In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalytic Evaluation
2.3. Characterization
3. Results and Discussion
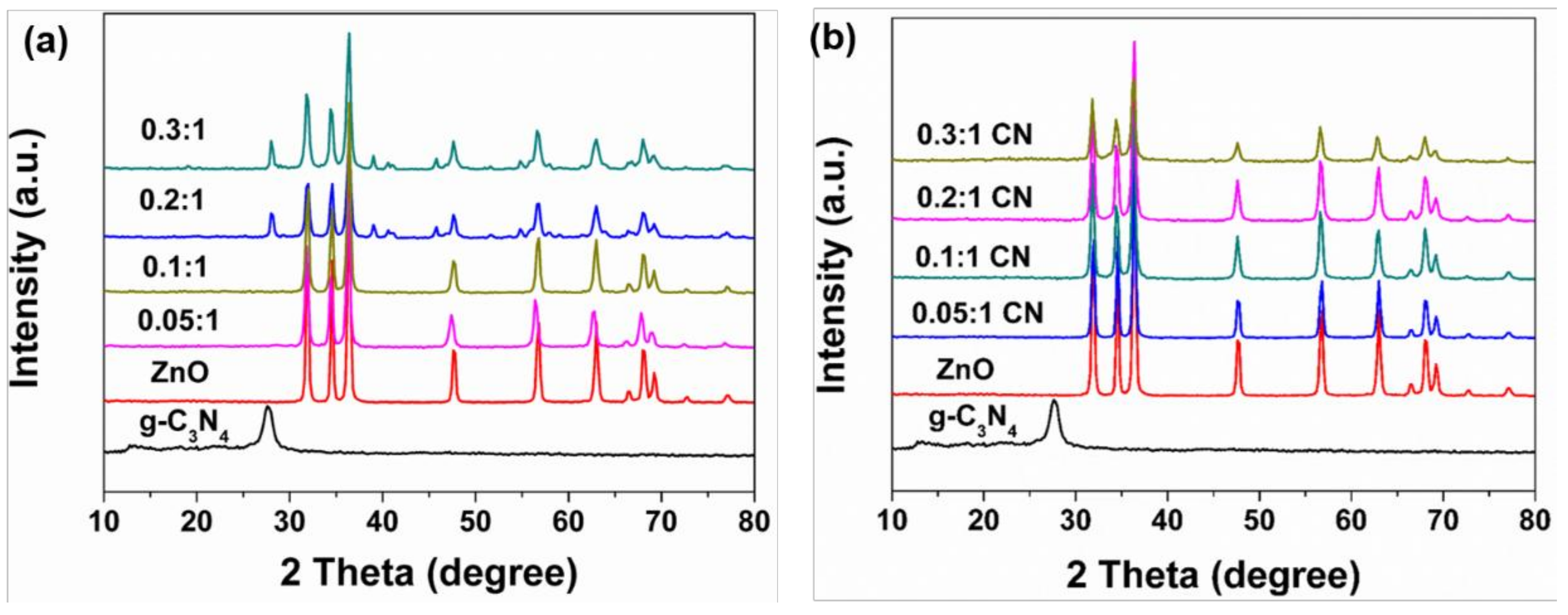
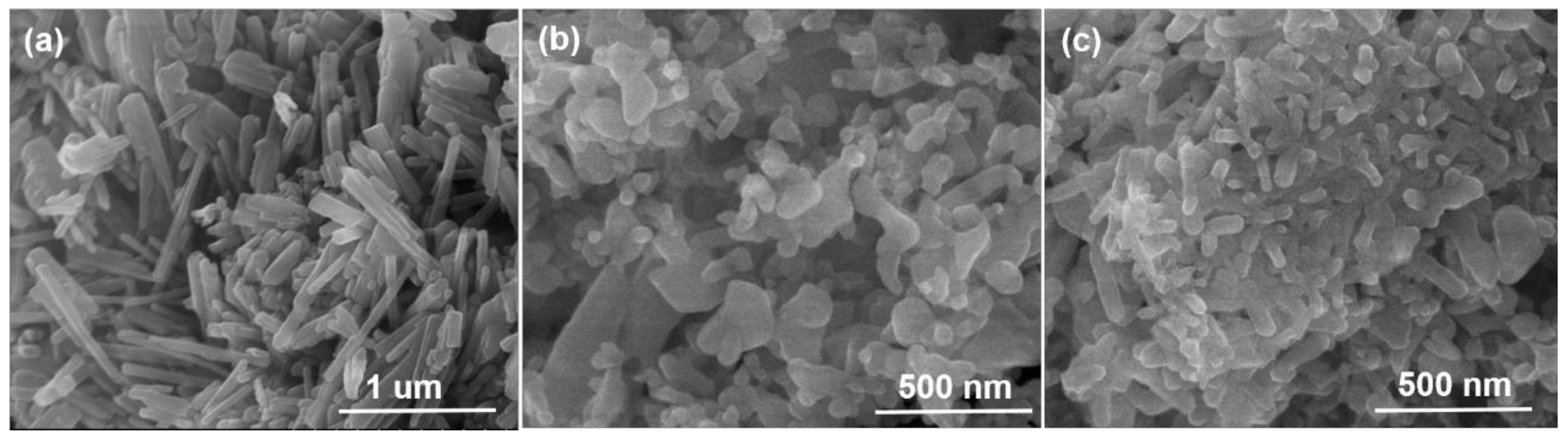
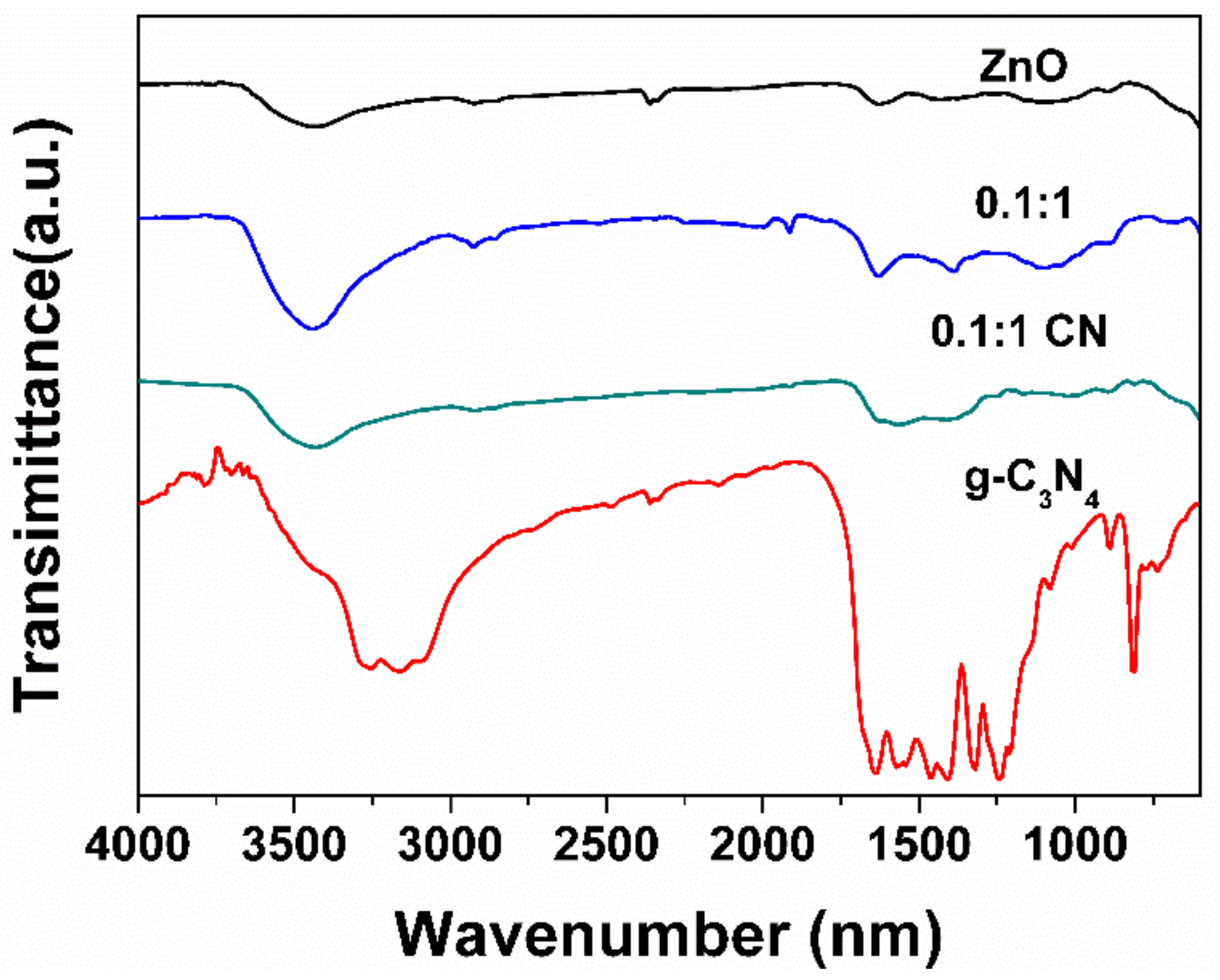
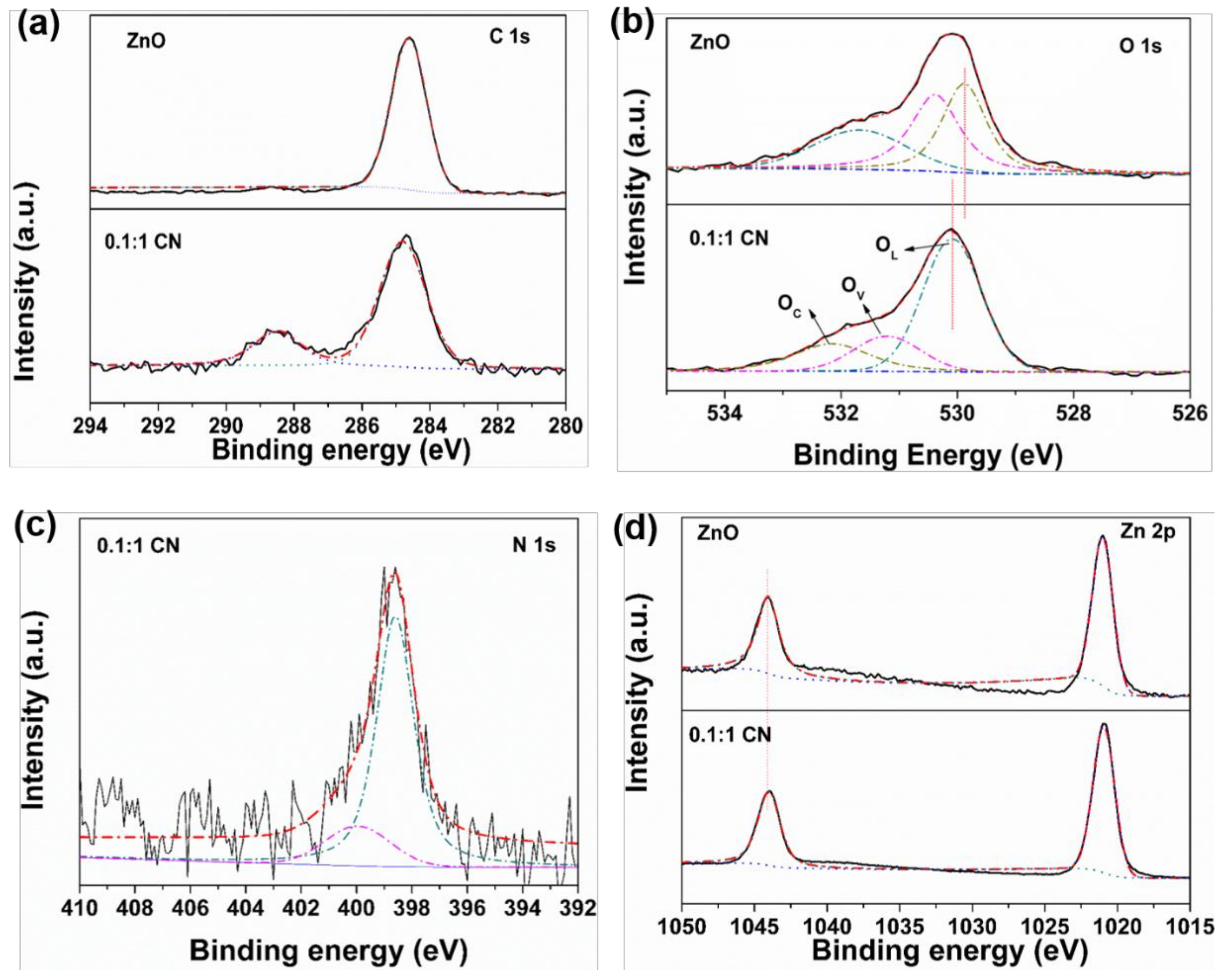
3.1. Physicochemical Properties
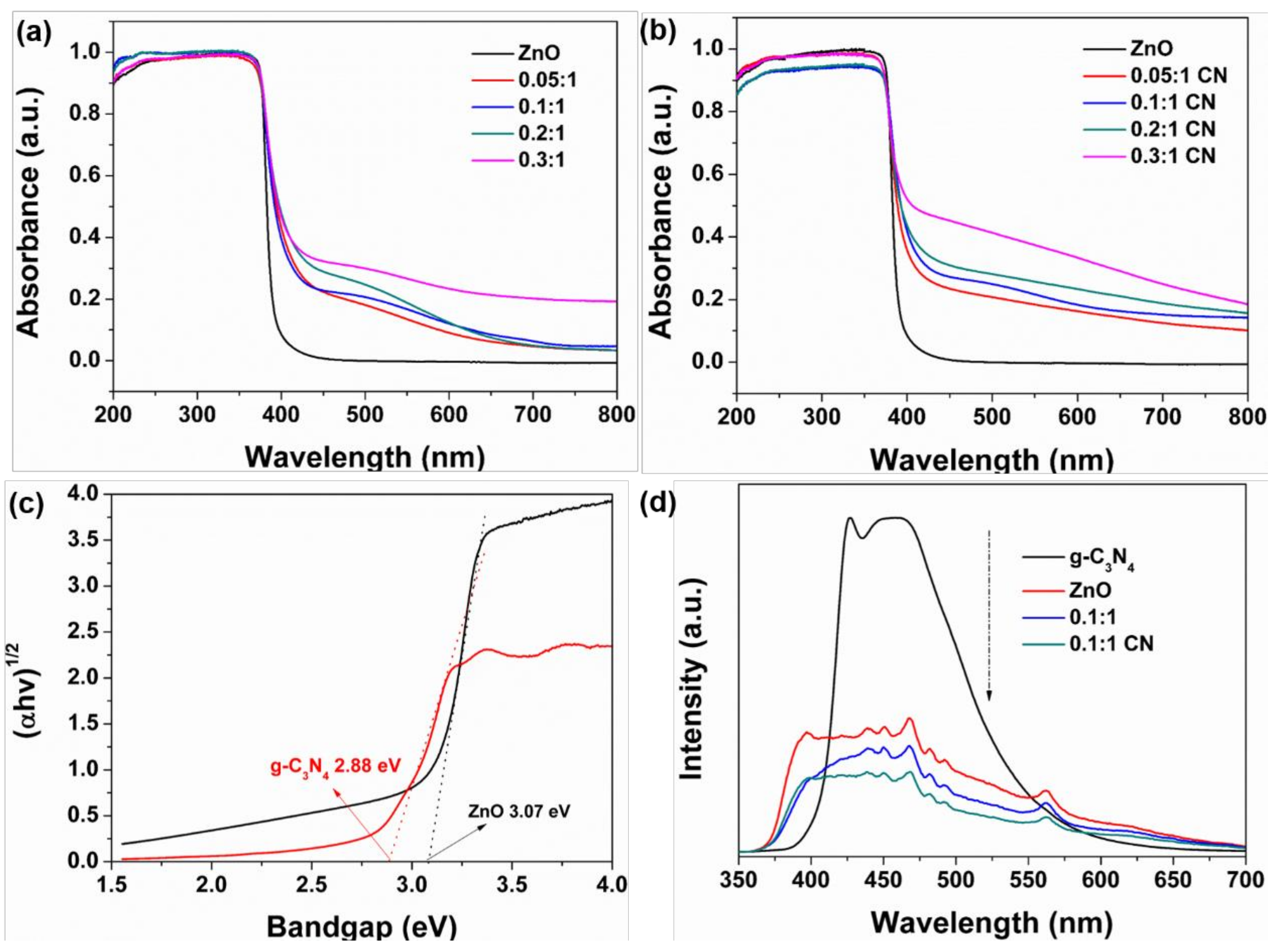
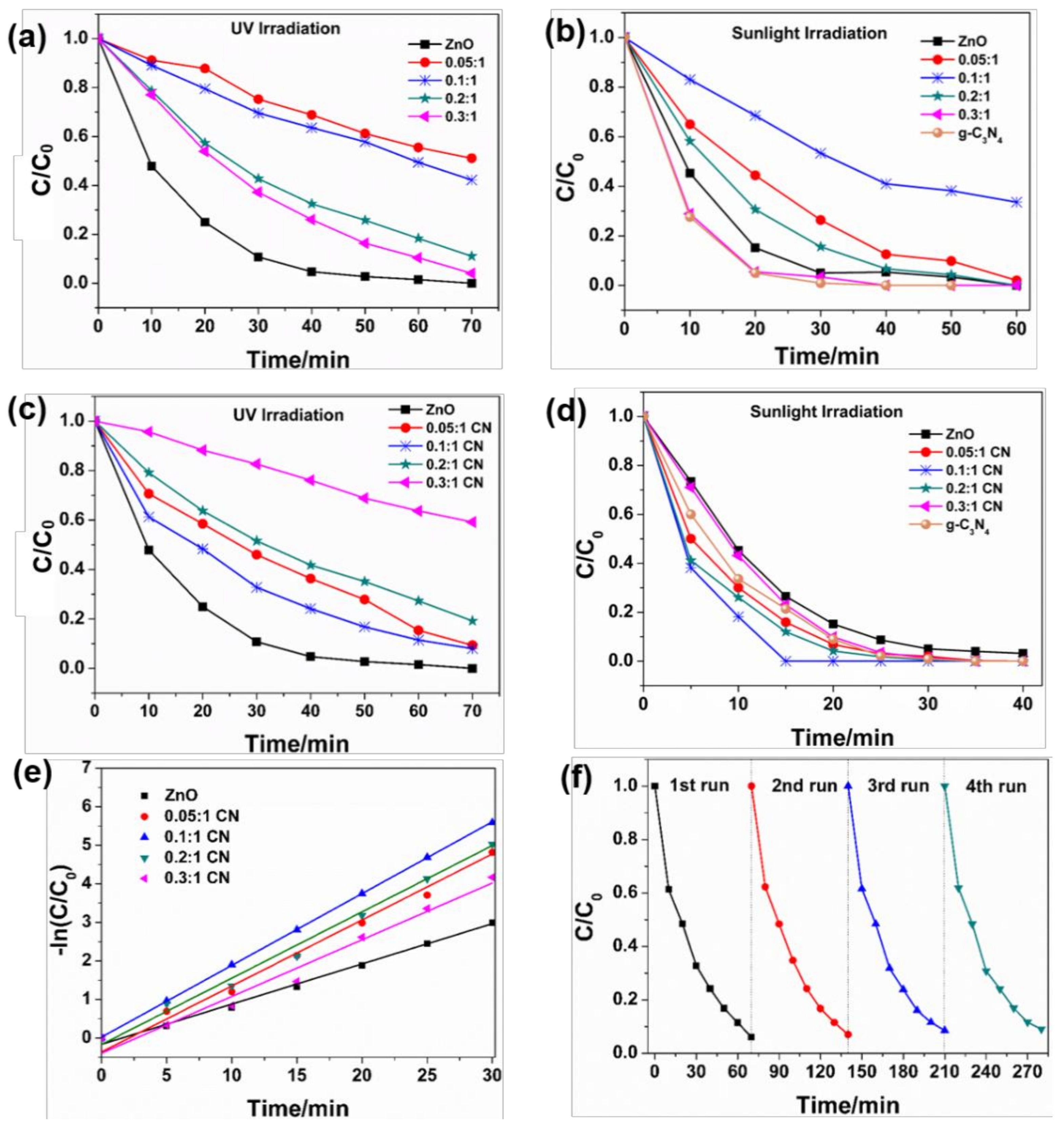
3.2. Photocatalytic Performance
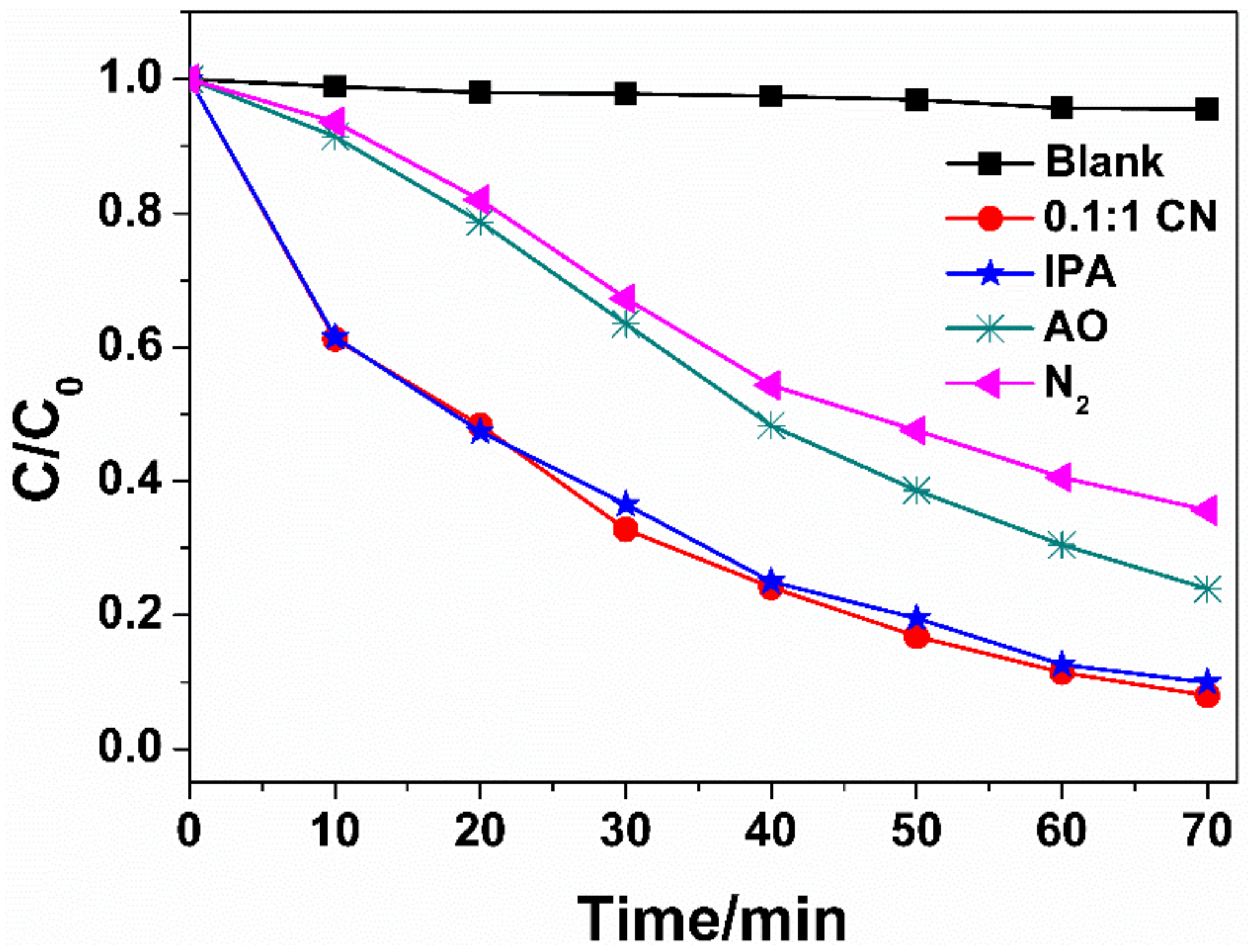
3.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Izumi, Y. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Q.; Wang, J.; Zhang, X.; Wei, X.; Wu, Z.; Li, K.; Meng, F.; Bao, D.; Yan, J. Engineering Ultrathin C3N4 Quantum Dots on Graphene as a Metal-Free Water Reduction Electrocatalyst. ACS Catal. 2018, 8, 3965–3970. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon Quantum Dot Implanted Graphite Carbon Nitride Nanotubes: Excellent Charge Separation and Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 130, 5867–5873. [Google Scholar] [CrossRef]
- Park, B.-G. Photocatalytic Behavior of Strontium Aluminates Co-Doped with Europium and Dysprosium Synthesized by Hydrothermal Reaction in Degradation of Methylene Blue. Catalysts 2018, 45, 227–236. [Google Scholar] [CrossRef]
- Sun, M.; Senthil, R.; Pan, J.; Osman, S.; Khan, A. A Facile Synthesis of Visible-Light Driven Rod-on-Rod like α-FeOOH/α-AgVO3 Nanocomposite as Greatly Enhanced Photocatalyst for Degradation of Rhodamine B. Catalysts 2018, 8, 392. [Google Scholar] [CrossRef]
- Yin, G.; Huang, X.; Chen, T.; Zhao, W.; Bi, Q.; Xu, J.; Han, Y.; Huang, F. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8, 1009–1017. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Bai, S.; Chen, S.; Long, R.; Song, L.; Li, Z.; Xiong, Y. Hydriding Pd cocatalysts: An approach to giant enhancement on photocatalytic CO2 reduction into CH4. Nano Res. 2017, 10, 3396–3406. [Google Scholar] [CrossRef]
- Yan, Y.; Crisp, R.W.; Gu, J.; Chernomordik, B.D.; Pach, G.F.; Marshall, A.R.; Turner, J.A.; Beard, M.C. Multiple exciton generation for photoelectrochemical hydrogen evolution reactions with quantum yields exceeding 100%. Nat. Energy 2017, 2, 17052–17058. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.C.; Xia, D.; Wong, P.K.; Li, Y. Graphene and g-C3N4 nanosheets cowrapped elemental alpha-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ. Sci. Technol. 2013, 47, 8724–8732. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B Environ. 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Stassi, S.; Cauda, V.; Ottone, C.; Chiodoni, A.; Pirri, C.F.; Canavese, G. Flexible piezoelectric energy nanogenerator based on ZnO nanotubes hosted in a polycarbonate membrane. Nano Energy 2015, 13, 474–481. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; He, C.; Qiu, L.; Cao, X. Uniform Carbon-Coated ZnO Nanorods: Microwave-Assisted Preparation, Cytotoxicity, and Photocatalytic Activity. Langmuir 2009, 25, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Zhou, L.; Liu, D.; Liu, F.; Liu, F.; Liang, X.; Yan, X.; Gao, Y.; Lu, G. The role of Ce doping in enhancing sensing performance of ZnO-based gas sensor by adjusting the proportion of oxygen species. Sens. Actuators B Chem. 2018, 273, 991–998. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Wei, C.; Liu, W.; Chen, Q.; Hou, H.; Liu, C.; Hou, C.; Xiong, W.; Zhang, D. Nitrogen-doped ZnO/Carbon hollow rhombic dodecahedral for photoelectrochemical sensing glutathione. Appl. Surf. Sci. 2018, 458, 872–879. [Google Scholar] [CrossRef]
- Liang, X.; Shi, X.; Zhang, F.; Li, Y.; Zhang, H.; Yuan, Y. Improved H2 Production by Ethanol Steam Reforming over Sc2O3-Doped Co-ZnO Catalysts. Catalysts 2017, 7, 241. [Google Scholar] [CrossRef]
- Hong, D.; Cao, G.; Zhang, X.; Qu, J.; Deng, Y.; Liang, H.; Tang, J. Construction of a Pt-modified chestnut-shell-like ZnO photocatalyst for high-efficiency photochemical water splitting. Electrochim. Acta 2018, 283, 959–969. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO Facets on Pd/ZnO Catalysts for Methanol Steam Reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Jo, W.K.; Selvam, N.C.S. Enhanced visible light-driven photocatalytic performance of ZnO-g-C3N4 coupled with graphene oxide as a novel ternary nanocomposite. J. Hazard. Mater. 2015, 299, 462–470. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Network Structured SnO2/ZnO Heterojunction Nanocatalyst with High Photocatalytic Activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Guo, Z.; Lv, Y.; Chen, W.; Chang, Z.; Yuan, Q.; Ming, H.; Wang, J. In-situ growth of g-C3N4 layer on ZnO nanoparticles with enhanced photocatalytic performances under visible light irradiation. Mater. Lett. 2017, 188, 347–350. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, M.; Du, Y.; Fan, X.; Wang, M.; Zhang, L.; Kong, Q.; Shi, J. Mesostructured CeO2/g-C3N4 nanocomposites: Remarkably enhanced photocatalytic activity for CO2 reduction by mutual component activations. Nano Energy 2016, 19, 145–155. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, L.; Wang, M.; Jin, X.; Zhou, Y.; Liu, J.; Shi, J. Remarkably enhanced H2 evolution activity of oxidized graphitic carbon nitride by an extremely facile K2CO3-activation approach. Appl. Catal. B Environ. 2018, 232, 322–329. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Mathew, S.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Duan, S.; Wu, G.; Sun, L.; Cao, G.; Li, D.; Xu, H.; Li, Q.; Xia, D. Enhanced catalytic ozonation performance of highly stabilized mesoporous ZnO doped g-C3N4 composite for efficient water decontamination. Appl. Catal. A-Gen. 2018, 551, 129–138. [Google Scholar] [CrossRef]
- Wang, Y.J.; Shi, R.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Eviron. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.L.; Xu, C.; Chen, S.; Fu, X. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J. Mol. Catal. A Chem. 2013, 368, 9–15. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, M.; Liu, Y.; Ren, T.; Yuan, Z. Carbon-Doped ZnO Hybridized Homogeneously with Graphitic Carbon Nitride Nanocomposites for Photocatalysis. J. Phys. Chem. C 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Park, T.J.; Pawar, R.C.; Kang, S.; Lee, C.S. Ultra-thin coating of g-C3N4 on an aligned ZnO nanorod film for rapid charge separation and improved photodegradation performance. RSC Adv. 2016, 6, 89944–89952. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, L.; Zhang, K.; Wang, J.; Cheng, F.; Tao, Z.; Chen, J. MoS2 Nanoflowers with Expanded Interlayers as High-Performance Anodes for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2014, 53, 12794–12798. [Google Scholar] [CrossRef]
- Hou, Y.; Laursen, A.B.; Zhang, J.; Zhang, G.; Zhu, Y.; Wang, X.; Dahl, S.; Chorkendorff, I. Layered Nanojunctions for Hydrogen-Evolution Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3621–3625. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev. 2013, 113, 2139–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Fan, X.; Zhou, Y.; Wu, M.; Shi, J. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, J.; Zhu, S.; Mao, L.; Ma, J.; Zhang, D. A high-performance Bi2WO6-graphene photocatalyst for visible light-induced H2 and O2 generation. Nanoscale 2014, 6, 2186–2193. [Google Scholar] [CrossRef]
- Yoon, M.; Seo, M.; Jeong, C.; Jang, J.H.; Jeon, K.S. Synthesis of Liposome-Templated Titania Nanodisks: Optical Properties and Photocatalytic Activities. Chem. Mater. 2005, 17, 6069–6079. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.H.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and Electron Paramagnetic Resonance of ZnO Tetrapod Structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Chang, C.; Fu, Y.; Hu, M.; Wang, C.; Shan, G.; Zhu, L. Photodegradation of bisphenol A by highly stable palladium-doped mesoporous graphite carbon nitride (Pd/mpg-C3N4) under simulated solar light irradiation. Appl. Catal. B Environ. 2013, 142–143, 553–560. [Google Scholar] [CrossRef]
- Zheng, J.H.; Jiang, Q.; Lian, J.S. Synthesis and optical properties of flower-like ZnO nanorods by thermal evaporation method. Appl. Surf. Sci. 2011, 257, 5083–5087. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Q.; Zhang, J.; Yang, Z.; Si, M.; Yan, Z.; Xue, D. Defect-related ferromagnetism in ultrathin metal-free g-C3N4 nanosheets. Nanoscale 2014, 6, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jing, L.; He, L.; Luan, Y.; Li, C. Phosphate-modified graphitic C3N4 as efficient photocatalyst for degrading colorless pollutants by promoting O2 adsorption. Chem. Commun. 2014, 50, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Yang, X.; Qian, F.; Zou, G.; Li, M.; Lu, J.; Li, Y.; Bao, M. Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation. Appl. Catal. B Environ. 2016, 193, 22–35. [Google Scholar] [CrossRef]
- An, H.; Su, C.; McGinn, P.J. Application of potash glass as a catalyst for diesel soot oxidation. Catal. Commun. 2009, 10, 509–512. [Google Scholar] [CrossRef]
- Su, C.; McGinn, P.J. The effect of Ca2+ and Al3+ additions on the stability of potassium disilicate glass as a soot oxidation catalyst. Appl. Catal. B Environ. 2013, 138, 70–78. [Google Scholar] [CrossRef]
- Su, C.; McGinn, P.J. Application of glass soot catalysts on metal supports to achieve low soot oxidation temperature. Catal. Commun. 2014, 43, 1–5. [Google Scholar] [CrossRef]
- Su, C. Stabilization of Potassium in Soot Oxidation Catalysts and Their Application on Diesel Particulate Filters. Ph.D. Thesis, University of Notre Dame, Notre Dame, IN, USA, 2011. [Google Scholar]
- Chen, X.; Kumar, A.; Klippstein, D.; Stafford, R.; Su, C.; Yuan, Y.; Zokoe, J.; McGinn, P. Development and Demonstration of a Soot Generator Integrated Bench Reactor; SAE Technical Paper 2014-01-1589; SAE: Warrendale, PA, USA, 2014. [Google Scholar]
- McGinn, P.J.; Su, C. Glass Catalysts for Soot Combustion and Methods of Manufacturing the Same. U.S. Patent 9,592,490, 14 March 2017. [Google Scholar]
- Su, C.; Wang, Y.; Kumar, A.; McGinn, P.J. Simulating real world soot-catalyst contact conditions for lab-scale catalytic soot oxidation studies. Catalysts 2018, 8, 247. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Clerc, J.; Harinath, A.; Rogoski, L. Experimental and Modeling Study of Ash Impact on DPF Backpressure and Regeneration Behaviors. SAE Int. J. Eng. 2015, 8, 1313–1321. [Google Scholar] [CrossRef]
- Su, C.; DeHart, T.; Anderson, M.; McGinn, P.J. Structured glass catalytic coating on wire mesh for particulate matter (PM) removal by modified sol-gel processing. Mater. Lett. 2019, 234, 168–171. [Google Scholar] [CrossRef]
- Su, C.; Brault, J.; Munnannur, A.; Liu, Z.; Milloy, S.; Harinath, A.; Dunnuck, D.; Federle, K. Model-Based Approaches in Developing an Advanced Aftertreatment System: An Overview; SAE Technical Paper, 2019-01-0026; SAE: Warrendale, PA, USA, 2019. [Google Scholar]
- Wang, Y.; Pan, Y.; Su, C.; Srinivasan, A.; Gong, J.; Kamp, C.J. Performance of Asymmetric Particulate Filter with Soot and Ash Deposits: Analytical Solution and Its Application. Ind. Eng. Chem. Res. 2018, 57, 15846–15856. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, Y.; Su, C.; Liu, Z.G. Lab Study of Urea Deposit Formation and Chemical Transformation Process of Diesel Aftertreatment System; SAE Technical Paper, 2017-01-0915; SAE: Warrendale, PA, USA, 2017. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Su, C.; Ren, H.; Li, M.; Zhu, L.; Ge, S.; Wang, M.; Zhang, Z.; Li, L.; Cao, X. In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials 2019, 9, 215. https://doi.org/10.3390/nano9020215
Zhang S, Su C, Ren H, Li M, Zhu L, Ge S, Wang M, Zhang Z, Li L, Cao X. In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials. 2019; 9(2):215. https://doi.org/10.3390/nano9020215
Chicago/Turabian StyleZhang, Shengqiang, Changsheng Su, Hang Ren, Mengli Li, Longfeng Zhu, Shuang Ge, Min Wang, Zulei Zhang, Lei Li, and Xuebo Cao. 2019. "In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter" Nanomaterials 9, no. 2: 215. https://doi.org/10.3390/nano9020215
APA StyleZhang, S., Su, C., Ren, H., Li, M., Zhu, L., Ge, S., Wang, M., Zhang, Z., Li, L., & Cao, X. (2019). In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials, 9(2), 215. https://doi.org/10.3390/nano9020215






