Preparation of Ce–Mn Composite Oxides with Enhanced Catalytic Activity for Removal of Benzene through Oxalate Method
Abstract
:1. Introduction
2. Experimental
2.1. The Preparation of Ce-Mn Composite Oxides
2.2. Characterization Technique
2.3. Catalytic Activity Tests
3. Results and Discussion
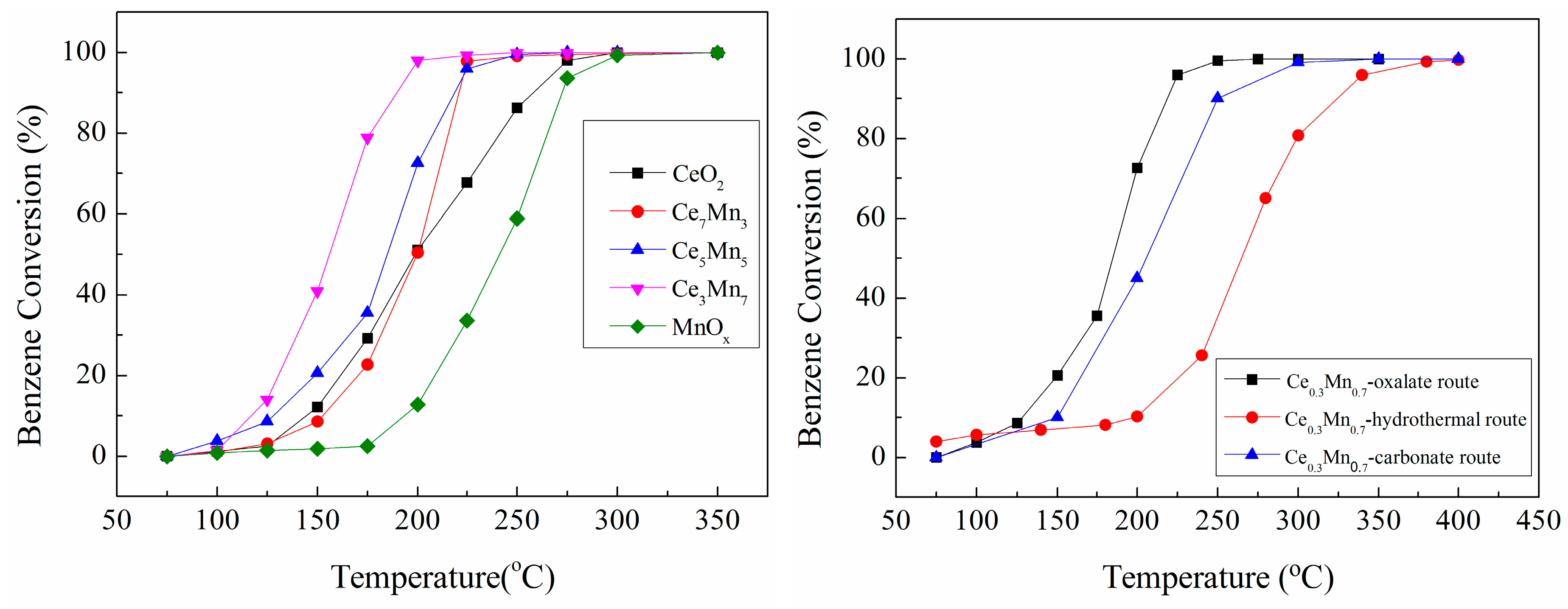
3.1. Catalytic Oxidation Activity of CexMn1−x Composite Oxides for Benzene
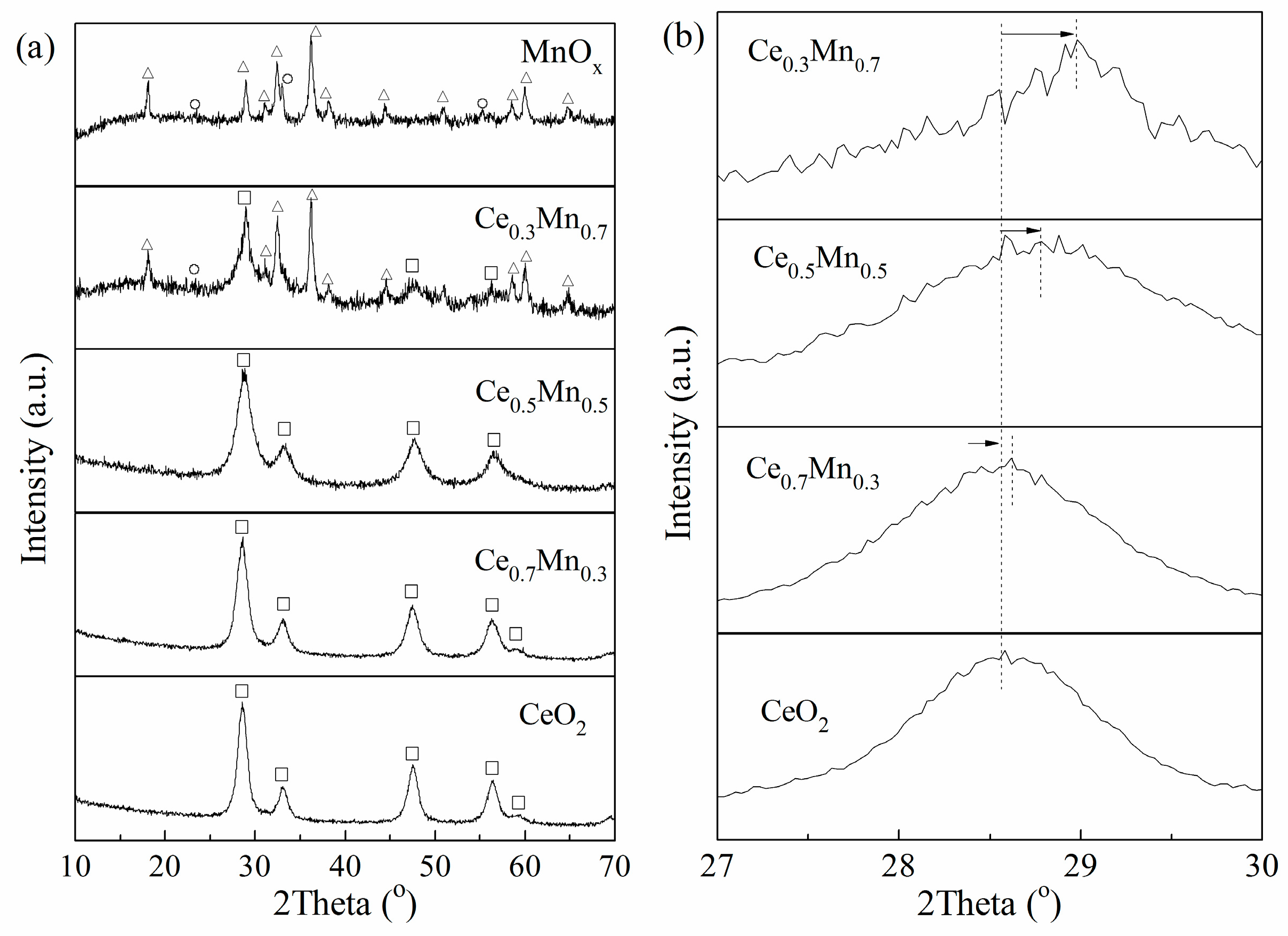
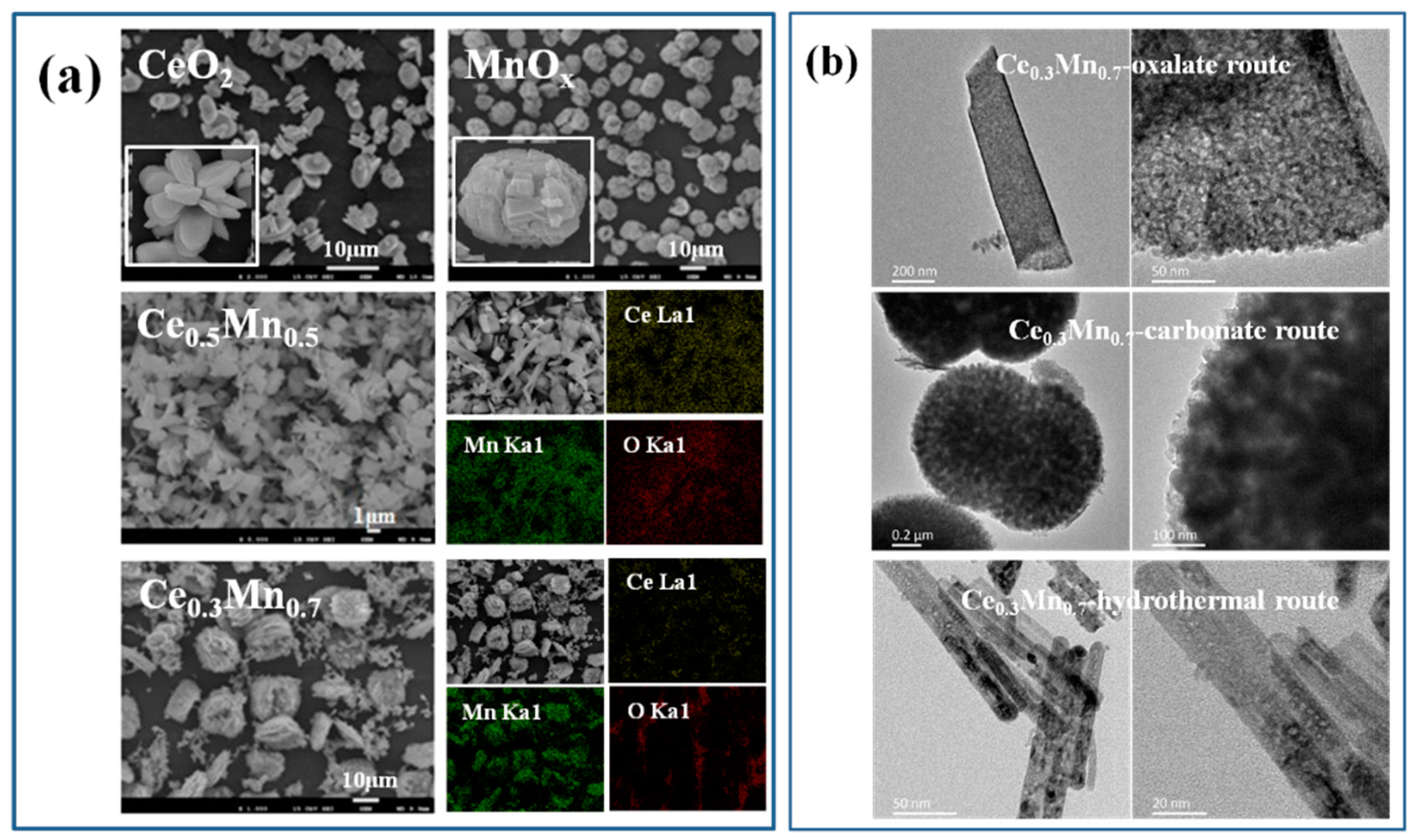
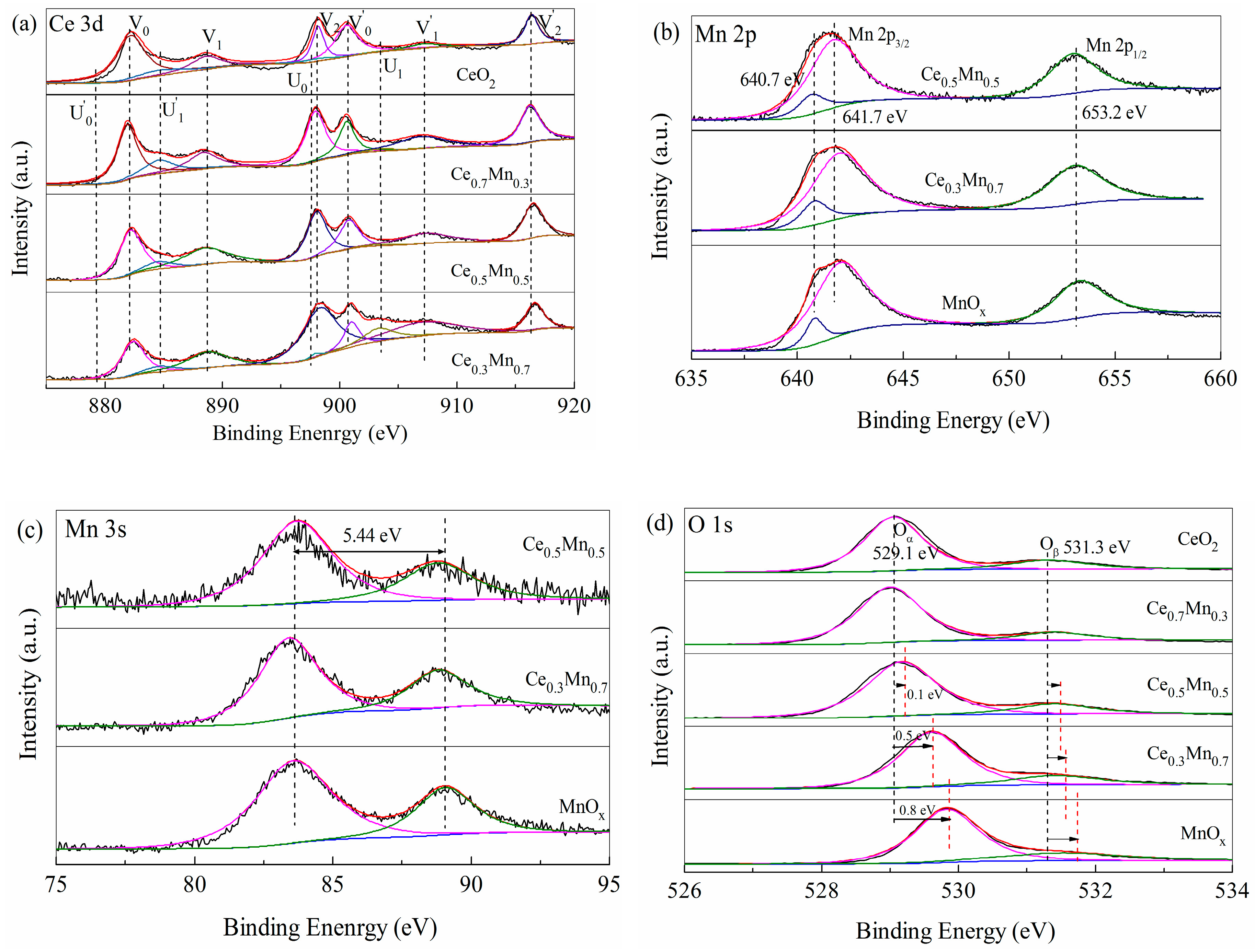
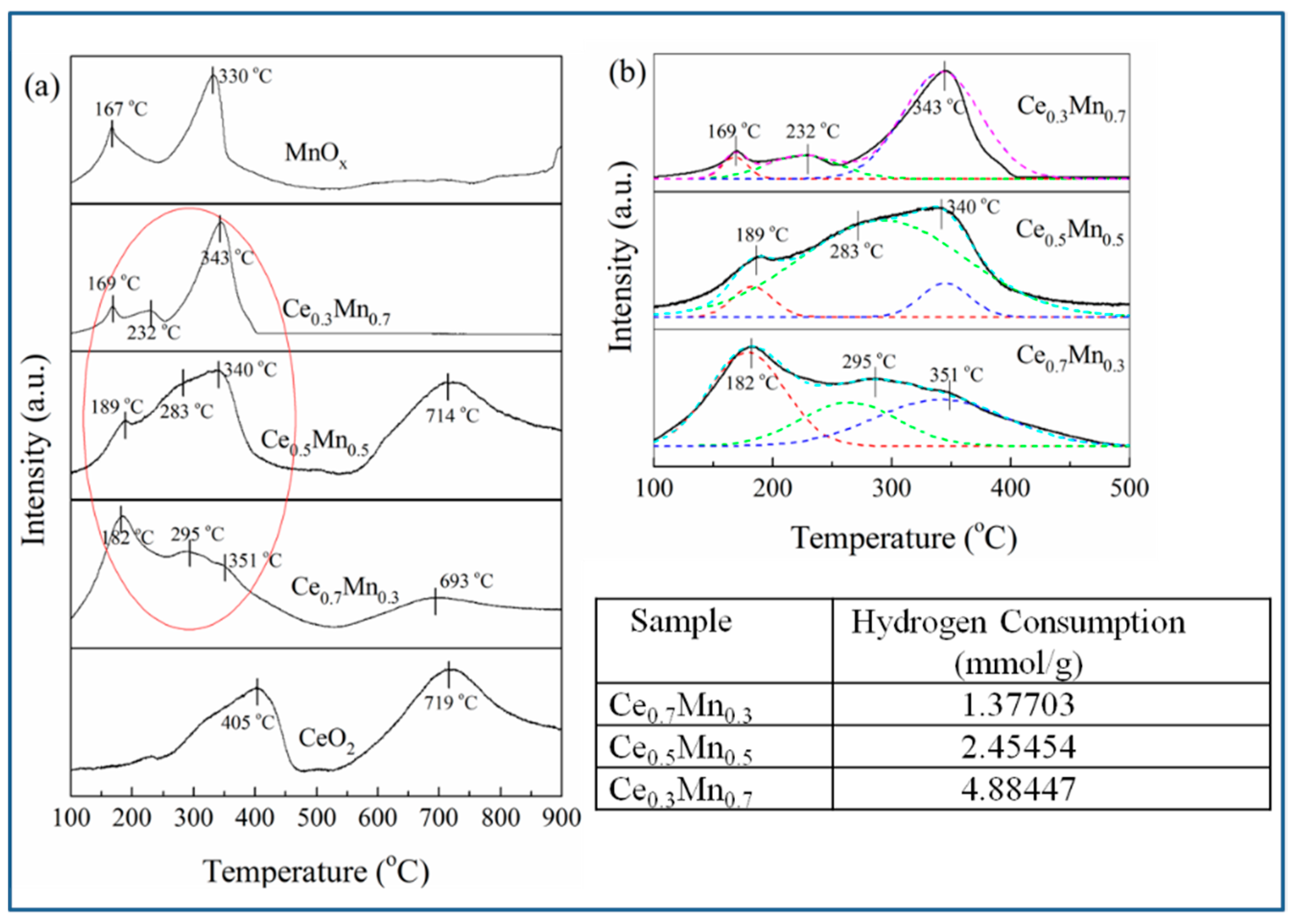
3.2. Characterization of CexMn1−x Catalysts
3.3. Factors Influencing the Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Garcia, T.; Solsona, B.; Cazorla-Amoros, D.; Linares-Solano, A.; Taylor, S.H. Total oxidation of volatile organic compounds by vanadium promoted palladium-titania catalysts: Comparison of aromatic and polyaromatic compounds. Appl. Catal. B Environ. 2006, 62, 66–76. [Google Scholar] [CrossRef]
- Tang, X.F.; Xu, Y.D.; Shen, W.J. Promoting effect of copper on the catalytic activity of MnOx-CeO2 mixed oxide for complete oxidation of benzene. Chem. Eng. J. 2008, 144, 175–180. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S. Comparative study on the catalytic activities of alumina-supported metal oxides for oxidation of benzene and cyclohexane with ozone. React. Kinet. Catal. Lett. 2004, 81, 121–128. [Google Scholar] [CrossRef]
- Einaga, H.; Ogata, A. Benzene oxidation with ozone over supported manganese oxide catalysts: Effect of catalyst support and reaction conditions. J. Hazard. Mater. 2009, 164, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Einaga, H.; Teraoka, Y.; Ogat, A. Benzene oxidation with ozone over manganese oxide supported on zeolite catalysts. Catal. Today 2011, 164, 571–574. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic Removal of Toluene over Co3O4-CeO2 Mixed Oxide Catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marci, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4-CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef]
- Lojewska, J.; Kolodziej, A.; Lojewski, T.; Kapica, R.; Tyczkowski, J. Structured cobalt oxide catalyst for VOC combustion. Part I: Catalytic and engineering correlations. Appl. Catal. A Gen. 2009, 366, 206–211. [Google Scholar] [CrossRef]
- Walerczyk, W.; Zawadzki, M. Structural and catalytic properties of Pt/ZnAl2O4 as catalyst for VOC total oxidation. Catal. Today 2011, 176, 159–162. [Google Scholar] [CrossRef]
- Zwinkels, M.F.M.; Jaras, S.G.; Menon, P.G.; Griffin, T.A. Catalytic Materials for High-Temperature Combustion. Catal. Rev.-Sci. Eng. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of gamma-MnO2 as a VOC removal catalyst: Comparison with a noble metal catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Dai, Q.G.; Wang, X.Y.; Lu, G.Z. Low-temperature catalytic combustion of trichloroethylene over cerium oxide and catalyst deactivation. Appl. Catal. B Environ. 2008, 81, 192–202. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.W.; Inyang, H.I. Catalytic oxidation of toluene in contaminant emission control systems using Mn-Ce/γ-Al2O3. Environ. Technol. 2008, 29, 559–569. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic Activity of Supported Platinum and Metal Oxide Catalysts for Toluene Oxidation. Top. Catal. 2009, 52, 517–527. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx-CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Marques, R.R.N.; Quinta-Ferreira, R.M. Catalysts based in cerium oxide for wet oxidation of acrylic acid in the prevention of environmental risks. Appl. Catal. B Environ. 2004, 47, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.F.; Li, Y.G.; Huang, X.M.; Xu, Y.D.; Zhu, H.Q.; Wang, J.G.; Shen, W.J. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, G.L.; Li, J.Q.; Liu, H.D.; Wang, Q.; Chen, Y.F. Catalytic removal of benzene over CeO2-MnOx composite oxides prepared by hydrothermal method. Appl. Catal. B Environ. 2013, 138, 253–259. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Shen, G.L.; Liu, H.D.; Chen, Y.F.; Wang, Q. Catalytic removal of benzene over CeO2-MnOx composite oxides with rod-like morphology supporting PdO. J. Nanopart. Res. 2014, 16, 2367. [Google Scholar] [CrossRef]
- Tang, W.X.; Wu, X.F.; Li, S.D.; Li, W.H.; Chen, Y.F. Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Dai, H.X.; Du, Y.C.; Zhang, L.; Deng, J.G.; Xia, Y.S.; Zhao, Z.X.; Meng, X.; Liu, Y.X. P123-PMMA Dual-Templating Generation and Unique Physicochemical Properties of Three-Dimensionally Ordered Macroporous Iron Oxides with Nanovoids in the Crystalline Walls. Inorg. Chem. 2011, 50, 2534–2544. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.F.; Suib, S.L.; O’Young, C.L. Characterization of manganese oxide octahedral molecular sieve (M-OMS-2) materials with different metal cation dopants. Chem. Mater. 2002, 14, 940–948. [Google Scholar] [CrossRef]
- Pfau, A.; Schierbaum, K.D. The Electronic-Structure of Stoichiometric and Reduced CeO2 Surfaces—An XPS, UPS and HREELS Study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Xiao, W.D.; Guo, Q.L.; Wang, E.G. Transformation of CeO2 (111) to Ce2O3 (0001) films. Chem. Phys. Lett. 2003, 368, 527–531. [Google Scholar] [CrossRef]
- Lenormand, F.; Hilaire, L.; Kili, K.; Krill, G.; Maire, G. Oxidation-State of Cerium in Cerium-Based Catalysts Investigated by Spectroscopic Probes. J. Phys. Chem. 1988, 92, 2561–2568. [Google Scholar] [CrossRef]
- Machida, M.; Uto, M.; Kurogi, D.; Kijima, T. MnOx-CeO2 binary oxides for catalytic NOx sorption at low temperatures. Sorptive removal of NOx. Chem. Mater. 2000, 12, 3158–3164. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Rao, T.; Shen, M.Q.; Jia, L.W.; Hao, J.J.; Wang, J. Oxidation of ethanol over Mn-Ce-O and Mn-Ce-Zr-O complex compounds synthesized by sol-gel method. Catal. Commun. 2007, 8, 1743–1747. [Google Scholar] [CrossRef]
- Dula, R.; Janik, R.; Machej, T.; Stoch, J.; Grabowski, R.; Serwicka, E.M. Mn-containing catalytic materials for the total combustion of toluene: The role of Mn localisation in the structure of LDH precursor. Catal. Today 2007, 119, 327–331. [Google Scholar] [CrossRef]
- Han, Y.F.; Chen, F.X.; Zhong, Z.Y.; Ramesh, K.; Chen, L.W.; Widjaja, E. Controlled synthesis, characterization, and catalytic properties of Mn2O3 and Mn3O4 nanoparticles supported on mesoporous silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.Q.; Mao, W.; Yang, X.J.; Zhu, K.K.; Xu, J.; Han, Y.F. Probing the Surface Structure of α-Mn2O3 Nanocrystals during CO Oxidation by Operando Raman Spectroscopy. J. Phys. Chem. C 2012, 116, 20975–20981. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Demeter, M.; Bartkowski, S.; Neumann, M.; Ovechkina, N.A.; Kurmaev, E.Z.; Logachevskaya, N.I.; Mukovskii, Y.M.; Mitchell, J.; Ederer, D.L. Mn 3s exchange splitting in mixed-valence manganites. Phys. Rev. B 2002, 65, 113102. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.Q.; Qin, Z.F.; Liang, F.X.; Wang, G.F.; Wang, J.G. Deactivation of a Au/CeO2-Co3O4 catalyst during CO preferential oxidation in H2-rich stream. J. Catal. 2009, 264, 154–162. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhang, Y.X.; Liu, M.; Ma, Z.C.C. Complete catalytic oxidation of o-xylene over Mn-Ce oxides prepared using a redox-precipitation method. Catal. Today 2010, 153, 170–175. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloy Compd. 2006, 408, 1096–1102. [Google Scholar] [CrossRef]
- Tana; Zhang, M.L.; Li, J.; Li, H.J.; Li, Y.; Shen, W.J. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Tang, X.F.; Chen, J.L.; Li, Y.G.; Li, Y.; Xu, Y.D.; Shen, W.J. Complete oxidation of formaldehyde over Ag/MnOx-CeO2 catalysts. Chem. Eng. J. 2006, 118, 119–125. [Google Scholar] [CrossRef]
- Tang, W.X.; Wu, X.F.; Li, D.Y.; Wang, Z.; Liu, G.; Liu, H.D.; Chen, Y.F. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
| Catalyst | Benzene Oxidation Activity | ||
|---|---|---|---|
| T10% (°C) | T50% (°C) | T90% (°C) | |
| CeO2 | 145 | 200 | 257 |
| Ce0.7Mn0.3 | 153 | 200 | 220 |
| Ce0.5Mn0.5 | 128 | 185 | 218 |
| Ce0.3Mn0.7 | 117 | 156 | 190 |
| MnOx | 194 | 241 | 273 |
| Ce0.3Mn0.7 (Hydrothermal) | 200 | 265 | 350 |
| Ce0.3Mn0.7 (Carbonate) | 150 | 220 | 250 |
| Sample | O | Oα/(Oα + Oβ) (%) | Ce (%) | Mn (%) | |
|---|---|---|---|---|---|
| Oα | Oβ | Ce3+/(Ce3+ + Ce4+) | Mn2+/(Mn2+ + Mn3+) | ||
| CeO2 | 529.0 | 531.3 | 77.16 | 11.05 | - |
| Ce0.7Mn0.3 | 529.1 | 531.3 | 81.01 | 10.89 | 16.23 |
| Ce0.5Mn0.5 | 529.1 | 531.3 | 82.04 | 10.05 | 15.12 |
| Ce0.3Mn0.7 | 529.2 | 531.5 | 83.18 | 5.65 | 13.42 |
| MnOx | 529.6 | 531.6 | 78.47 | - | 10.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Shen, G.; Liu, M.; Chen, Y.; Wang, Z.; Wang, Q. Preparation of Ce–Mn Composite Oxides with Enhanced Catalytic Activity for Removal of Benzene through Oxalate Method. Nanomaterials 2019, 9, 197. https://doi.org/10.3390/nano9020197
Yang M, Shen G, Liu M, Chen Y, Wang Z, Wang Q. Preparation of Ce–Mn Composite Oxides with Enhanced Catalytic Activity for Removal of Benzene through Oxalate Method. Nanomaterials. 2019; 9(2):197. https://doi.org/10.3390/nano9020197
Chicago/Turabian StyleYang, Min, Genli Shen, Mi Liu, Yunfa Chen, Zhen Wang, and Qi Wang. 2019. "Preparation of Ce–Mn Composite Oxides with Enhanced Catalytic Activity for Removal of Benzene through Oxalate Method" Nanomaterials 9, no. 2: 197. https://doi.org/10.3390/nano9020197
APA StyleYang, M., Shen, G., Liu, M., Chen, Y., Wang, Z., & Wang, Q. (2019). Preparation of Ce–Mn Composite Oxides with Enhanced Catalytic Activity for Removal of Benzene through Oxalate Method. Nanomaterials, 9(2), 197. https://doi.org/10.3390/nano9020197






