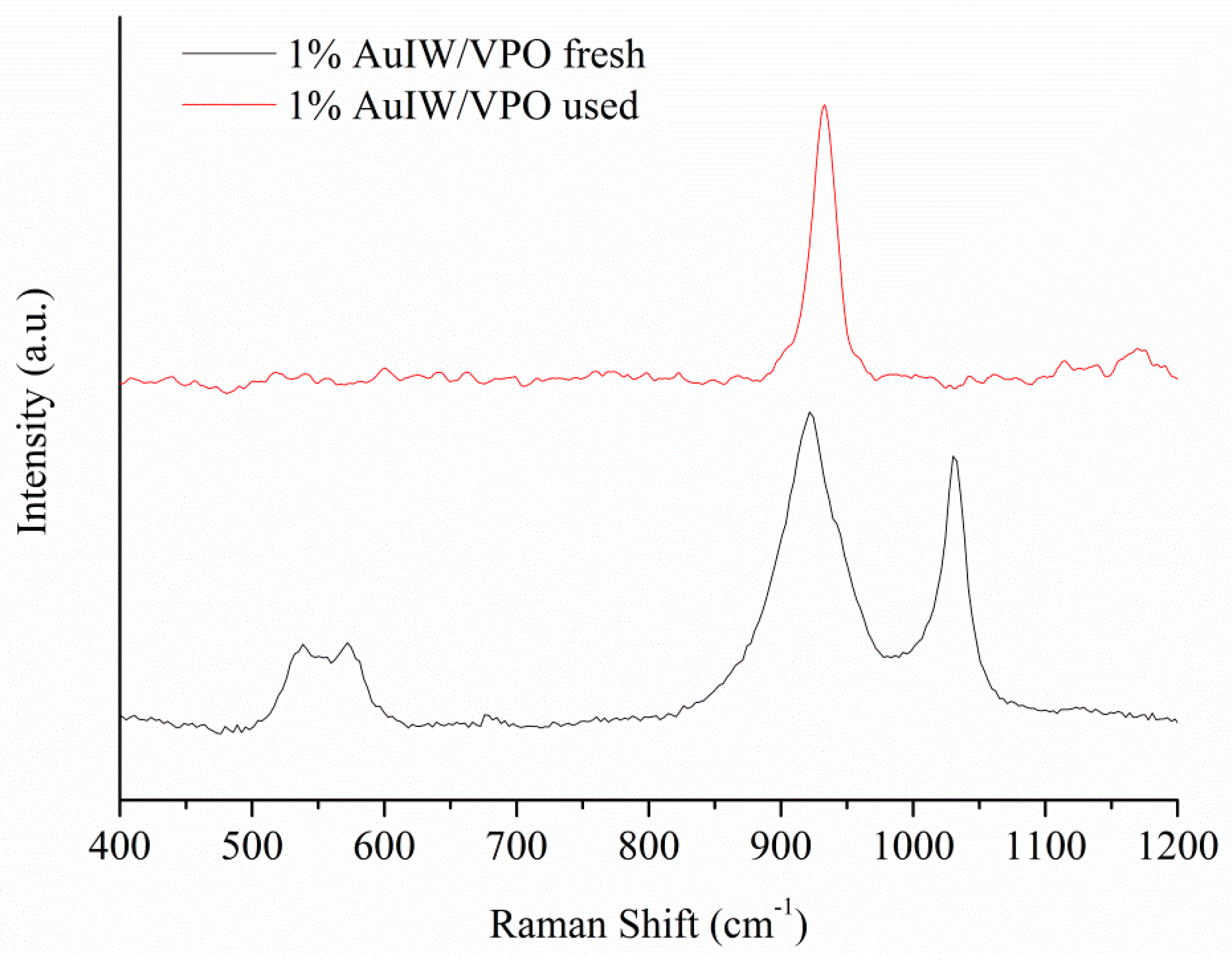
| Catalyst 1 | Au Size (nm) | Acid sites 2 | Activity 3 | Activity Ns 4 | Selectivity (%) 5 | |||
|---|---|---|---|---|---|---|---|---|
| (mmol g−1) | Benzal-Dehyde | Benzyl Ether | Benzyl Benzoate | Benzoic Acid | ||||
| VPO 6 | - | 0.485 (>99%) | 2 | - | 16 | 4 | 78 | - |
| 1%AuIW/VPO | 19.1 | 0.324 (92%) | 120 | 1639 | 8 | 6 | 76 | - |
| 1%AuSI/AC | 3.6 | - | 27 | 98 | 82 | 1 | 7 | 7 |
| 1%AuIW/AC | 23.1 | - | 8 | 114 | 83 | - | 6 | 9 |
| Samples | V2P | P1s | O1s | Au4f | Au %at | P/V | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| V4+ | V5+ | |||||||||
| VPO | BE eV | 517.0 | 518.2 | 134.0 | 531.3 | 532.1 | 533.3 | - | - | 3.3 |
| % | 58.2 | 41.8 | 100 | 54.7 | 29.6 | 15.7 | ||||
| AuIW/VPO fresh | BE eV | 517.0 | 518.4 | 134.1 | 531.3 | 532.0 | 533.2 | 84.3 | 0.14 | 3.4 |
| % | 57.6 | 42.4 | 100 | 55.1 | 30.6 | 14.3 | 100 | |||
| AuIW/VPO used | BE eV | 516.9 | - | 133.8 | 531.2 | 532.0 | 533.0 | 84.2 | 0.03 | 2.9 |
| % | 100 | - | 100 | 60.0 | 21.3 | 18.7 | 100 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campisi, S.; Ferri, M.; Chan-Thaw, C.E.; Sanchez Trujillo, F.J.; Motta, D.; Tabanelli, T.; Dimitratos, N.; Villa, A. Metal-Support Cooperative Effects in Au/VPO for the Aerobic Oxidation of Benzyl Alcohol to Benzyl Benzoate. Nanomaterials 2019, 9, 299. https://doi.org/10.3390/nano9020299
Campisi S, Ferri M, Chan-Thaw CE, Sanchez Trujillo FJ, Motta D, Tabanelli T, Dimitratos N, Villa A. Metal-Support Cooperative Effects in Au/VPO for the Aerobic Oxidation of Benzyl Alcohol to Benzyl Benzoate. Nanomaterials. 2019; 9(2):299. https://doi.org/10.3390/nano9020299
Chicago/Turabian StyleCampisi, Sebastiano, Michele Ferri, Carine E. Chan-Thaw, Felipe J. Sanchez Trujillo, Davide Motta, Tommaso Tabanelli, Nikolaos Dimitratos, and Alberto Villa. 2019. "Metal-Support Cooperative Effects in Au/VPO for the Aerobic Oxidation of Benzyl Alcohol to Benzyl Benzoate" Nanomaterials 9, no. 2: 299. https://doi.org/10.3390/nano9020299
APA StyleCampisi, S., Ferri, M., Chan-Thaw, C. E., Sanchez Trujillo, F. J., Motta, D., Tabanelli, T., Dimitratos, N., & Villa, A. (2019). Metal-Support Cooperative Effects in Au/VPO for the Aerobic Oxidation of Benzyl Alcohol to Benzyl Benzoate. Nanomaterials, 9(2), 299. https://doi.org/10.3390/nano9020299









