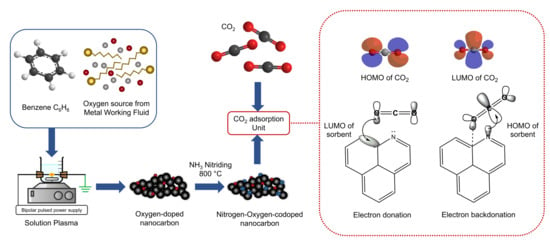
Nitriding an Oxygen-Doped Nanocarbonaceous Sorbent Synthesized via Solution Plasma Process for Improving CO2 Adsorption Capacity
Abstract
1. Introduction
2. Materials and Methods
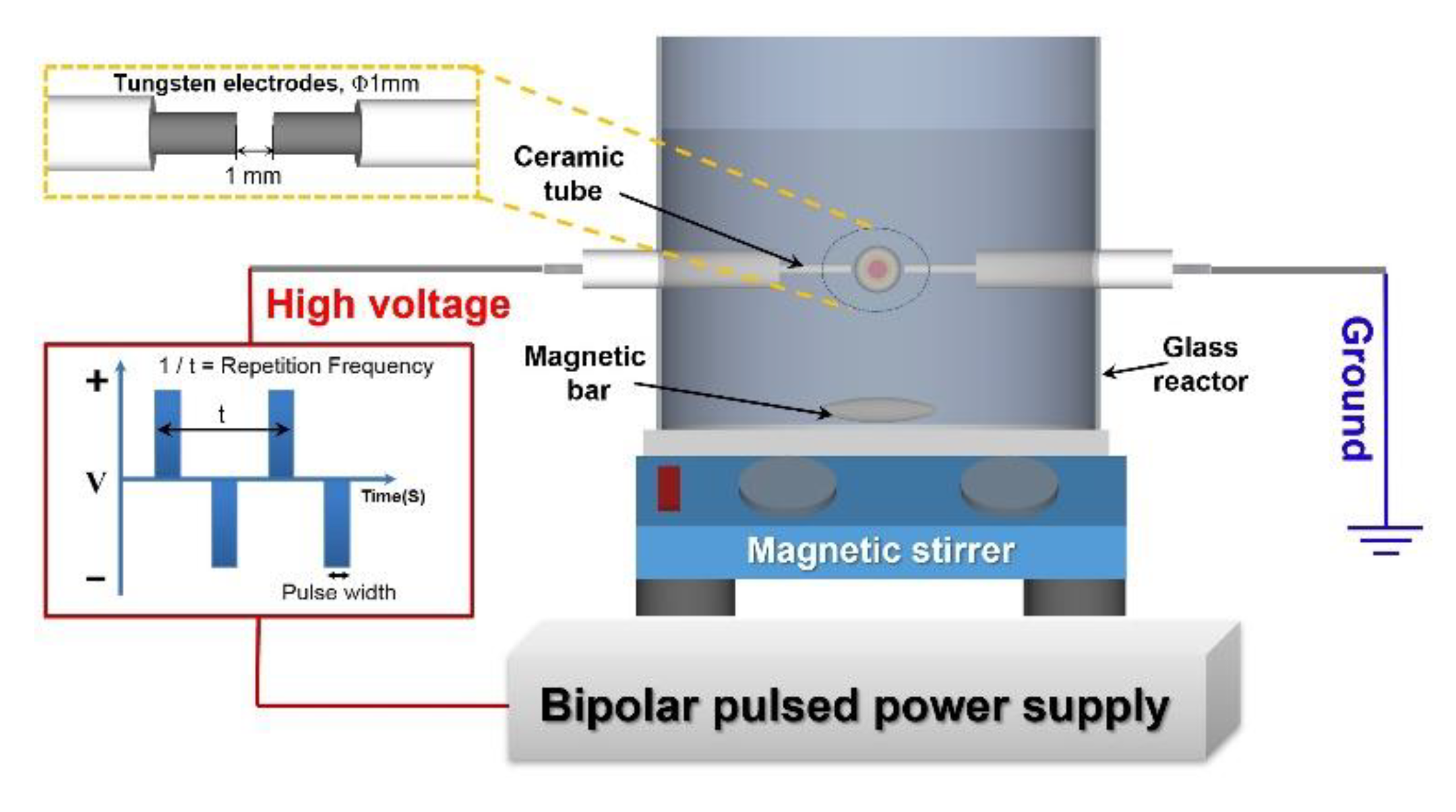
2.1. Sorbent Preparation
2.2. Measurement of CO2 Adsorption
2.3. Characterization of the Sorbents
3. Results and Discussion
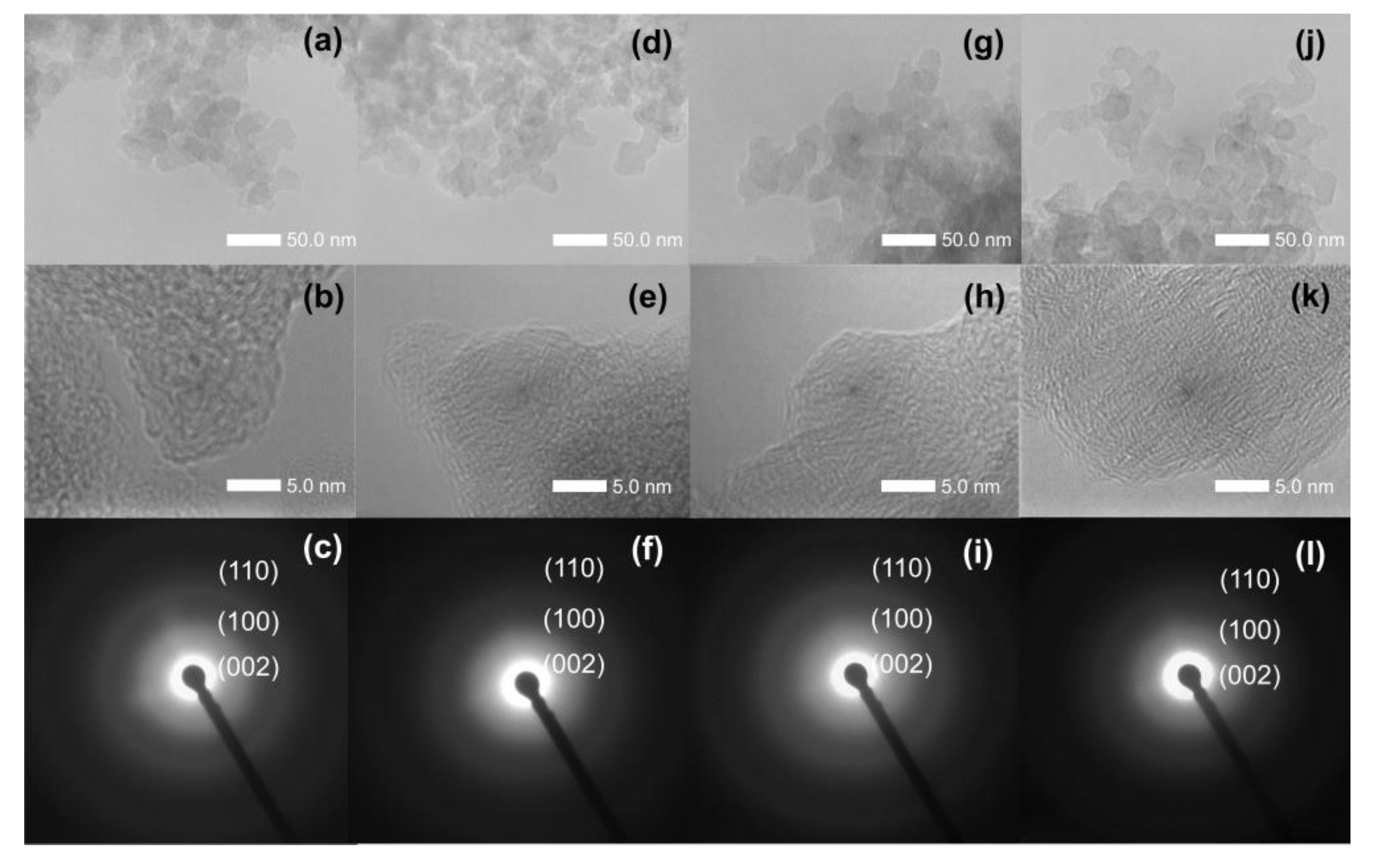
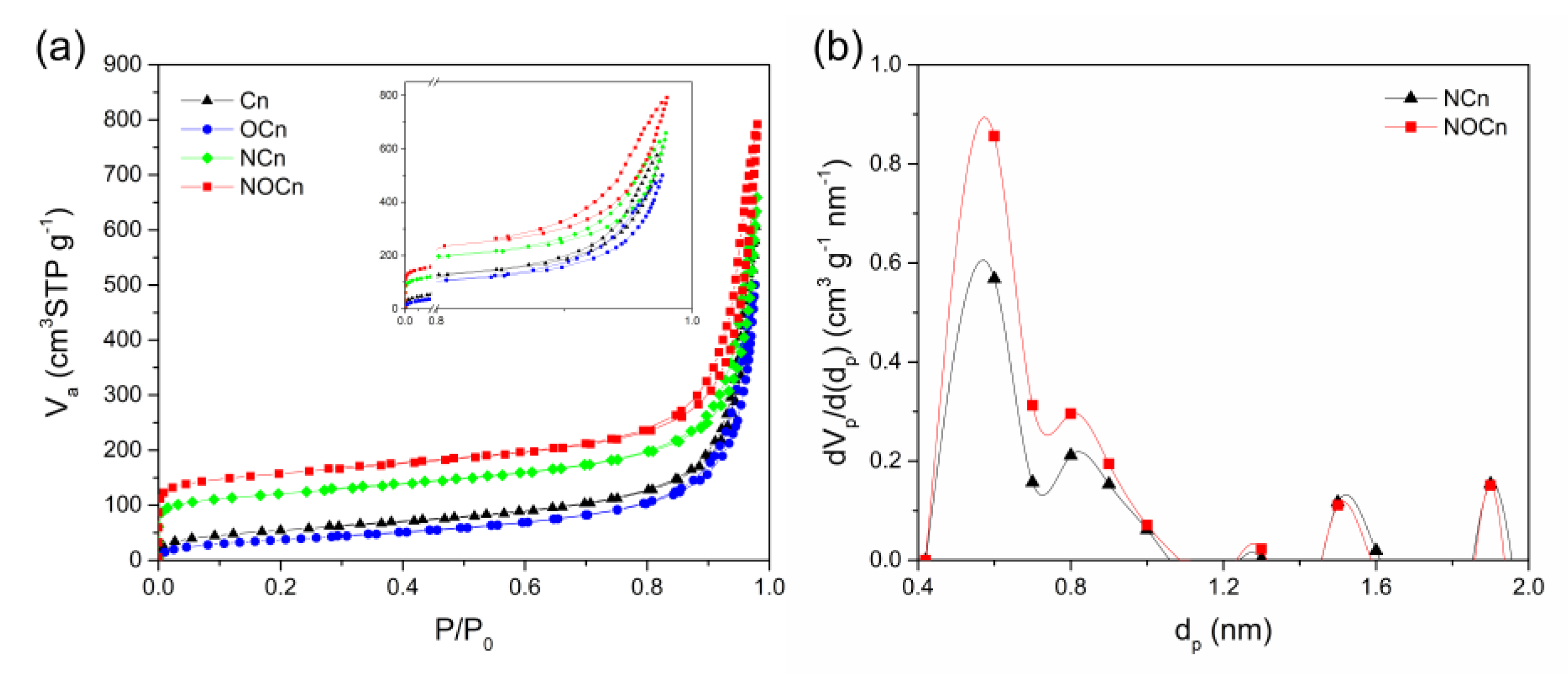
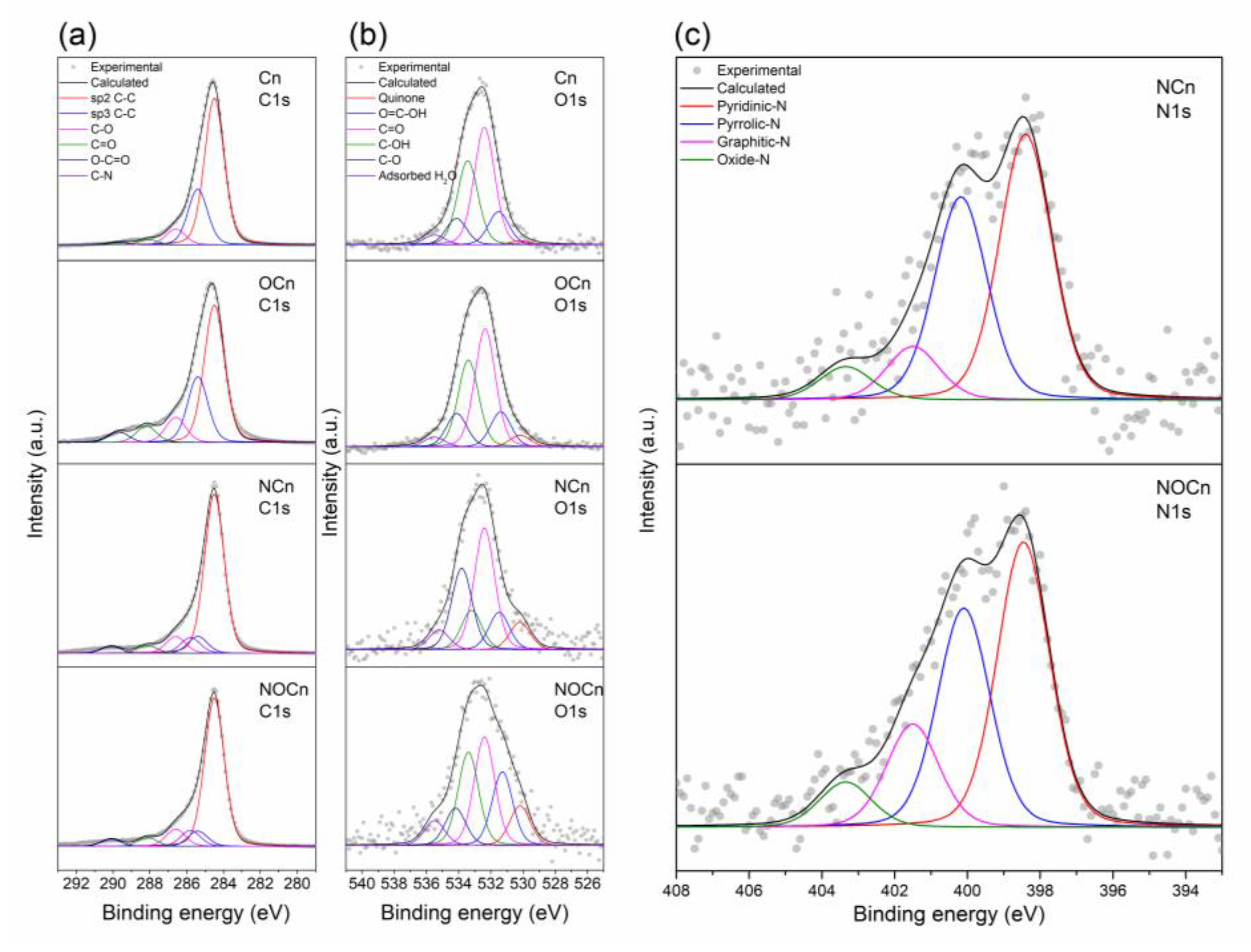
3.1. Characterization of Sorbents
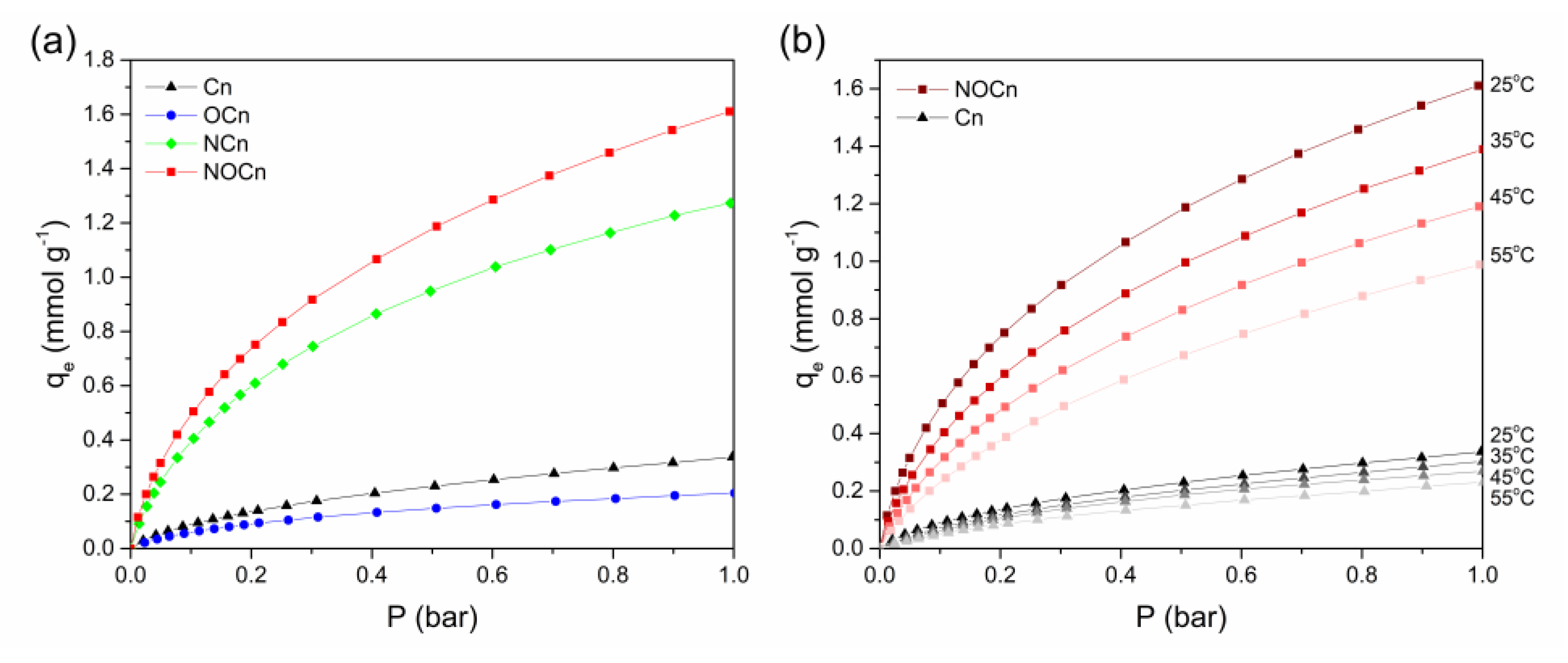
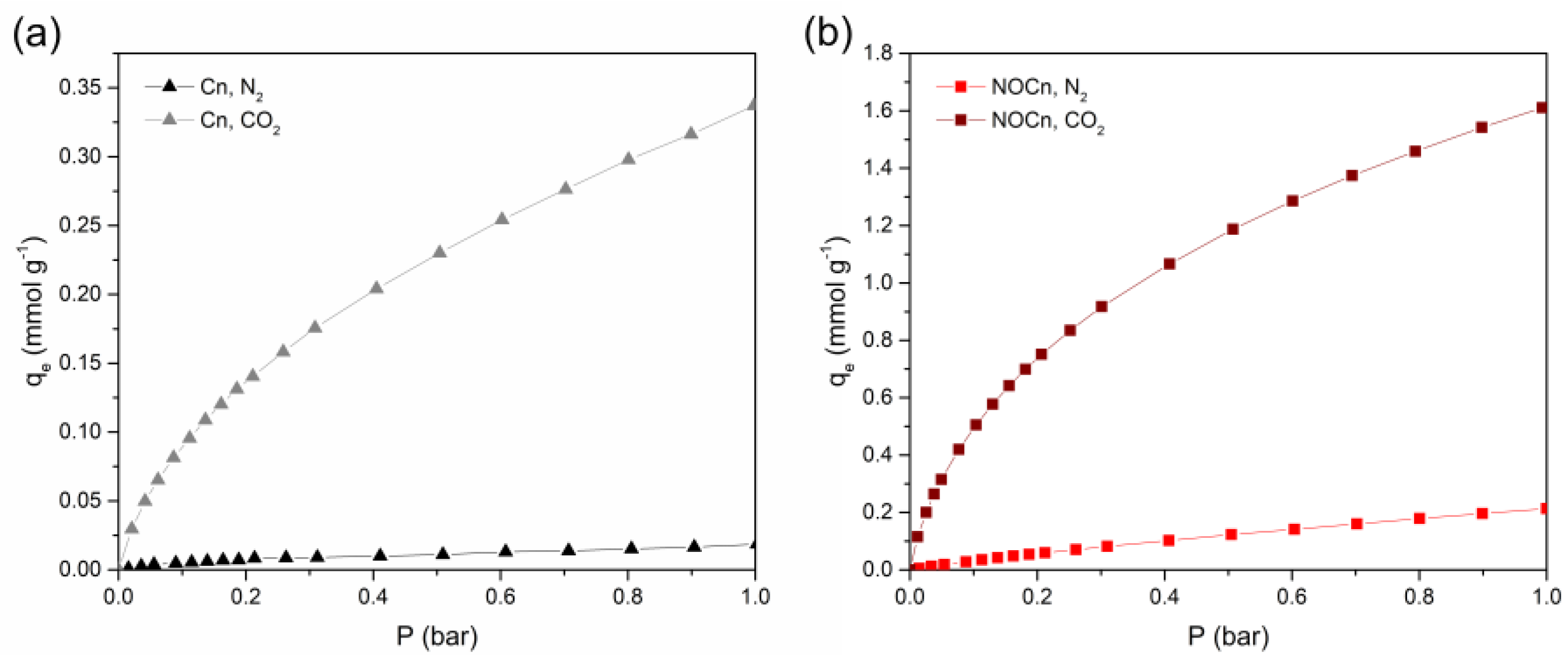
3.2. Performance of CO2 Adsorption
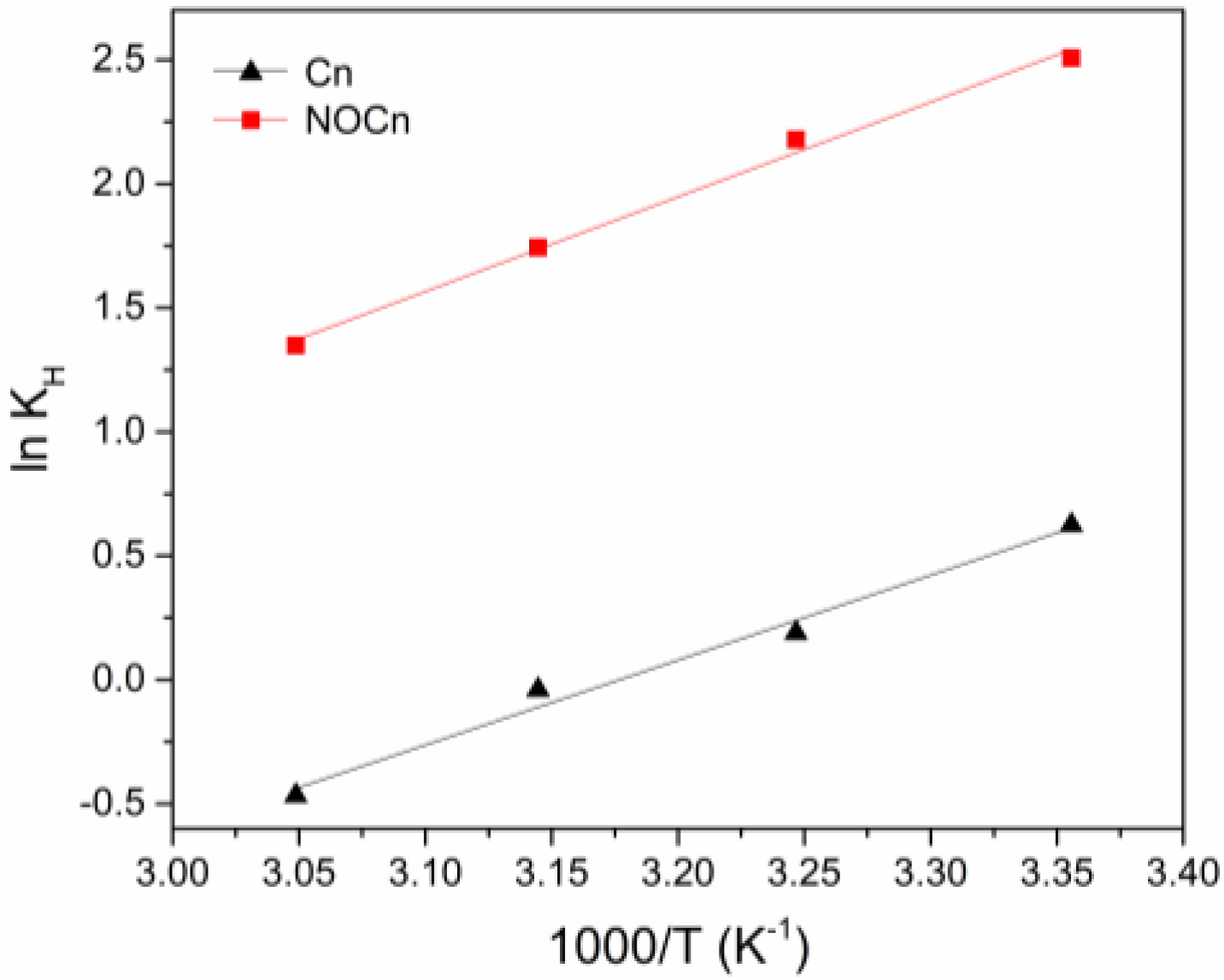
3.3. Thermodynamic Studies
3.4. The Selectivity of CO2 Adsorption
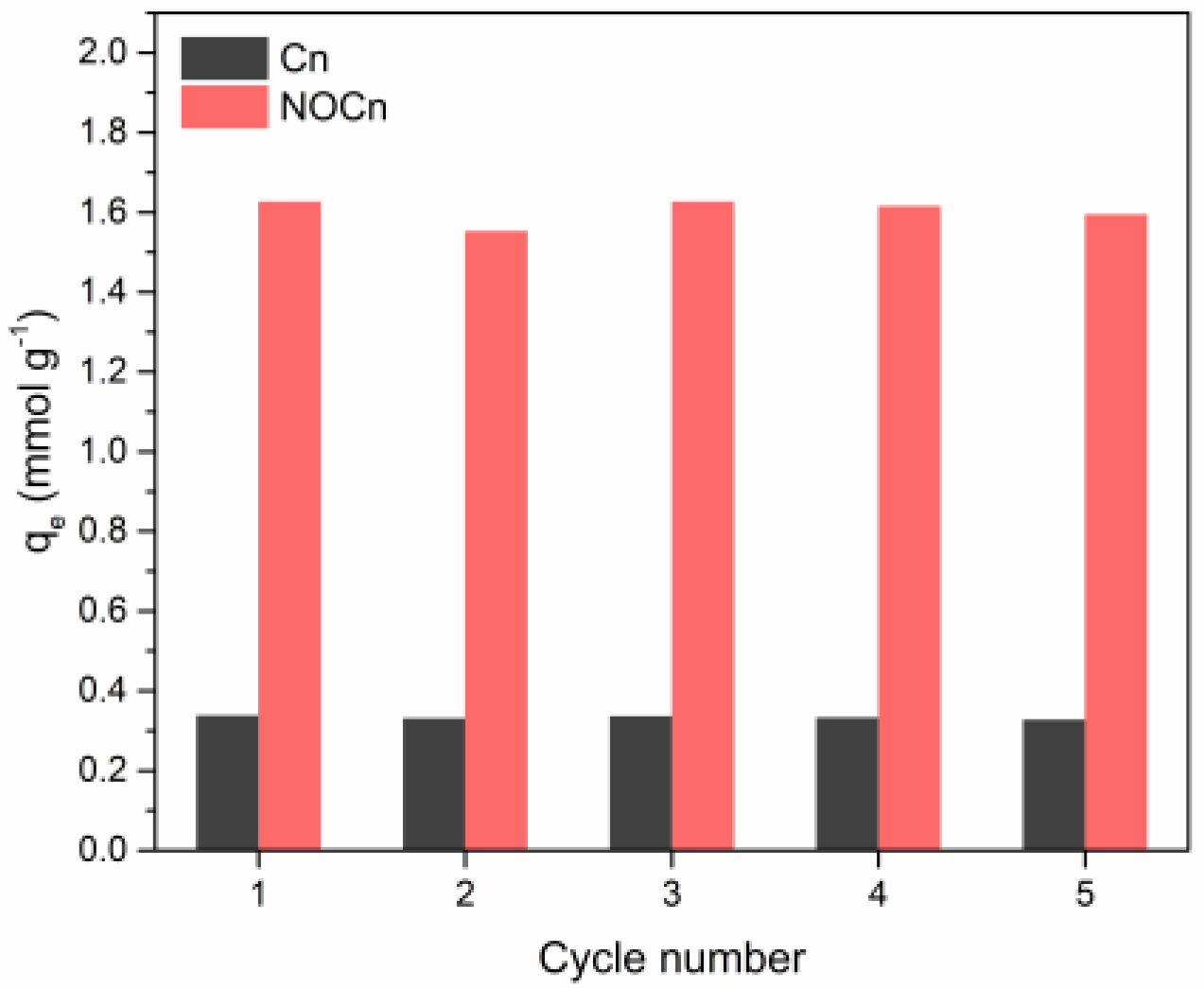
3.5. Cyclic Adsorption/Regeneration
3.6. Adsorption Isotherm Studies
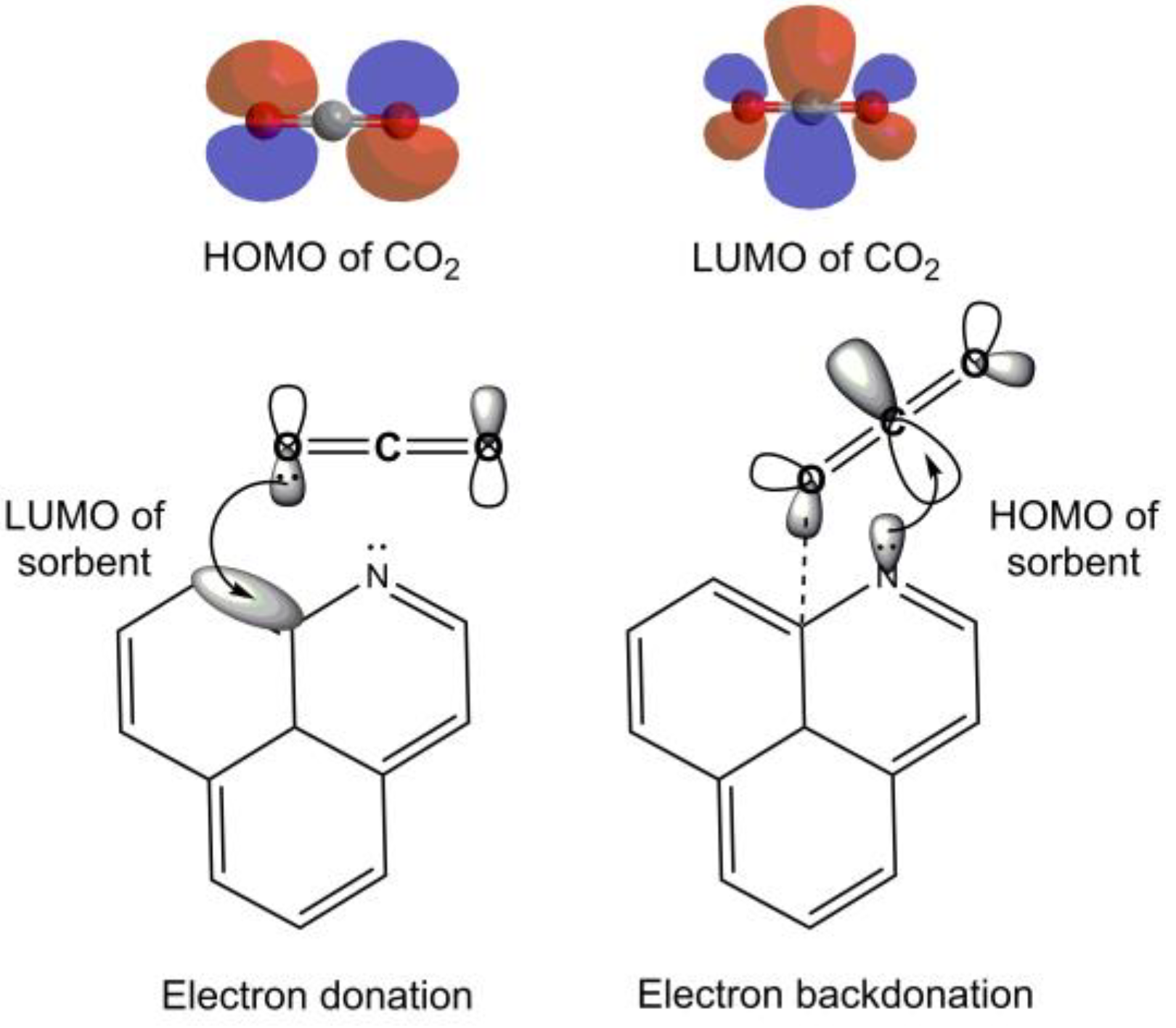
3.7. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-Modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]
- Su, F.S.; Lu, C.Y.; Kuo, S.C.; Zeng, W.T. Adsorption of CO2 on amine-functionalized Y-Type zeolites. Energy Fuels 2010, 24, 1441–1448. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Yun, L.J.; Anuradha, R.; Jang, H.T. Synthesis of chloropropylamine grafted mesoporous MCM-41, MCM-48 and SBA-15 from rice husk ash: Their application to CO2 chemisorption. J. Porous Mater. 2009, 17, 475–484. [Google Scholar] [CrossRef]
- Xu, X.C.; Song, C.S.; Miller, B.G.; Scaroni, A.W. Influence of moisture on CO2 separation from gas mixture by a nanoporous adsorbent based on polyethylenimine-modified molecular sieve MCM-41. Ind. Eng. Chem. Res. 2005, 44, 8113–8119. [Google Scholar] [CrossRef]
- Houshmand, A.; Daud, W.M.A.W.; Lee, M.G.; Shafeeyan, M.S. Carbon dioxide capture with amine-Grafted activated carbon. Water Air Soil Pollut. 2011, 223, 827–835. [Google Scholar] [CrossRef]
- Gray, M.L.; Hoffman, J.S.; Hreha, D.C.; Fauth, D.J.; Hedges, S.W.; Champagne, K.J.; Pennline, H.W. Parametric study of solid amine sorbents for the capture of carbon dioxide. Energy Fuels 2009, 23, 4840–4844. [Google Scholar] [CrossRef]
- Veneman, R.; Li, Z.S.; Hogendoorn, J.A.; Kersten, S.R.A.; Brilman, D.W.F. Continuous CO2 capture in a circulating fluidized bed using supported amine sorbents. Chem. Eng. J. 2012, 207, 18–26. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 Capture by As-Prepared SBA-15 with an Occluded Organic Template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A Controllable Synthesis of Rich Nitrogen-Doped Ordered Mesoporous Carbon for CO2 Capture and Supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Zhao, X.C.; Wang, A.Q.; Yan, J.W.; Sun, G.Q.; Sun, L.X.; Zhang, T. Synthesis and Electrochemical Performance of Heteroatom-Incorporated Ordered Mesoporous Carbons. Chem. Mater. 2010, 22, 5463–5473. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Wang, S.; Wang, G.H.; Qi, L.; Lu, A.H. Lysine-Assisted rapid synthesis of crack-Free hierarchical carbon monoliths with a hexagonal array of mesopores. Carbon 2011, 49, 3762–3772. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Zhang, F.; Li, Q.; Zhu, G. One-Pot synthesis of highly ordered nitrogen-Containing mesoporous carbon with resorcinol–Urea–Formaldehyde resin for CO2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Yu, G.; Ma, H.; Zhu, G. Simple fabrication of an ordered nitrogen-Doped mesoporous carbon with resorcinol–Melamine–Formaldehyde resin. Microporous Mesoporous Mater. 2014, 190, 117–127. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Xu, Y.Y.; Sun, Q.; Lu, A.H. Evidence of Microporous Carbon Nanosheets Showing Fast Kinetics in both Gas Phase and Liquid Phase Environments. Small 2015, 11, 5151–5156. [Google Scholar] [CrossRef]
- Hao, G.P.; Jin, Z.Y.; Sun, Q.; Zhang, X.Q.; Zhang, J.T.; Lu, A.H. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties. Energy Environ. Sci. 2013, 6, 3740–3747. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.B.; Ham, H.C. Effect of N-Containing Functional Groups on CO2 Adsorption of Carbonaceous Materials: A Density Functional Theory Approach. J. Phys. Chem. C 2016, 120, 8087–8095. [Google Scholar] [CrossRef]
- Kiuchi, H.; Shibuya, R.; Kondo, T.; Nakamura, J.; Niwa, H.; Miyawaki, J.; Kawai, M.; Oshima, M.; Harada, Y. Lewis Basicity of Nitrogen-Doped Graphite Observed by CO2 Chemisorption. Nanoscale Res. Lett. 2016, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Arami-Niya, A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation. Appl. Surf. Sci. 2011, 257, 3936–3942. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Kaneko, Y.; Panomsuwan, G. Electrocatalytic activity for the oxygen reduction reaction of oxygen-Containing nanocarbon synthesized by solution plasma. J. Mater. Chem. A 2014, 2, 10589–10598. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chiba, S.; Kaneko, Y.; Saito, N.; Ishizaki, T. In situ solution plasma synthesis of nitrogen-doped carbon nanoparticles as metal-free electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 18677–18686. [Google Scholar] [CrossRef]
- Lee, H.; Ueno, T.; Saito, N. The Effect of Electrode Gap Distance on the Synthesis of Carbon Materials by Using Solution Plasma Process. Jom 2015, 67, 2550–2556. [Google Scholar] [CrossRef]
- Pansare, S.S.; Torres, W.; Goodwin, J.G. Ammonia decomposition on tungsten carbide. Catal. Commun. 2007, 8, 649–654. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhaes-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lippens, B. Studies on pore systems in catalysts V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- De Boer, J.H.; Lippens, B.C.; Linsen, B.G.; Broekhoff, J.C.P.; Van Den Heuvel, A.; Osinga, T.J. The t-Curve of multimolecular N2-Adsorption. J. Colloid Interface Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Stohr, B.; Boehm, H.P.; Schlogl, R. Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon 1991, 29, 707–720. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-Magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540–552. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergstrom, L.; Yan, J. Adsorbents for the post-Combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley Sons: New York, NJ, USA, 1984; p. 433. [Google Scholar]
- Balsamo, M.; Silvestre-Albero, A.; Silvestre-Albero, J.; Erto, A.; Rodriguez-Reinoso, F.; Lancia, A. Assessment of CO2 adsorption capacity on activated carbons by a combination of batch and dynamic tests. Langmuir 2014, 30, 5840–5848. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Xing, W. CHAPTER 1 Carbon-Based CO2 Adsorbents. In Post-Combustion Carbon Dioxide Capture Materials; The Royal Society of Chemistry: London, UK, 2019; pp. 1–75. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Yue, Q.; Tao, K.; Kong, C.; Chen, L. N-Rich porous carbon with high CO2 capture capacity derived from polyamine-Incorporated metal–Organic framework materials. RSC Adv. 2016, 6, 53017–53024. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, H.; Tang, X.; Deng, H.; Liu, H. Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem. Eng. J. 2012, 200, 399–404. [Google Scholar] [CrossRef]
- Czepirski, L.; JagieLLo, J. Virial-Type thermal equation of gas—Solid adsorption. Chem. Eng. Sci. 1989, 44, 797–801. [Google Scholar] [CrossRef]
- Neti, V.S.P.K.; Wang, J.; Deng, S.; Echegoyen, L. High and selective CO2 adsorption by a phthalocyanine nanoporous polymer. J. Mater. Chem. A 2015, 3, 10284–10288. [Google Scholar] [CrossRef]
- Jousten, K. Handbook of Vacuum Technology, 2nd ed.; Jousten, K., Ed.; Wiley-VCH: Weinheim, Gemany, 2016. [Google Scholar] [CrossRef]
- Golden, T.C.; Sircar, S. Gas Adsorption on Silicalite. J. Colloid Interface Sci. 1994, 162, 182–188. [Google Scholar] [CrossRef]
- Madyal, R.S.; Arora, J.S. DFT studies for the evaluation of amine functionalized polystyrene adsorbents for selective adsorption of carbon dioxide. RSC Adv. 2014, 4, 20323–20333. [Google Scholar] [CrossRef]
- Aresta, M.; Angelini, A. Carbon Dioxide and Organometallics, 1st ed.; Lu, X.B., Ed.; Springer International Publishing: New York, NJ, USA, 2016; p. 308. [Google Scholar] [CrossRef]
- Chai, G.L.; Guo, Z.X. Highly effective sites and selectivity of nitrogen-Doped graphene/CNT catalysts for CO2 electrochemical reduction. Chem. Sci. 2016, 7, 1268–1275. [Google Scholar] [CrossRef]
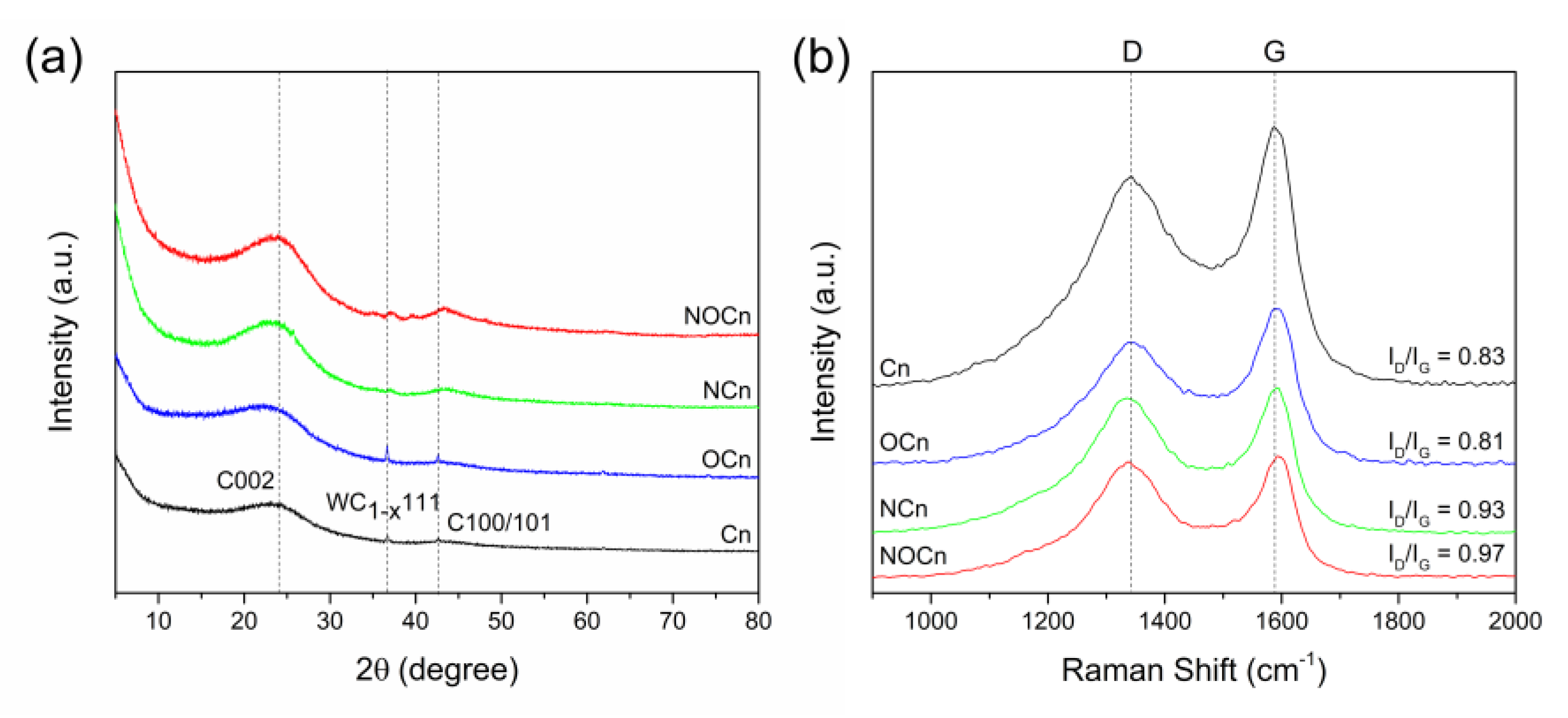
| Sample | XRD | Raman Spectroscopy | |
|---|---|---|---|
| d002 (nm) | ID/IG | La (nm) | |
| Cn | 0.370 | 0.83 | 23.18 |
| Ocn | 0.383 | 0.81 | 23.75 |
| NCn | 0.375 | 0.93 | 20.69 |
| NOCn | 0.372 | 0.97 | 19.83 |
| Sample | BET | t-Plot | |||
|---|---|---|---|---|---|
| Specific Surface Area | Average Pore Size | Specific Surface Area | MicroporeArea | Micropore Volume | |
| (m2 g−1) | (nm) | (m2 g−1) | (m2 g−1) | (cm3 g−1) | |
| Cn | 190.8 | 19.7 | 191.4 | - | - |
| OCn | 138.4 | 22.4 | 137.5 | - | - |
| NCn | 437.8 | 9.3 | 521.3 | 262.7 | 0.06 |
| NOCn | 569.7 | 8.6 | 679.8 | 469.7 | 0.15 |
| Sample | Bulk Elemental Analysis, EA (wt %) | Surface Elemental Analysis, XPS (at %) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | C | N | O | O/C Ratio | N/C Ratio | |
| Cn | 92.99 | 1.37 | - | 95.82 | - | 4.18 | 0.044 | - |
| OCn | 88.13 | 2.76 | - | 92.79 | - | 7.21 | 0.078 | - |
| NCn | 89.21 | 0.99 | 1.77 | 97.05 | 1.29 | 1.66 | 0.017 | 0.013 |
| NOCn | 82.35 | 1.50 | 2.76 | 95.35 | 2.29 | 2.36 | 0.025 | 0.024 |
| Region | Bonding | Position (eV) | Relative Percentage (%) | |||
|---|---|---|---|---|---|---|
| Cn | OCn | NCn | NOCn | |||
| C 1s | C–C sp2 | 284.5 | 65.0 | 53.7 | 71.5 | 69.7 |
| C–C sp3 | 285.4 | 24.7 | 25.8 | 7.5 | 7.2 | |
| C–N | 285.8 | - | - | 7.0 | 7.5 | |
| C–O | 286.4–286.6 | 7.0 | 9.9 | 7.5 | 8.1 | |
| C=O | 287.9–288.3 | 2.3 | 6.5 | 3.4 | 4.2 | |
| O–C=O | 289.7–290.1 | 1.0 | 4.2 | 3.1 | 3.4 | |
| O 1s | Quinone | 530.2 | 1.5 | 4.0 | 8.4 | 10.5 |
| O=C–OH | 531.2–531.6 | 12.1 | 11.9 | 11.4 | 19.5 | |
| C=O | 532.2–532.4 | 42.6 | 40.0 | 37.2 | 28.8 | |
| C–OH | 533.2–533.4 | 30.6 | 29.4 | 12.1 | 24.7 | |
| C–O | 533.8–534.2 | 9.6 | 11.4 | 24.9 | 9.9 | |
| Water adsorbed | 535 | 3.6 | 3.3 | 6.1 | 6.6 | |
| N 1s | Pyridinic-N | 398.4–398.6 | - | - | 47.8 | 43.7 |
| Pyrrolic-N | 400.1–400.3 | - | - | 36.6 | 33.6 | |
| Graphitic-N | 401.1–401.5 | - | - | 9.6 | 15.8 | |
| Pyridinic N-oxide | 403.3–403.7 | - | - | 6.0 | 6.9 | |
| Adsorption Temperature (°C) | Cn | NOCn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ln KH | KH 1 | ln KH | KH 1 | |||||||
| 25 | 0.62 | 1.86 | −1.52 | −28.45 | −90.39 | 2.51 | 12.26 | −6.30 | −31.73 | −85.36 |
| 35 | 0.19 | 1.21 | −0.61 | 2.18 | 8.82 | −5.44 | ||||
| 45 | −0.04 | 0.96 | 0.29 | 1.74 | 5.71 | −4.59 | ||||
| 55 | −0.47 | 0.63 | 1.20 | 1.35 | 3.85 | −3.74 | ||||
| Sample | Selectivity | ||
|---|---|---|---|
| Cn | 1.86 | 0.11 | 16.29 |
| NOCn | 12.26 | 0.42 | 29.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pornaroontham, P.; Panomsuwan, G.; Chae, S.; Saito, N.; Thouchprasitchai, N.; Phongboonchoo, Y.; Pongstabodee, S. Nitriding an Oxygen-Doped Nanocarbonaceous Sorbent Synthesized via Solution Plasma Process for Improving CO2 Adsorption Capacity. Nanomaterials 2019, 9, 1776. https://doi.org/10.3390/nano9121776
Pornaroontham P, Panomsuwan G, Chae S, Saito N, Thouchprasitchai N, Phongboonchoo Y, Pongstabodee S. Nitriding an Oxygen-Doped Nanocarbonaceous Sorbent Synthesized via Solution Plasma Process for Improving CO2 Adsorption Capacity. Nanomaterials. 2019; 9(12):1776. https://doi.org/10.3390/nano9121776
Chicago/Turabian StylePornaroontham, Phuwadej, Gasidit Panomsuwan, Sangwoo Chae, Nagahiro Saito, Nutthavich Thouchprasitchai, Yuththaphan Phongboonchoo, and Sangobtip Pongstabodee. 2019. "Nitriding an Oxygen-Doped Nanocarbonaceous Sorbent Synthesized via Solution Plasma Process for Improving CO2 Adsorption Capacity" Nanomaterials 9, no. 12: 1776. https://doi.org/10.3390/nano9121776
APA StylePornaroontham, P., Panomsuwan, G., Chae, S., Saito, N., Thouchprasitchai, N., Phongboonchoo, Y., & Pongstabodee, S. (2019). Nitriding an Oxygen-Doped Nanocarbonaceous Sorbent Synthesized via Solution Plasma Process for Improving CO2 Adsorption Capacity. Nanomaterials, 9(12), 1776. https://doi.org/10.3390/nano9121776