Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Lignin Hollow NPs
2.3. Preparation of Magnetic NPs (MLHNPs)
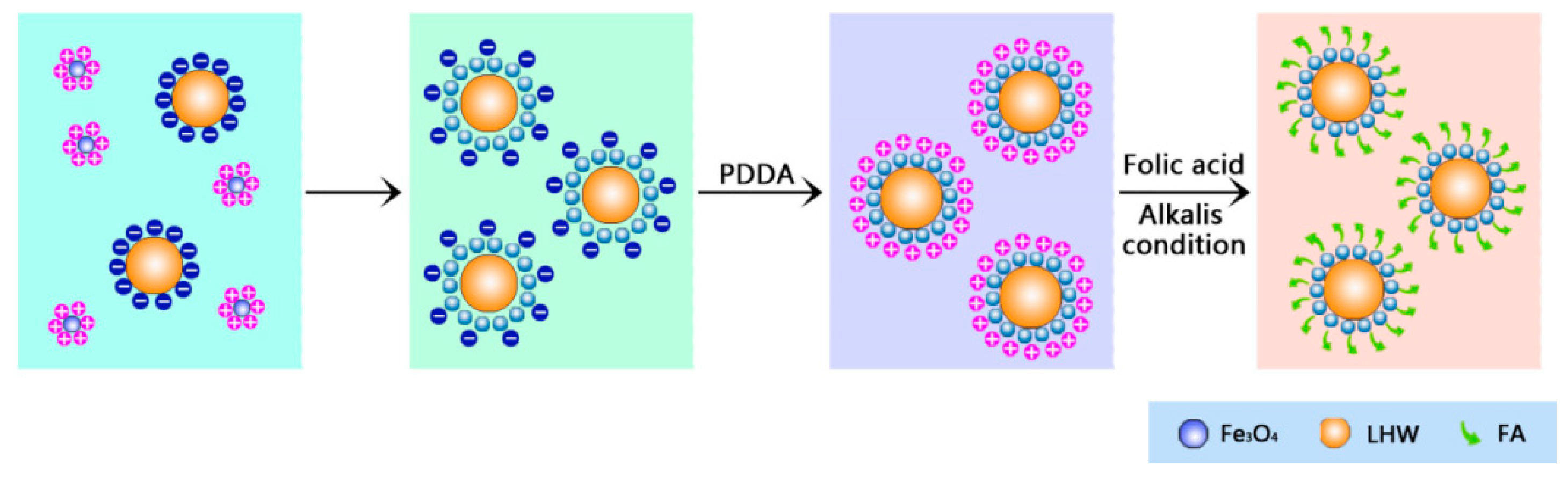
2.4. Synthesis of LHNPs Functionalized with Magnetic NPs and Folic Acid Molecule
2.5. Drug Loading
2.6. In Vitro Release Studies
2.7. Cellular Uptake of Modified LHNPs Assays
2.8. In Vitro Cytotoxicity Studies
3. Results and Discussion
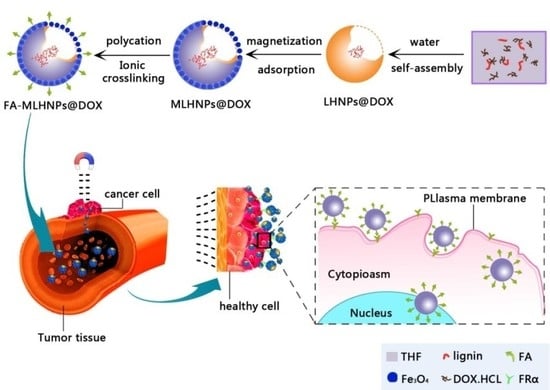
3.1. The Preparation of Folic-Magnetic-Functionalized Lignin Hollow Nanoparticles (FA-MLHNPs) and Formation Mechanism of NPs
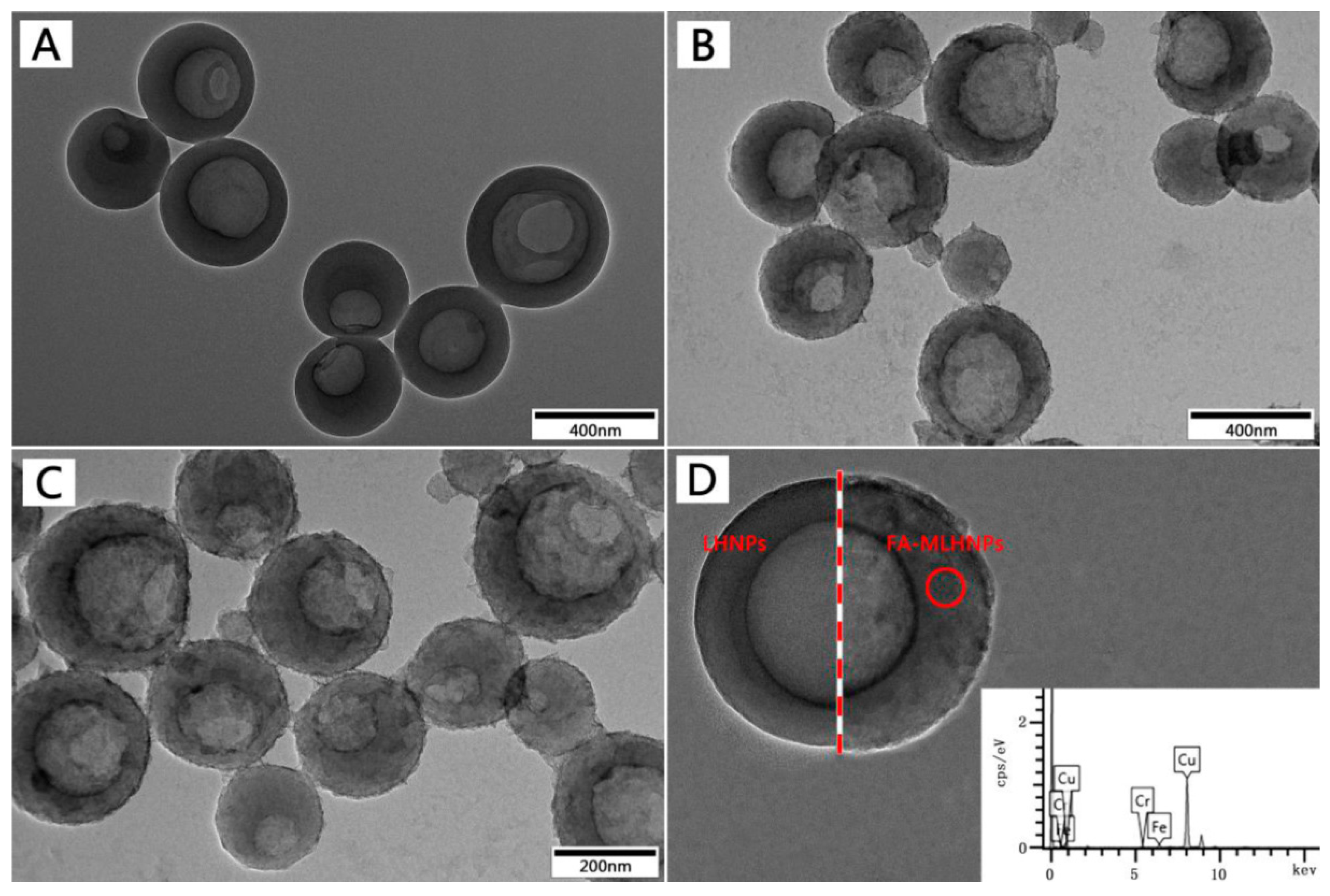
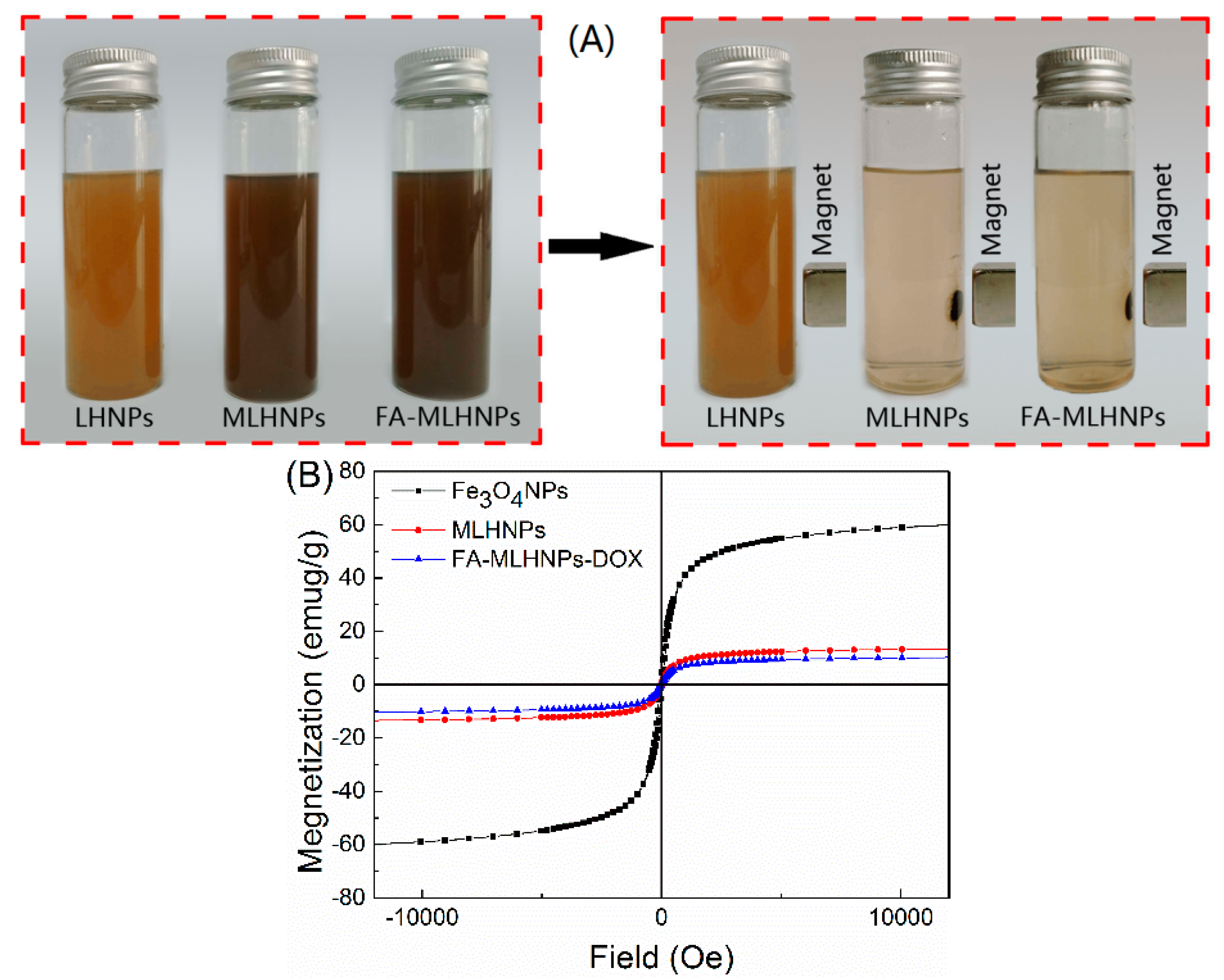
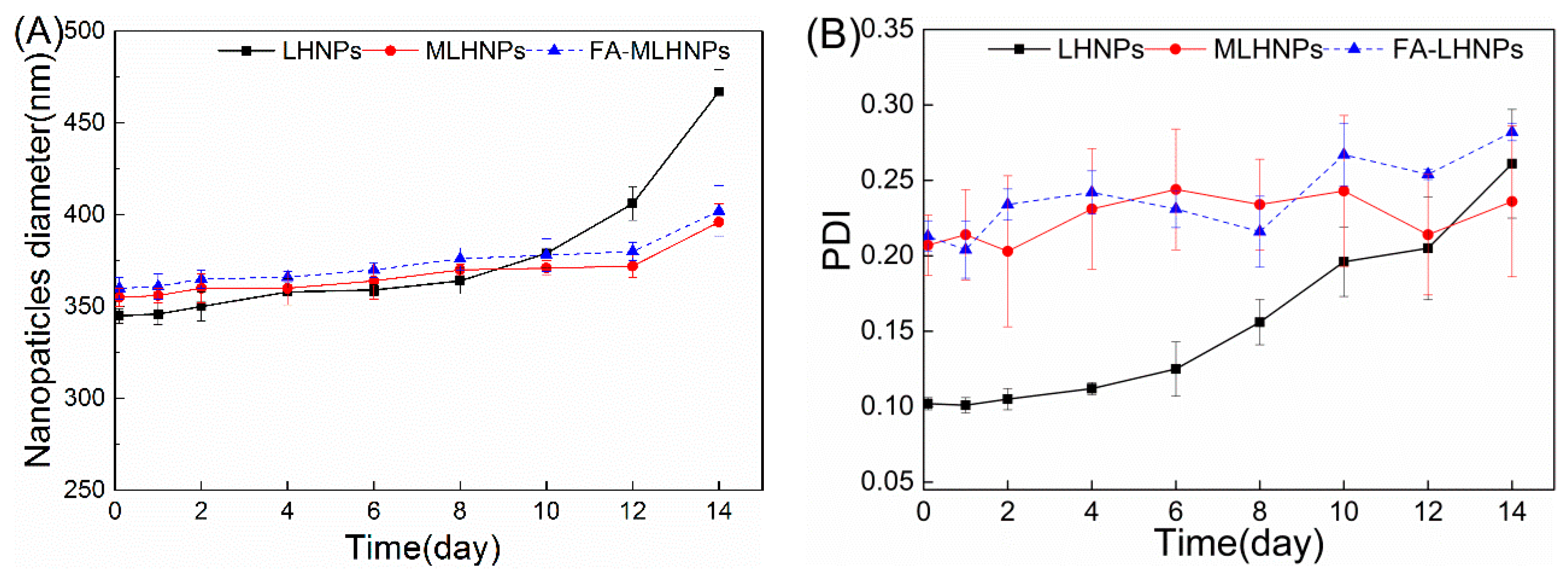
3.2. FA-MLHNPs Characterization
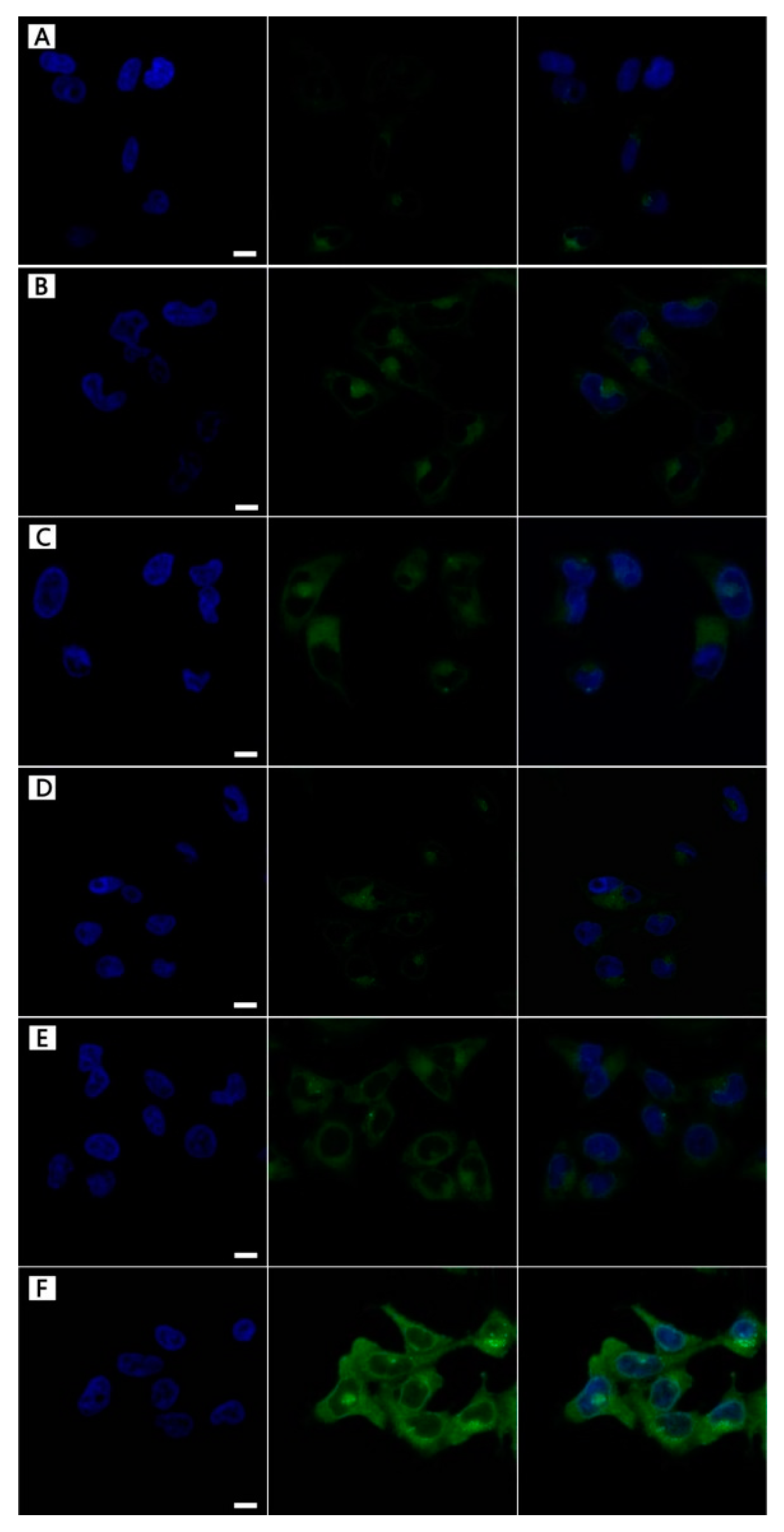
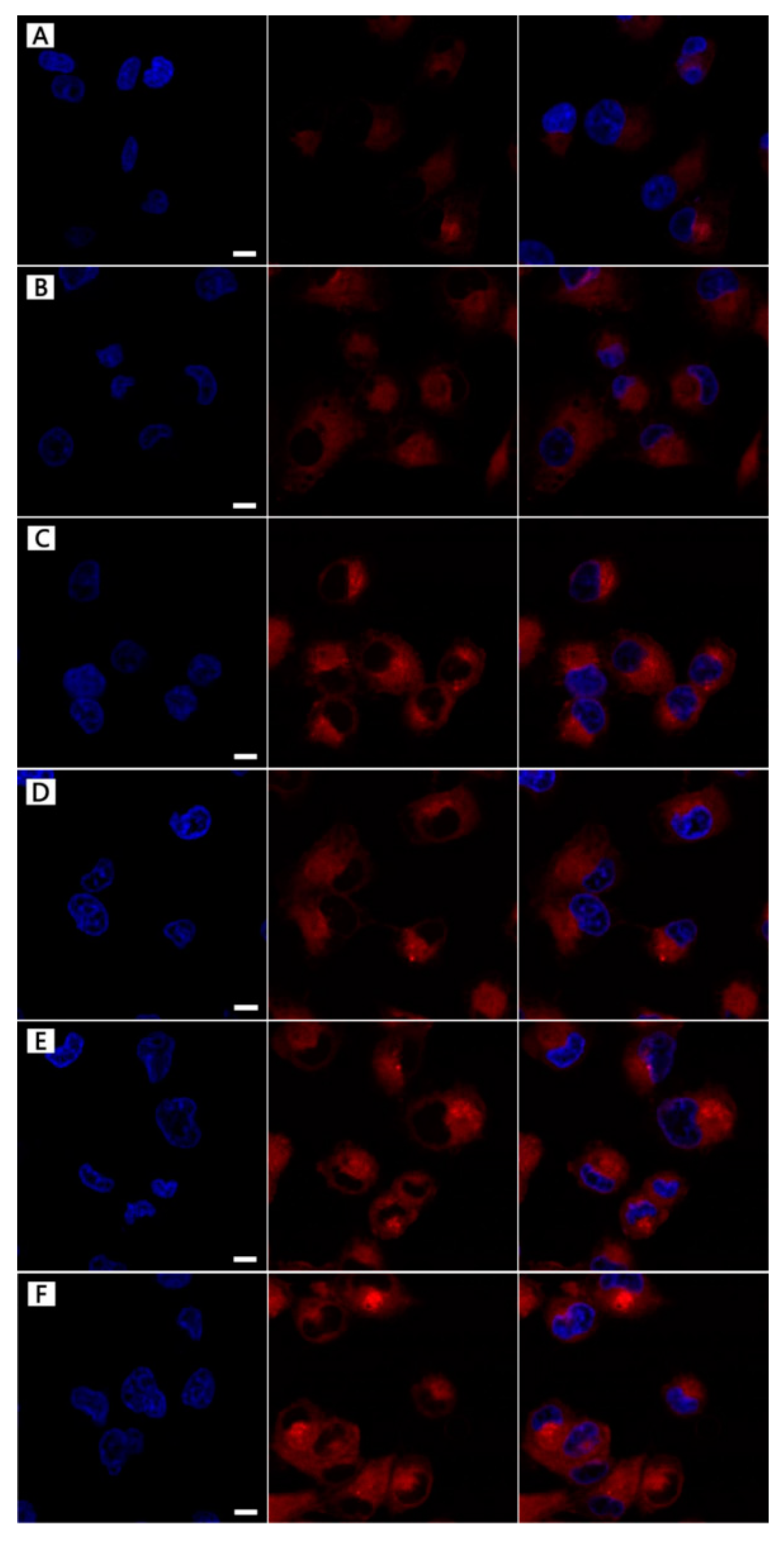
3.3. NPs Cellular Uptake
3.4. In Vitro Drug Loading
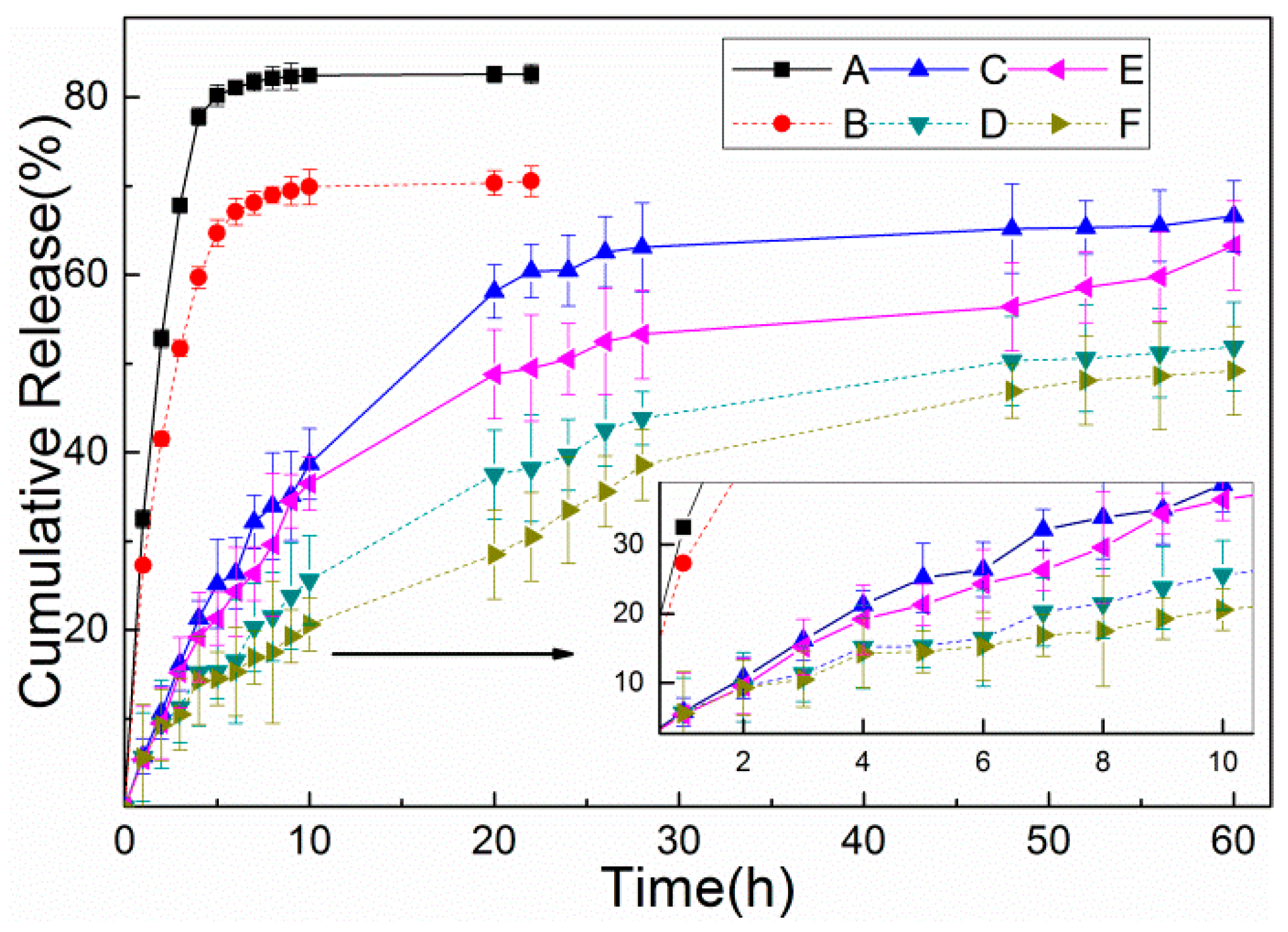
3.5. In Vitro Release Studies
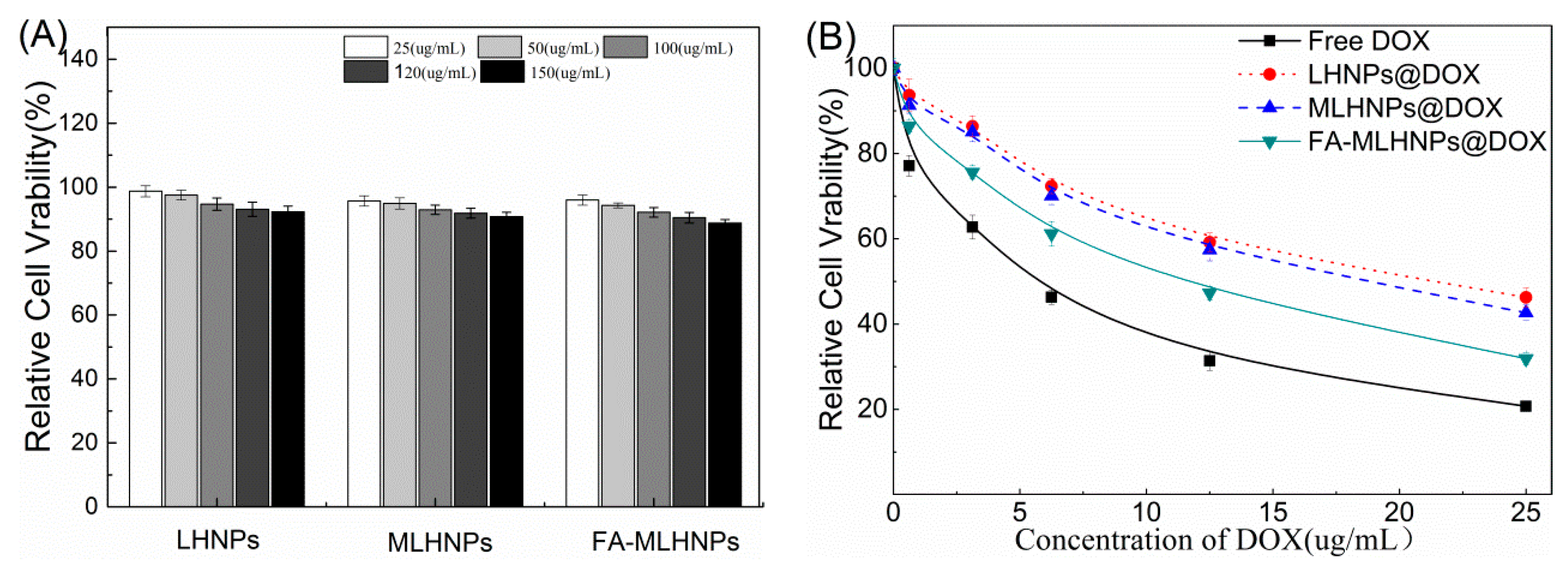
3.6. In Vitro Cytotoxicity Studies
3.7. DOX-Loading NPs Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmark of cancer. Cell 2000, 100, 57–71. [Google Scholar] [CrossRef]
- Roizen, M.F. Hallmarks of Cancer: The Next Generation. Yearb. Anesthesiol. Pain Manag. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Shrestha, N.; Mäkilä, E.; Araújo, F.; Correia, A.; Ramos, T.; Sarmento, B.; Salonen, J.; Hirvonen, J.; Santos, H.A. A prospective cancer chemo-immunotherapy approach mediated by synergistic CD326 targeted porous silicon nanovectors. Nano Res. 2015, 8, 1505–1521. [Google Scholar] [CrossRef]
- Herranzblanco, B.; Shahbazi, M.A.; Correia, A.R.; Balasubramanian, V.; Kohout, T.; Hirvonen, J.; Santos, H.A. Targeted Cancer Therapy: pH-Switch Nanoprecipitation of Polymeric Nanoparticles for Multimodal Cancer Targeting and Intracellular Triggered Delivery of Doxorubicin (Adv. Healthcare Mater. 15/2016). Adv. Healthc. Mater. 2016, 5, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Balasubramanian, V.; Shahbazi, M.A.; Correia, A.; Wu, D.; Palivan, C.G.; Hirvonen, J.T.; Santos, H.A. Angiopep2-functionalized Polymersomes for Targeted Doxorubicin Delivery to Glioblastoma Cells. Int. J. Pharm. 2016, 511, 794–803. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, X.; Zhang, H.; Qu, X.; Chen, D.; Servos, M.; Mäkilä, E.; Salonen, J.; Santos, H.A.; Hai, M. Inhibition of Multidrug Resistance of Cancer Cells by Co− Delivery of DNA Nanostructures and Drugs Using Porous Silicon Nanoparticles@Giant Liposomes. Adv. Funct. Mater. 2015, 25, 3330–3340. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Calvoflores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Pei, L.C.; Yun, K.C.; Yong, L.Y.; Xian, J.L. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Casas, A.; Alonso, M.V.; Oliet, M.; Rojo, E.; Rodríguez, F. FTIR analysis of lignin regenerated from Pinus radiata and Eucalyptus globulus woods dissolved in imidazolium-based ionic liquids. J. Chem. Technol. Biotechnol. 2012, 87, 472–480. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. Simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-Based Microsphere: Preparation and Performance on Encapsulating the Pesticide Avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-responsive Lignin Based Nanocapsules for Controlled Release of Hydrophobic Molecules. ACS Sustain. Chem. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.; Zou, Z.; Si, C. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 5204–5211. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustain. Chem. Eng. 2017, 5, 97–108. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Leu, A.J.; Berk, D.A.; Lymboussaki, A.; Alitalo, K.; Jain, R.K. Absence of functional lymphatics within a murine sarcoma: A molecular and functional evaluation. Cancer Res. 2000, 60, 4324–4327. [Google Scholar]
- Wang, C.; Yan, J.; Cui, X.; Cong, D.; Wang, H. Preparation and characterization of magnetic hollow PMMA nanospheres via in situ emulsion polymerization. Colloids Surf. A Phys. Eng. Asp. 2010, 363, 71–77. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of Magnetic Lignin-Based Hollow Microspheres: A Highly Adsorptive and Reusable Adsorbent Derived from Renewable Resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.H.; Fang, G.Z.; Su, L.; Li, J.M. Preparation and characterization of hydroxycamptothecin loated folate-chitosan nanoparticles as a sustained-release drugs carrier. J. Harbin Univ. Commer. 2012, 684, 57–62. [Google Scholar]
- Sahana, D.K.; Mittal, G.; Bhardwaj, V.; Kumar, M.N.V.R. PLGA nanoparticles for oral delivery of hydrophobic drugs: Influence of organic solvent on nanoparticle formation and release behavior In Vitro and In Vivo using estradiol as a model drug. J. Pharm. Sci. 2010, 97, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Shafi, K.V.P.M.; Ulman, A.; Dyal, A.; Yan, X.; Yang, N.L.; Estournès, C.; Fournès, L.; Wattiaux, A.; White, H.; Rafailovich, M. Magnetic Enhancement of γ-Fe2O3 Nanoparticles by Sonochemical Coating. Chem. Mater. 2012, 14, 1778–1787. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 2012, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kamalapuram, S.K.; Krishnakumar, S.; Kanwar, R.K. Multimodal iron oxide (Fe3O4)-saturated lactoferrin nanocapsules as nanotheranostics for real-time imaging and breast cancer therapy of claudin-low, triple-negative (ER-/PR-/HER2-). Nanomedicine 2016, 11, 249–268. [Google Scholar] [CrossRef]
- Yu, P.; Xia, X.M.; Wu, M.; Cui, C.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.X.; Zhang, L.J.; Zhou, X. Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drug delivery. Colloids Surf. B Biointerfaces 2014, 120, 142–151. [Google Scholar] [CrossRef]
- Wang, H.; He, J.; Cao, D.; Zhang, M.; Li, F.; Tam, K.C.; Ni, P. Synthesis of acid-labile polymeric prodrug DOX-acetal-PEG-acetal-DOX with high drug loading content for pH-triggered intracellular drug release. Polym. Chem. 2015, 6, 4809–4818. [Google Scholar] [CrossRef]
- Choi, D.; Heo, J.; Hong, J. Controllable drug release from nano-layered hollow carrier by non-human enzyme. Nanoscale 2018, 10, 18228–18237. [Google Scholar] [CrossRef] [PubMed]
- Yiamsawas, D.; Beckers, S.; Hao, L.; Landfester, K.; Wurm, F.R.; Yiamsawas, D.; Beckers, S.; Hao, L.; Landfester, K.; Wurm, F.R. Morphology-Controlled Synthesis of Lignin Nanocarriers for Drug Delivery and Carbon-Materials. ACS Biomater. Sci. Eng. 2017, 3, 2375–2383. [Google Scholar] [CrossRef]
- Javed, K.R.; Ahmad, M.; Ali, S.; Butt, M.Z.; Nafees, M.; Butt, A.R.; Nadeem, M.; Shahid, A. Comparison of Doxorubicin Anticancer Drug Loading on Different Metal Oxide Nanoparticles. Medicine 2015, 94, e617. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2011, 14, 1–16. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef]

| Sample | ζ-Potential (mV) | Average Size (nm) | PDI |
|---|---|---|---|
| Fe3O4 NPs | 34.7 ± 17 | 10 ± 4 | 0.186 |
| LHNPs | −38.2 ± 9 | 286 ± 8 | 0.208 |
| MLHNPs | −27.1 ± 7 | 302 ± 14 | 0.243 |
| FA-MLHNPs | −20.2 ± 5 | 314 ± 11 | 0.294 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Xiong, F.; Chu, F. Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials 2019, 9, 188. https://doi.org/10.3390/nano9020188
Zhou Y, Han Y, Li G, Yang S, Xiong F, Chu F. Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials. 2019; 9(2):188. https://doi.org/10.3390/nano9020188
Chicago/Turabian StyleZhou, Yu, Yanming Han, Gaiyun Li, Sheng Yang, Fuquan Xiong, and Fuxiang Chu. 2019. "Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin" Nanomaterials 9, no. 2: 188. https://doi.org/10.3390/nano9020188
APA StyleZhou, Y., Han, Y., Li, G., Yang, S., Xiong, F., & Chu, F. (2019). Preparation of Targeted Lignin–Based Hollow Nanoparticles for the Delivery of Doxorubicin. Nanomaterials, 9(2), 188. https://doi.org/10.3390/nano9020188