Abstract
Photocatalytic H2 production is a promising strategy toward green energy and alternative to carbon-based fuels which are the root cause of global warming and pollution. In this study, carbon nanotubes (CNTs) incorporated Z-scheme assembly of AgBr/TiO2 was developed for photocatalytic H2 production under visible light irradiations. Synthesized photocatalysts were characterized through transmission electron microscope (TEM), X-ray photoelectron spectra (XPS), X-ray diffractometer (XRD), Fourier transform infrared (FTIR), photoluminescence spectra (PL), Brunauer Emmet-Teller(BET), and UV-vis spectroscopy analysis techniques. The composite photocatalysts exhibited a H2 production of 477 ppm which was three-folds higher than that produced by TiO2. The good performance was attributed to the strong interaction of three components and the reduced charge recombination, which was 89 and 56.3 times lower than the TiO2 and AgBr/TiO2. Furthermore, the role of surface acidic and basic groups was assessed and the photocatalytic results demonstrated the importance of surface functional groups. In addition, the composites exhibited stability and reusability for five consecutive cycles of reaction. Thus, improved performance of the photocatalyst was credited to the CNTs as an electron mediator, surface functional groups, higher surface area, enhanced charge separation and extended visible light absorption edge. This work provides new development of Z-scheme photocatalysts for sustainable H2 production.
1. Introduction
Photocatalytic H2 production is an attractive approach to satisfy the worldwide energy requirements and to reduce the global warming. However, development of efficient photocatalyst with the ability to function under visible light irradiations and good charge separation is still a great challenge. State-of-the art Z-scheme photocatalysts offer a two-fold advantage of efficient charge separation and visible light solar energy utilization [1,2]. In Z-scheme photocatalytic system, compatible semiconductors with respect to their band positions establish efficient charge transfer mechanism which lessen the charge recombination. Further, the efficiency of charge separation is even higher in indirect Z scheme structure owing to the presence of electron mediators. The electron mediator expedites the process of charge transfer and reduce the recombination of electrons-holes pairs. These parted electrons and holes with high redox potential increase the efficiency of photocatalytic production [2,3].
The Z-scheme assembly of TiO2 with compatible semiconductors is considered as a promising strategy to enhance the charge transfer and light absorption capability [4,5]. Silver bromide (AgBr) with an indirect bandgap energy of 2.6 eV have hardly been used in photocatalysis because of decomposition problem under light irradiations. However, the coupling of AgBr with suitable semiconductors like TiO2 enhances the stability by developing heterojunction which fastens the transport of electrons [6] and prevent the degradation. The conduction band potential of AgBr is −0.30 eV allowing it to develop Z-scheme assembly with TiO2 whose conduction band is −0.5 eV [7]. Asi et al. [8] studied the AgBr/TiO2 photocatalysts for CO2 conversion into CH3OH and reported the stable activity for continuous four cycles. Similarly, AgBr particles supported over amine functionalized rGO were reported by Shah et al. [6] for the decomposition of methylene blue and they noticed stable activity for continuous 12 cycles. Further, electron mediator plays an important role in the efficiency of indirect Z-scheme system. Noble metals such as Ag, Au, and Pt have been reported as electron mediators for Z-scheme photocatalysts [9,10,11]. However, noble metals-based Z-scheme systems are costly and require complex synthesis protocols.
Nowadays, there is an increasing trend to develop carbon-based electron mediators such as graphene and carbon nanotubes owing to their high electrical conductivity [12,13,14]. Numerous studies have reported the use of graphene as an electron mediator because of high charge carrier separation capability. Similarly, carbon nanotubes have been used as an electron mediator owing to extraordinary electrical, optical, and physical properties. Recently, Mohamad et al. [13] have reported the Z-scheme assembly of g-C3N4/CNTs/TiO2 composites for the photocatalytic degradation of dyes. The composite exhibited almost six times higher degradation of phenol because of intimate surface contact between the two semiconductor through CNTs which reduced the charge recombination. Similarly, Boon-Joon et al. [3] reported CNTs as an electron mediator in Zn0.5Cd0.5S-MWCNT-TiO2 nanocomposites for H2 production. The CNTs efficiently transported the electrons from TiO2 to Zn0.5Cd0.5S. Yet, there are few studies which focus on carbon nanotubes as an electron mediator. Further, effective bonding is essential to achieve the desired charge separation and transportation through an electron mediator. The strength of bonding depends on the surface functional groups that promote the interaction between semiconductors. The surface functional groups play a dynamic role in enhancing the interaction of reactants with surface of photocatalyst and photocatalytic stability. For example, Nasir et al. [5] reported that AgBr/rGO/TiO2 exhibited higher efficiency in the basic medium rather than acidic. Acidic and basic groups can be introduced on the surface of the composite by functionalization. Additionally, the difficulty of pH control during the photoreaction experiment could also be avoided by functionalization of materials with different solvents which would be an added advantage to the stability of photocatalyst. However, there is limited study on coupling of CNTs as an electron mediator and the role of the functional groups in Z-scheme structures.
In this study, carbon nanotubes-incorporated Z-scheme assembly of AgBr/TiO2 was studied. The AgBr/bCNTs/TiO2 photocatalysts were synthesized by deposition of AgBr and TiO2 on CNTs followed by reflux treatment to develop effective interfacial bonding. Obtained photocatalysts were calcined and analyzed through several characterization techniques including TEM, XRD, XPS, Raman, PL, UV-vis spectroscopy, N2 adsorption and desorption technique. Performance of the AgBr/CNTs/TiO2 was studied for H2 production under visible light irradiations. Furthermore, role of functional groups was assessed by treating the CNTs with NaOH and NaOCl, and with H2SO4, HNO3 and CH3COOH solvents and integrated with AgBr/TiO2. In the end, the mechanism of photocatalytic H2 generation was proposed.
2. Experimental Section
2.1. Materials
Multi-wall carbon nanotubes (length 5 µm, Diameter 20 nm) were obtained from Carbon Nano-material Technology Co., Ltd. Pohang, South Korea. The cetyltrimethylammonium bromide (CTAB, C19H42BrN, ≥98%) and anatase TiO2 (99.50%) were obtained from Sigma-Aldrich, St. Louis, MO, USA. Hydrochloric acid (HCl, 37%), sodium hydroxide (NaOH) and sodium oxychloride (NaOCl) were obtained from R&M chemicals, Essex, U.K. Silver nitrate (AgNO3 99%) and ethanol (C2H5OH, 99.99%) were obtained from Merck, KGaA, Darmstadt, Germany.
2.2. Preparation of Functionalized Carbon Nanotubes
The carbon nanotubes (CNTs) were dispersed into 1M NaOH and were boiled at 120 °C for 15 min. After that 30% NaOCl was added into dispersion followed by boiling for 2 h. The suspension was then cooled down to room temperature, filtered and neutralized by dispersing into the HCl solution. Obtained CNTs were washed with 0.1 M HCl and water to remove residual acid and base contents, followed by drying at 80 °C for 8 h. The product was denoted as bCNTs. Similarly, CNTs functionalization was carried out in acidic media containing 0.1 M H2SO4, 0.1 M HNO3, and 0.1 M CH3COOH at 120 °C for 4 h. The mixture was then filtered and washed several times with water to remove residual acid contents and denoted as aCNTs.
2.3. Preparation of AgBr/CNTs/TiO2
The AgBr/bCNTs/TiO2 was prepared by employing a two-steps approach which includes growth of AgBr over CNTs and bonding with TiO2 particles followed by reflux. A particular amount of bCNTs were distributed in absolute ethanol with the help of sonication for 1 h. The desired quantity of CTAB was added to the bCNTs mixture. The required weight of AgNO3 was dissolved in NH3 solution and mixed into the above suspension. The obtained mixture was stirred for 1 h for the uniform deposition of AgBr nanoparticles on the surface of bCNTs. Simultaneously, the desired quantity of TiO2 was distributed in absolute ethanol, sonicated and stirred for 1 h. The TiO2 suspension was mixed with AgBr/bCNTs solution and the resulting mixture was shifted to round neck flask and refluxed for 2 h at 80 °C to strengthen the interfacial bonding among AgBr, bCNTs and TiO2. The obtained product was filtered, dried in an oven at 100 °C and calcined at 300 °C for 2 h. A similar method was repeated to synthesize the AgBr/aCNTs/TiO2, where AgBr/TiO2 was synthesized using the same approach without the addition of the CNTs. Likewise, aCNTs/TiO2 and bCNTs/TiO2 were also prepared without the addition of AgNO3 and CTAB.
2.4. Characterizations of Photocatalyst
The surface morphology of the synthesized photocatalysts was studied employing a transmission electron microscope (TEM HITACHI HT7700, Tokyo, Japan) on different magnifications. Crystalline structure photocatalysts were investigated using an X-ray diffractometer (XRD, PANalytical X’pert3 powder, Malvern WR14 1XZ, U.K) with a diffraction angle range of 5° to 90° employing Cu α radiations (40 kV and 30 mA) at a scanning rate of 0.07878°. The surface chemical composition was investigated using X-ray photoelectron spectra (XPS) (Thermo scientific K-alpha (Kα) spectrometer, East Grinstead, U.K). The XPS spectrometer was equipped with Al Kα radiations with corrected calibration binding energies against the C1 of carbon fixed at 284.6 eV. Raman microscope (Lab RAM HR Evolution, HORIBA, Kyoto, Japan) with laser excitation at 325 nm in the range of 100–1000 cm−1 was used to record the PL spectra. The functional groups present on the surface of different photocatalysts were observed using Fourier transform infrared (FTIR) spectroscopy (Thermo Nicolet FTIR 6700, Thermo Scientific, Waltham, MA, USA) with wavelengths of 600 to 4000 cm−1. Optical properties of photocatalysts were studied using UV-visible spectrometer (Agilent Cary technologies 100 UV-vis spectrometer Model G9821A, Santa Clara, CA USA) in the range of 200 to 800 nm. Bandgap energies of photocatalysts were estimated from Tauc plots of Kubelka–Munk Function versus photon energy.
2.5. Photocatalytic H2 Production
The performance of the photocatalysts was evaluated for H2 generation from water using a slurry reactor as reported in our previous study [5]. The glass photoreactor consisted of two chambers with 35 W HID Xe lamp (intensity 20 mWcm−2, Philips, Selangor, Malaysia) as a source of light. Methanol was added as a sacrificial agent. Typically, 100 mg of photocatalyst was dispersed into a 20% methanol solution. Photoreactor setup was positioned on a magnetic stirrer for good dispersion of photocatalyst in the slurry. Prior to photoreaction, N2 gas was purged for 1 h to remove gaseous impurities from the reactor and also used as a carrier gas at the flow rate of 20 mL/min. Photoreaction was triggered by switching on Xe lamp. Gaseous samples were collected after an interval of 1 h in gas collecting bags and H2 was detected using Intelligent H2 analyser (BROTIE Technology Co., Ltd., Haidian District, Beijing China).
3. Results and Discussions
3.1. Characterization of Photocatalysts
Crystallinity and phase structures of aCNTs, bCNTs, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts were studied using XRD analysis as shown in Figure 1. Debye–Scherrer equation and Bragg’s Law were employed to measure the particles size and interlayer spacing. Diffractogram of aCNTs and bCNTs have peaks at 26.2° and 45.68° corresponding to the (002) and (100) planes respectively. In aCNTs/TiO2 and bCNTs/TiO2 spectrum, peaks at 25.33°, 37.83°, 48.07°, 53.9°, 55.08°, 62.70°, 68.79°, 70.31° and 75.04° indicated the (101), (004), (200), (105), (211), (204), (116) and (220) crystalline planes of TiO2 (JCPDS-98-008-2084) [15]. The particles size was 44.79 nm measured from the prominent peak at 25.33°. The pattern of both aCNTs/TiO2 and bCNTs/TiO2 did not show any peaks for CNTs owing to the very low quantity of CNTs. In AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 spectra, there were additional new peaks at 31.21° and 44.54° representing the AgBr nanoparticles with face-centred cubic structure with crystal planes of (200) and (220) crystal plane, respectively [16]. No peak was observed for silver (Ag°) indicating the formation of only the AgBr/bCNTs/TiO2 photocatalyst. Similarly, no peak was found for CNTs because of marginal quantity, even dispersion and shielding effect of TiO2 peak at 2θ = 25.33° [17]. These results showed the successful formation of AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 by the two-steps method.
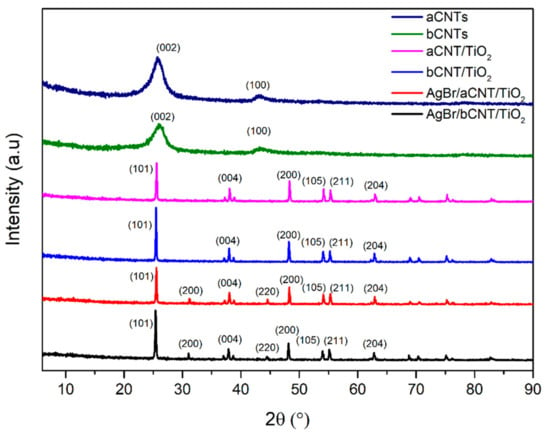
Figure 1.
X-ray diffractometer (XRD) spectra of aCNTs, bCNTS, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
Morphology of AgBr/bCNTs/TiO2 was examined by TEM as shown in Figure 2. Uniform distribution of different particles without significant agglomeration is evident in Figure 2a–c. Uniform dispersion of particles supported and endorsed the importance of functionalization and formation of the composite. Figure 2d,e demonstrate higher magnification images showing the unvarying distribution of TiO2, AgBr and multi-wall carbon nanotubes. Multiwall structure and thickness of each carbon nanotubes could be clearly observed. Deposition of TiO2 and AgBr particles on the walls of CNTs can also be observed, which shows the strong integration of particles with CNTs. The strong bonding among particles would help in efficient charge separation across Z-scheme assembly of the photocatalysts and consequently higher H2 production. Furthermore, Figure 2f shows selected area electron diffraction (SAED) image of AgBr/bCNTs/TiO2 having several bright spots circles which shows the various crystalline planes of AgBr/bCNTs/TiO2. Additionally, TEM images showed that CNTs were uniformly dispersed and no carbon peak in the XRD analysis was observed which was due to the lower amount and even dispersion of CNTs.
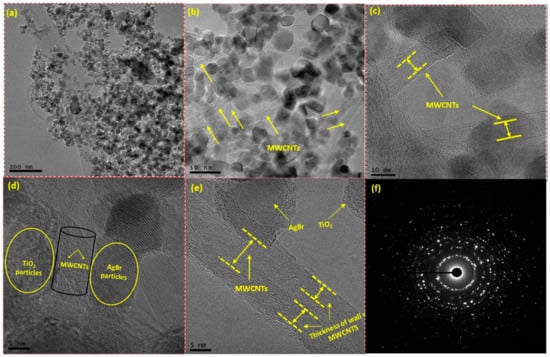
Figure 2.
Transmission electron microscope (TEM) images of AgBr/bCNTs/TiO2 at different resolutions (a) 200 nm; (b) 50 nm; (c) 10 nm; (d) 5 nm; (e) 5 nm and (f) SAED pattern.
Surface chemical composition of AgBr/bCNTs/TiO2 was investigated through XPS spectroscopy as shown in Figure 3. Survey spectra of AgBr/bCNTs/TiO2 exhibited various peaks for silver (Ag), titanium (Ti), bromide (Br), carbon (C) and oxygen (O) as shown in Figure 3a and deconvoluted spectra of individual elements were further plotted to analyse the chemical species in depth as in Figure 3a–d. The Ag spectra presented two major peaks at 367.78 eV and 373.88 eV predicting the Ag3d1/2 and Ag3d3/2 matching to Ag+1 [18]. Valency of Ag was additionally confirmed from the spectra of Br which has peaks at 68.58 and 69.68 eV of Br3d5/2 and Br3d3/2, corresponding to Br−1 in AgBr [19]. The Ti2p spectra has three different peaks at 459.28, 464.98 and 472.68 eV corresponding to Ti4+ of Ti2p3/2 and Ti 2p1/2, respectively [20]. Similarly, C1s spectra produced two peaks at 285.18 and 289.58 eV, respectively. The peaks at 289.58 eV represented the chemical bonding of carbon with oxygen in the form of C–O and C–O–C functional groups. On the other hand, an intense peak at 285.18 eV indicated the sp2 characteristics of carbon nanotubes corresponding to C=C, C–C, and C–H bonding [21]. Importantly, the bigger area of peak at 285.18 eV showed that functional groups from the surface of CNTs were eliminated during heat treatment and calcination process. Likewise, O1s spectra of AgBr/bCNTs/TiO2 demonstrated the main peak at 530.38 indicating the Ti–O–Ti, and Ti–O–C bonding [22]. Therefore, findings showed the strong bonding of AgBr, CNTs and TiO2.
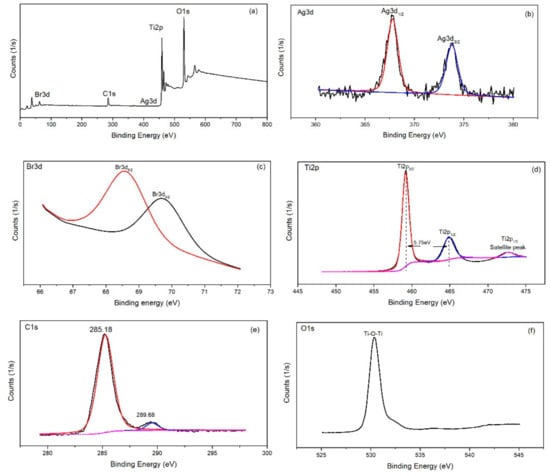
Figure 3.
X-ray photoelectron spectra (XPS) analysis of AgBr/bCNTs/TiO2 (a) Survey spectra; (b) Ag3d; (c) Br3d; (d) Ti2p; (e) C1s and (f) O1s.
Figure 4 shows the FTIR spectra of aCNTs, bCNTs, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2, AgBr/bCNTs/TiO2. Spectra have several peaks at different region corresponding to various surface moieties that helped in the development of effective bonding in final composites. In aCNTs and bCNTs, peaks at 3300 cm−1 correspond to the OH functional groups. However, intensities of peaks are higher in aCNTs compared to bCNTs. Peaks appearing at 2923 cm−1 in bCNTs, and 2890 cm−1 in aCNTs represent the –CH stretching. Peaks at 2302 cm−1 in aCNTs and peaks at 2183 cm−1 in bCNTs showed the COOH bonding [23]. Intensity of COOH bonding is higher in bCNTs compared to aCNTs/TiO2. Similarly, bCNTs exhibited peaks at 1493, 1307, 1132 and 943 cm−1 in the C=C and –COH groups, whereas peaks were observed at 1556 and 1278 as well 1005 cm−1 for C=C and –COH groups in aCNTs [23]. Intensities of these groups are obviously higher in bCNTs which shows that it would help in more effective bonding in composites. In rest of the samples including aCNTs/TiO2 and bCNTs/TiO2, AgBr/bCNTs/TiO2 and AgBr/bCNTs/TiO2 most of peaks disappeared because of thermal treatment during the synthesis at high temperature. Only dominant peaks near the 600 cm−1 was due to the Ti–O–Ti bonding.
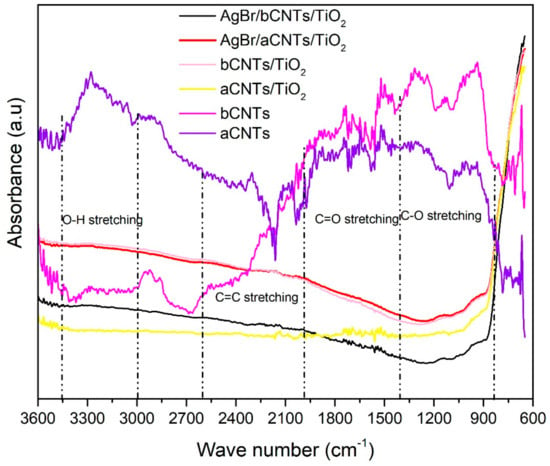
Figure 4.
Fourier transform infrared (FTIR) spectra of aCNTs, bCNTs, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
Photogenerated charge carriers trapping, separation, transportation and recombination was estimated by PL spectroscopy as shown in Figure 5. It is obvious that charge recombination intensity of AgBr/aCNTs/TiO2 was higher than other photocatalysts owing to the poor bonding between the two semiconductors. The luminance intensity of TiO2 compared to aCNTs/TiO2, bCNTs/TiO2 and AgBr/bCNTs/TiO2 was very high because of lower charge separation efficiency. The charge recombination intensity of AgBr/TiO2 (8978 a.u) was lower compared to TiO2 (14,541 a.u). This shows the better separation of charges in direct Z-scheme assembly of AgBr/TiO2 which would be helpful for higher photocatalytic H2 production. In addition, aCNTs/TiO2, bCNTs/TiO2, and AgBr/bCNTs/TiO2 exhibited significantly lower intensities compared to that of aCNTs/TiO2, AgBr/aCNTs/TiO2, AgBr/TiO2 and TiO2. Remarkable reduced charge recombination intensity indicated the important role of carbon nanotubes as an electron mediator in AgBr/bCNTs/TiO2 photocatalysts [24]. Thus, improved charge separation was ascribed to multiwall carbon nanotubes as a solid-state electron mediator in AgBr/TiO2.
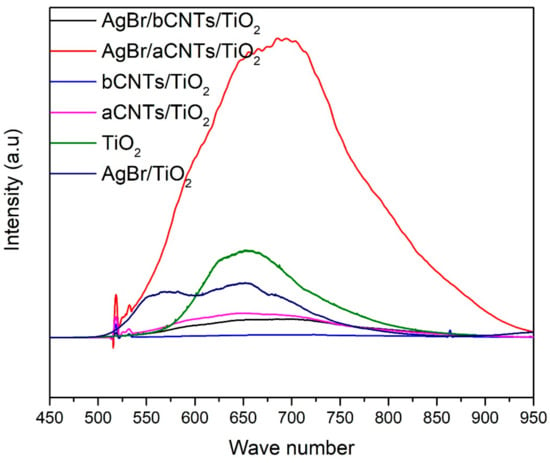
Figure 5.
PL spectra of TiO2, AgBr/TiO2, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
The optical behaviour of photocatalyst shows the threshold wavelength of light photons required to initiate the photoreaction. Figure 6 shows the light absorption profile of photocatalysts examined by UV-vis spectrophotometer. All the photocatalysts showed higher absorption in the UV light spectrum owing to the transition of energized electrons from O2p to Ti3d of TiO2 [25]. The aCNTs/TiO2 and bCNTs/TiO2 exhibited little extended light absorption edges compared to TiO2 and also higher absorption coefficient. The AgBr/bCNTs/TiO2 and AgBr/aCNTs/TiO2 exhibited stretched light absorption in the visible light region as compared to bCNTs/TiO2 and aCNTs/TiO2 and TiO2 showing that optical response was greatly enhanced. The light absorption edge of AgBr/bCNTs/TiO2 is more stretched into the visible light region compared to the AgBr/aCNTs/TiO2 which makes the former more efficient compared to the latter. This will help to produce greater number of photoelectrons and eventually yield of H2 production. Moreover, the indirect bandgap energy of AgBr is 2.60 eV which helped to improve the optical response and excitation of photogenerated electrons from valence band (VB) to the conduction band (CB) in AgBr/CNTs/TiO2 [26,27]. Thus, improved UV-Vis spectrum of AgBr/bCNTs/TiO2 can be regarded as a superposition of AgBr and bCNTs/TiO2 optical absorption spectra.
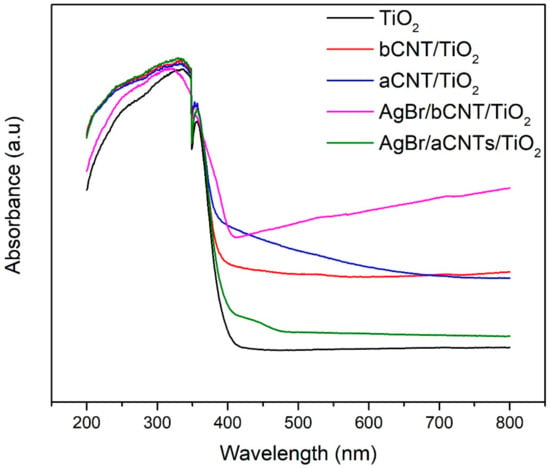
Figure 6.
UV-vis spectra TiO2, aCNTs/TiO2, bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
Surface areas of TiO2, bCNTs/TiO2 and AgBr/bCNTs/TiO2 were estimated by BELSORP-mini as shown in Figure 7. The N2 adsorption and desorption isotherms of TiO2, bCNTs/TiO2 and AgBr/bCNTs/TiO2 resembled type IV isotherm of IUPAC having hysteresis loop in the range 0.8–1 of P/Po and designated the mesoporous structures of photocatalysts. The surface area of TiO2 was 45 m2 which was increased to 49 m2 because of the addition of bCNTs. However, the surface area of final composite, AgBr/bCNTs/TiO2, was 47 which was lower than bCNTs/TiO2. This decrease in the area was due to the lower surface area of AgBr, and small agglomeration of particles [28].
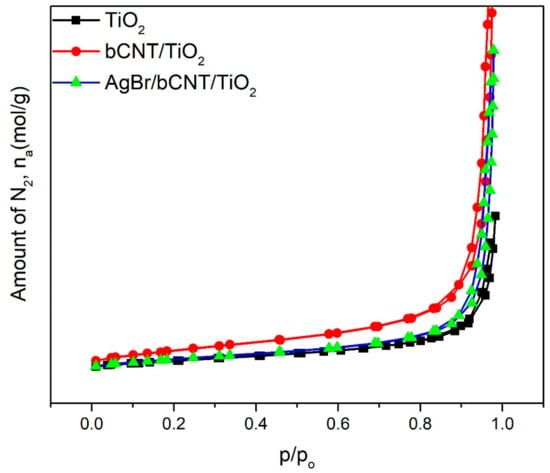
Figure 7.
N2 adsorption and desorption isotherms of TiO2, bCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
Further, bandgap structure of AgBr/bCNTs/TiO2 photocatalyst were investigated from XPS spectra and Tauc plot of modified Kubelka–Munk function. To measure VB, XPS data were standardized with reference to carbon C1s position at 284.6 eV and were plotted as shown in Figure 8a. The value of VB was measured from the intersection of a tangent line drawn from the curve and straight lines from the initial point of curves. The measured value of VB was 2.42 eV, whereas reported VB potential of AgBr and TiO2 are 2.30 eV and 2.7 eV, respectively [29]. Modified VB value indicated the stronger bonding among TiO2, CNTs and AgBr. Furthermore, Figure 8b shows the Tauc plot of modified Kubelka–Munk (KM) function vs. photoenergy/bandgap to estimate the bandgap energies of different photocatalysts. Bandgap energy of AgBr/bCNTs/TiO2 was 2.62 eV which is lower than TiO2 (3.2 eV). Lower bandgap energy of photocatalyst designated the higher expected light absorption and photocatalytic efficiency. The value of CB was calculated using Equation (1) given as follows.
where , and are the bandgap energy, VB and CB potentials of semiconductors. The of AgBr/bCNTs/TiO2 was found to be −0.2 eV, whereas stated of AgBr and TiO2 are −0.30 and −0.5 eV, respectively. Altered CB position of AgBr/bCNTs/TiO2 was ascribed to the bCNTs and AgBr.
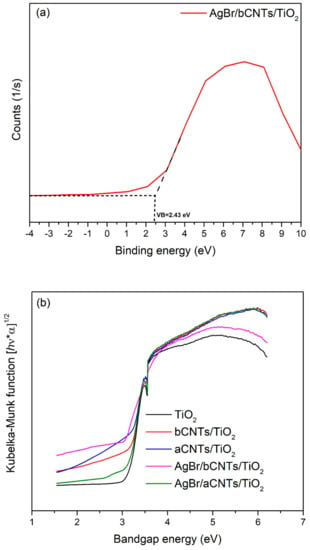
Figure 8.
(a) VB edge of AgBr/CNTs/TiO2. (b) Bandgap energy of TiO2, aCNTs/TiO2 bCNTs/TiO2, AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2 photocatalysts.
3.2. Photocatalytic H2 Production
The photocatalytic performance of all the composites was assessed for H2 generation from water using methanol as hole scavenger under visible light irradiations. Production of H2 using different photocatalysts is shown in Figure 9. Pure TiO2 produced 151 ppm of H2, which was lower than other photocatalysts owing to its large bandgap energy and severe charge recombination. The AgBr/TiO2 photocatalyst exhibited 289 ppm of H2 almost twice than benchmark TiO2. The AgBr/bCNTs/TiO2 and AgBr/aCNTs/TiO2 photocatalysts exhibited higher yield of 477 ppm and 376 ppm of H2 which were greater than TiO2. The significant increase in yield of the H2 was due to lower bandgap energy and efficient charge recombination. Also, incorporation of CNTs boosted the amount of H2 production owing to higher surface area, electrical, and optical properties. Therefore, enhanced efficiency of AgBr/bCNTs/TiO2 could be credited to efficient charge separation, light absorption, and indirect Z-scheme assembly developed between AgBr and TiO2 through bridge of CNTs.
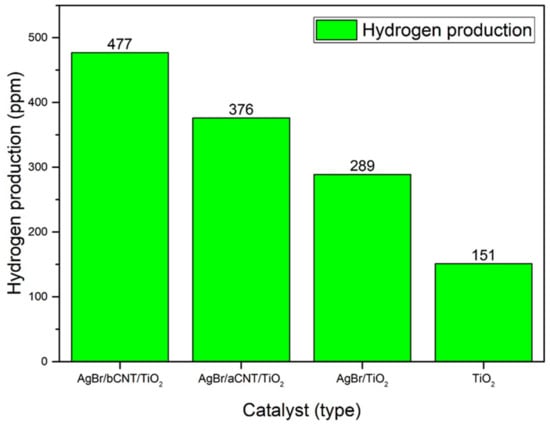
Figure 9.
Photocatalytic H2 production over various photocatalysts.
Evidently, the optical behaviour of AgBr/bCNTs/TiO2 was far better than TiO2 which played a pivotal role in the enhancement of photocatalytic activity under visible light irradiations. Bandgap energy of AgBr/bCNTs/TiO2 was 2.62 eV, lower than pure TiO2 because of the incorporation of CNTs and AgBr. The modified optical response enabled the composites to work efficiently under visible light irradiations and led to 477 ppm of H2 production. Moreover, the conduction band position of composite was −0.2 eV, which favoured the water splitting. It is due to the fact that the reduction potential for H2 is 0 eV, whereas CB of AgBr/bCNTs/TiO2 was −0.20 eV, higher than overall reduction potential required for water splitting and sufficient to produce the H2 [30]. In addition, the dominant factor for the enhancement in H2 production was efficient charge separation and transportation capability of photocatalyst. Charge recombination intensity of composites was 89 times lower than simple TiO2 owing to the development of Z-scheme heterojunction. Incorporation of CNTs increased the surface area and enhanced the charge separation because of the excellent electrical conductivity [24]. Separated electrons and holes on the surface of photocatalyst carried out redox reaction and exhibited a remarkable improvement in H2 production. The AgBr/aCNTs/TiO2 charge separation was very poor compared to the AgBr/bCNTs/TiO2 and led to the lower H2 production. Further, the dual role of CNTs was very important owing to the generation of greater active sites because of the greater area, surface functional groups and higher diffusion capability. Diffusion of charge through tubular structure lowered the charge recombination and led to good separation and transportation [24,31,32].
Moreover, as an electron mediator, the activity of acid functionalized CNTs was quite different from the basic one. The H2 production rate of acid-functionalised aCNTs-based photocatalyst was 376 ppm whereas base-functionalised bCNTs-based photocatalyst exhibited 477 ppm of H2, which was 1.27 times higher than the former. The difference of yield was attributed to functional groups attached on the surface of bCNTs. The functional moieties on the bCNTs surface affected the interfacial bonding between AgBr/TiO2 and bCNTs. Strength of bonding was responsible for charge recombination [33]. Higher efficiency of the base-functionalised CNTs-based composites showed that there was uniform dispersion and strong bonding among CNTs, TiO2 and AgBr which led to efficient charge separation and transportation. Quickly migrated electrons and holes attacked the H+ and reduced it into H2. On the other hand, the comparatively lower yield of H2 over aCNTs-based photocatalyst revealed that AgBr and TiO2 interfacial bonding strength was weaker than the former which led to relatively lower production of solar fuel. Also, functional groups present on the surface of the photocatalysts help in redox reaction and intermediate formation which further enhance the H2 production. In addition to the interfacial bonding strength, basic and acidic moieties on the surface of photocatalyst affected the dispersibility of the photocatalyst. It was visualised that dispersion of bCNTs-based photocatalyst was better and uniform as compared to aCNTs-based photocatalyst. Uniform dispersion of active photocatalyst particles enable the efficient harnessing of visible light irradiations and led to higher H2 production. This can be further better explained by considering the affinity of AgBr particles. The AgBr particle is highly soluble in basic solution. The solubility of AgBr particles strongly affects the deposition process. When basic solvents were attached on the surface of the bCNTs, they promoted the interaction of depositing particles of AgBr on the surface of bCNTs. Uniform distribution and strong interaction of AgBr and bCNTs fortified the effective bonding of light-sensitive element with electron mediator. In the case of acid solvents, which is hydrophobic to the AgBr particles lead to poor dispersion and weaker interfacial bonding and eventually lower comparative efficiency of the photocatalyst. The difference in the behaviour of both the composites was due to the different nature of the functional groups present on the surface. Therefore, surface chemical species on electron mediator strongly influence the interfacial bonding and hence, yield of solar fuels [33,34,35].
In addition, effect of time was observed for the acid- and base-functionalised CNTs-based composites by performing the reaction for 5 h continuously as plotted in Figure 10. Both the photocatalysts demonstrated smooth production of H2. The yield was initially increased with time and then become almost constant. Further, recyclability analysis was performed for consecutive five cycles and photocatalyst exhibited almost the same yield of H2 without losing activity as shown in Figure 11. Notable stability of photocatalyst was due to good interfacial bonding, generation and transportation of the photogenerated electrons, resulting in enhanced spatial charge separation [21,36]. The AgBr is highly sensitive to light and immediately decomposes to Ag and Br. Reason of decomposition could be the inability of generated charge carriers to move to other elements because of lower electrical conductivity or poor bonding. The stable activity was observed for five consecutive cycles owing to the smooth transfer of electrons across the electronic interface between AgBr and TiO2 through bCNTs. Similarly, Xin et al. [37] reported that the activity of Ag/AgBr/GdVO4 was stable without any significant loss for five consecutive cycles. Likewise, Xu et al. [38] used the AgBr/AgIn(MoO4)2 photocatalysts for the degradation of dyes and observed stable and smooth performance for six cycles. Thus, the stable photocatalytic performance of AgBr/bCNTs/TiO2 was credited to the bCNTs as an electron mediator and stronger bonding because of the surface functional which enabled efficient separation of charges and reduced the recombination intensity. In addition, comparison was made with other studies reported in literature as shown in Table 1. It shows that photocatalytic H2 in current study is comparable and even higher than many photocatalysts. However, true comparison cannot be developed till process parameters and reactor geometry are identical.
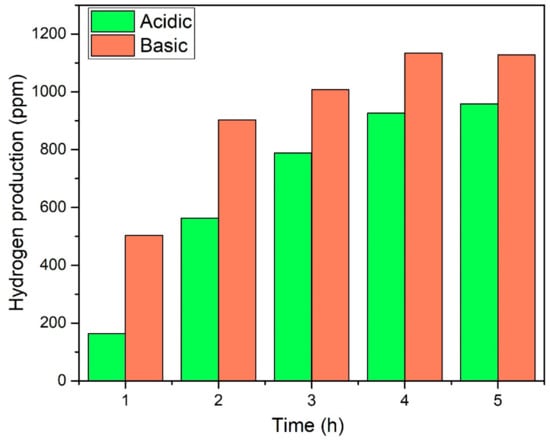
Figure 10.
Effect of time on yield of H2 over AgBr/aCNTs/TiO2 and AgBr/bCNTs/TiO2.
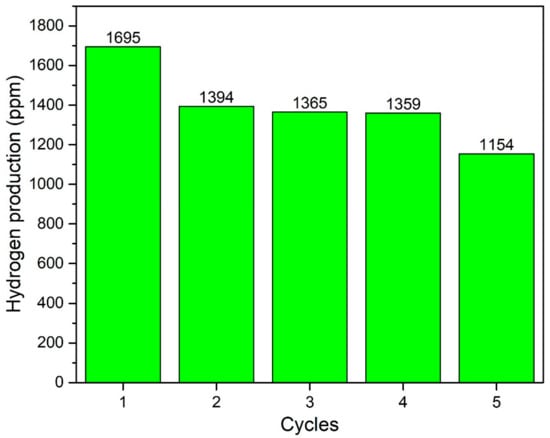
Figure 11.
Stability analysis of AgBr/bCNTs/TiO2 for H2.

Table 1.
Comparison of yield of H2 production over various photocatalysts.
3.3. Mechanism of H2 Generation on AgBr/bCNTs/TiO2
Water splitting involves complex redox reaction on the surface of photocatalyst and therefore it is crucial to understand the mechanism of H2 production using photocatalysis. Figure 12 illustrates the mechanism of H2 production over AgBr/bCNTs/TiO2. It could be seen that when light irradiations strike the surface of photocatalyst, electrons started moving from the VB to CB of AgBr. Photogenerated electrons jumped to the carbon nanotubes which were acting as a bridge in transferring the charge carriers from AgBr to TiO2. Separated electrons take part into redox reaction thereby reducing the H+ into H2 and holes left on AgBr attacked the adsorbed H2O molecules to oxidize it to H+ and O2. Further, CB potentials of TiO2 and AgBr are −0.5 and −0.3 eV, respectively, which determine the mechanism of transfer of electrons and hence redox reaction [7,46,47]. In AgBr/bCNTs/TiO2, AgBr is a light-sensitive element and emits electrons under visible light irradiations. These electrons are transferred to the TiO2 for H2 production else could recombine on the surface of AgBr leading to its decomposition and loss of activity. The CB of TiO2 is higher than AgBr, therefore, the excited electrons move to the VB of TiO2 only, resulting in the formation of Z-scheme assembly of AgBr/TiO2. As observed in PL spectra and H2 production results, the efficiency of AgBr/TiO2 was better than pure TiO2 but considerably lower than AgBr/bCNTs/TiO2. These results indicated that the presence of CNTs was very important to enhance the efficiency of Z-scheme AgBr/TiO2 because of its outstanding electrical conductivity as it reduced the charge recombination and also enhance the light absorption capability. Photocatalytic reduction of H+ into H2 depends upon the CB potential of a photocatalyst. For AgBr/bCNTs/TiO2, overall CB was −0.2 and VB was 2.42 which was sufficient to convert H2O into H2 and O2. Further, the standard thermodynamics redox potential of H2 is 0 eV, while theoretically redox potential of O2 is 1.23 eV [48]. Similarly, individual CB positions of AgBr and TiO2 are −0.30 and 0.5 eV, respectively [7,46,47]. Therefore, indirect Z-scheme assembly of AgBr/bCNTs/TiO2 was an efficient photocatalyst for photocatalytic H2 production form H2O.
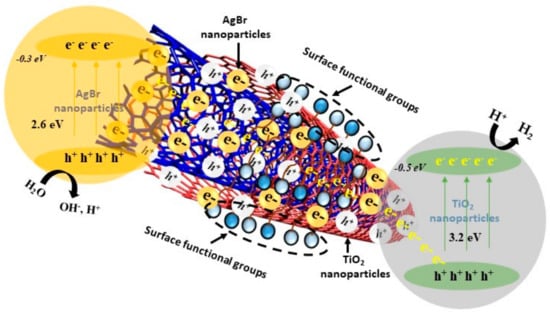
Figure 12.
Mechanism of H2 production over AgBr/bCNTs/TiO2 photocatalyst.
4. Conclusions
Carbon nanotubes-incorporated Z-scheme assembly of AgBr/TiO2 photocatalysts were successfully prepared by facile deposition and reflux method. The photocatalysts exhibited great potential for H2 production with maximum yield of 477 ppm of H2. The performance of AgBr/bCNTs/TiO2 was higher than AgBr/aCNTs/TiO2, AgBr/TiO2 and TiO2, respectively. The charge recombination intensity of AgBr/bCNTs/TiO2 was 89 times lower than TiO2 and bandgap energy was 2.62 eV showing that properties of TiO2 were greatly improved. Further, the photocatalyst exhibited stability for consecutive five cycles showing its potential for continuous H2 production under visible light irradiations. Remarkable activity was ascribed to strong interfacial bonding, high surface area, reduced charge recombination and improved visible light response because of the incorporation of CNTs and surface functional groups. Thus, this study would be helpful to develop new Z-scheme photocatalyst with enhanced efficiency for various photocatalytic applications.
Author Contributions
Conceptualization, N.S.; methodology, N.S., M.S., H.A. and A.R.; software, N.S. and A.H.; formal analysis, N.S.; investigation, N.S., A.H. and P.A.; resources, I.M.M., M.T., K.J. and M.H.; data curation, N.S., M.T., M.H. and K.J.; Writing—Original draft preparation, N.S., P.A. and A.H.; Writing—Review and editing, N.S., A.H., P.A., M.T., M.H. and I.M.M.; visualization, N.S.; supervision, K.J., M.T., M.H. and I.M.M.; project administration, K.J., M.T., M.H. and I.M.M.; funding acquisition, K.J., M.T., M.H. and I.M.M.
Funding
Research University Grant (RUG, UTM vot no. Q.J130000.2651.16J58).
Acknowledgments
The authors would like to thank Chemical Engineering Department, COMSATS University Islamabad, Lahore Campus, Chemical Engineering Department, Universiti Teknologi PETRONAS (UTP), Malaysia, Chemical Reaction Engineering group, Universiti Teknologi Malaysia (UTM), Malaysia for providing facilities, and Department of Chemical Engineering, Jazan University, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2—based Z—scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Tan, L.L.; Pasbakhsh, P.; Chai, S.P. All-solid-state Z-scheme photocatalyst with carbon nanotubes as an electron mediator for hydrogen evolution under simulated solar light. Chem. Eng. J. 2017, 316, 41–49. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Cheng, B.; Yu, J. Direct Z-Scheme TiO2/NiS Core–Shell Hybrid Nanofibers with Enhanced Photocatalytic H2-Production Activity. ACS Sustain. Chem. Eng. 2018, 6, 12291–12298. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. Fabrication of highly efficient and stable indirect Z-scheme assembly of AgBr/TiO2 via graphene as a solid-state electron mediator for visible light induced enhanced photocatalytic H2 production. Appl. Surf. Sci. 2019, 463, 445–455. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Kim, W.J.; Park, J.; Rhee, D.K.; Jang, I.H.; Park, N.G.; Lee, J.Y.; Yoo, P.J. Highly efficient and recyclable nanocomplexed photocatalysts of AgBr/N-doped and amine-functionalized reduced graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 20819–20827. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z- Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Asi, M.A.; He, C.; Su, M.; Xia, D.; Lin, L.; Deng, H.; Xiong, Y.; Qiu, R.; Li, X.Z. Photocatalytic reduction of CO2 to hydrocarbons using AgBr/TiO2 nanocomposites under visible light. Catal. Today 2011, 175, 256–263. [Google Scholar] [CrossRef]
- Lin, H.; Cao, J.; Luo, B.; Xu, B.; Chen, S. Synthesis of novel Z-scheme AgI/Ag/AgBr composite with enhanced visible light photocatalytic activity. Catal. Commun. 2012, 21, 91–95. [Google Scholar] [CrossRef]
- Liang, S.; Han, B.; Liu, X.; Chen, W.; Peng, M.; Guan, G.; Deng, H.; Lin, Z. 3D spatially branched hierarchical Z-scheme CdS-Au nanoclusters- ZnO hybrids with boosted photocatalytic hydrogen evolution. J. Alloys Compd. 2018, 754, 105–113. [Google Scholar] [CrossRef]
- Akple, M.S.; Chimmikuttanda, S.P. A ternary Z-scheme WO3–Pt–CdS composite for improved visible-light photocatalytic H 2 production activity. J. Nanopart. Res. 2018, 20, 231. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Z.; Wang, G.; Gengenbach, T.R.; McCarthy, D.T.; Deletic, A.; Yu, J.; Zhang, X. Highly dispersed TiO2 nanocrystals and WO3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 2017, 218, 163–173. [Google Scholar] [CrossRef]
- Fakhrul, M.; Samsudin, R.; Bacho, N.; Su, S.; Hau, Y. Photocatalytic degradation of phenol wastewater over Z-scheme g-C3N4/CNT/BiVO4 heterostructure photocatalyst under solar light irradiation. J. Mol. Liq. 2019, 277, 977–988. [Google Scholar]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. Improved interfacial bonding of graphene-TiO2 with enhanced photocatalytic reduction of CO2 into solar fuel. J. Environ. Chem. Eng. 2018, 6, 6947–6957. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Hierarchical Nanotubular Anatase/Rutile/TiO2 (B) Heterophase Junction with Oxygen Vacancies for Enhanced Photocatalytic H2 Production. Langmuir 2018, 2, 1883–1889. [Google Scholar] [CrossRef]
- Feng, B.; Wu, Z.; Liu, J.; Zhu, K.; Li, Z.; Jin, X.; Hou, Y.; Xi, Q.; Cong, M.; Liu, P.; et al. Combination of ultrafast dye-sensitized-assisted electron transfer process and novel Z-scheme system: AgBr nanoparticles interspersed MoO3 nanobelts for enhancing photocatalytic performance of RhB. Appl. Catal. B Environ. 2017, 206, 242–251. [Google Scholar] [CrossRef]
- Song, S.; Meng, A.; Jiang, S.; Cheng, B.; Jiang, C. Construction of Z-scheme Ag2CO3/N-doped graphene photocatalysts with enhanced visible-light photocatalytic activity by tuning the nitrogen species. Appl. Surf. Sci. 2017, 396, 1368–1374. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Synthesis, characterization and photocatalysis of heterostructure. Mater. Lett. 2018, 216, 92–96. [Google Scholar] [CrossRef]
- Mahmoud, Z.H.; Falih, M.S.; Khalaf, O.E.; Farhan, M.A.; Ali, F.K. Photosynthesis of AgBr Doping TiO 2 Nanoparticles and degradation of reactive red 120 dye. J. Adv. Pharm. Educ. Res. 2018, 8, 51–55. [Google Scholar]
- Alzamly, A.; Hamed, F.; Ramachandran, T.; Bakiro, M.; Ahmed, S.H.; Mansour, S.; Salem, S. Tunable band gap of Bi3þ-doped anatase TiO2 for enhanced photocatalytic removal of acetaminophen under UV-visible light irradiation. J. Water Reuse Desal. 2019, 9, 31–46. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Thangavel, N.; Yusuf, H.; Neppolian, B. Highly active and stable multi-walled carbon nanotubes-graphene-TiO2 nanohybrid: An efficient non-noble metal photocatalyst for water splitting. Catal. Today 2019, 321–322, 120–127. [Google Scholar] [CrossRef]
- Koli, V.B.; Mavengere, S.; Sik, J. Boron-doped-TiO2–CNTs nanocomposites for photocatalytic application. J. Mater. Sci. Mater. Electron. 2018, 29, 16660–16672. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Appusamy, A.; Gnanasundaram, N. Surface modi fi cation and characterization of carbonaceous adsorbents for the e ffi cient removal of oil pollutants. J. Hazard. Mater. 2019, 379, 120673. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wu, J.; Gao, M.; Xin, Y.; Chai, C. Enhanced debromination and degradation of 2,4-dibromophenol by an Z-scheme Bi2MoO6/CNTs/g-C3N4 visible light photocatalyst. Chem. Eng. J. 2017, 316, 461–470. [Google Scholar] [CrossRef]
- de Brito, J.F.; Tavella, F.; Genovese, C.; Ampelli, C.; Zanoni, M.V.B.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, C.; Zhuang, J.; Zhong, Y.; Wang, D. AgBr/MgBi2O6 heterostructured composites with highly ef fi cient visible-light-driven photocatalytic activity. J. Phys. Chem. Solids 2018, 117, 94–100. [Google Scholar] [CrossRef]
- He, Y.; Yuan, R.; Leung, M.K.H. Highly efficient AgBr/BiVO4 photoanode for photocatalytic fuel cell. Mater. Lett. 2019, 236, 394–397. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.; Wen, X.; Zhang, L.; Liang, C.; Zhang, X.; Guan, D.; Tang, N.; Zeng, G. Journal of Colloid and Interface Science Construction of highly efficient and stable ternary AgBr/Ag/PbBiO2Br Z-scheme photocatalyst under visible light irradiation: Performance and mechanism insight. J. Colloid Interface Sci. 2018, 513, 852–865. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Jiang, W.; Zhang, M.; Ming, X.; Wang, S.; Zhang, Q. Strongly visible-light responsive plasmonic shaped AgX:Ag (X = Cl, Br) nanoparticles for reduction of CO2 to methanol. Nanoscale 2012, 4, 5646–5650. [Google Scholar] [CrossRef]
- Heyrovská, R. Tables of absolute potentials of aqueous redox couples of elements and of reference electrodes. Monatshefte für Chem. Chem. Mon. 2019, 150, 391–393. [Google Scholar] [CrossRef]
- Ali, A.; Ayoub, A.; Patil, S.A.; Mengal, N. Applied Catalysis A General Synthesis of solution processed f-CNT@Bi2S3 hybrid fi lm coated linen fabric as a free-standing textile structured photo catalyst. Appl. Catal. A, Gen. 2018, 566, 87–95. [Google Scholar]
- Wang, Y.; Liu, X.; Zheng, C.; Li, Y.; Jia, S.; Li, Z. Tailoring TiO2 Nanotube-Interlaced Graphite Carbon Nitride Nanosheets for Improving Visible-Light-Driven Photocatalytic Performance. Adv. Sci. 2018, 5, 1700844. [Google Scholar] [CrossRef] [PubMed]
- Rajender, G.; Kumar, J.; Giri, P.K. Interfacial charge transfer in oxygen de fi cient TiO2-graphene quantum dot hybrid and its in fl uence on the enhanced visible light photocatalysis. Appl. Catal. B Environ. 2018, 224, 960–972. [Google Scholar] [CrossRef]
- Thi, W.; Qun, Y.; Xueying, W.; Yang, F.; Xiaohong, L. Enhancement of photocatalytic performance in sonochemical synthesized ZnO–rGO nanocomposites owing to effective interfacial interaction. Environ. Chem. Lett. 2018, 16, 251–264. [Google Scholar]
- Xue, C.; Yan, X.; An, H.; Li, H.; Wei, J.; Yang, G. Bonding CdS-Sn2S3 eutectic clusters on graphene nanosheets with unusually photoreaction-driven structural recon fi guration e ff ect for excellent H2 evolution and Cr (VI) reduction. Appl. Catal. B Environ. 2018, 222, 157–166. [Google Scholar] [CrossRef]
- Gopannagari, M.; Kumar, D.P.; Park, H.; Kim, E.H.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Influence of surface-functionalized multi-walled carbon nanotubes on CdS nanohybrids for e ff ective photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 236, 294–303. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Yu, C.; Teng, B.; He, Y.; Zhao, L.; Fan, M. Application of Ag/AgBr/GdVO4 composite photocatalyst in wastewater treatment. J. Environ. Sci. 2017, 63, 1–8. [Google Scholar] [CrossRef]
- Yan, X.; Wang, X.; Gu, W.; Wu, M.M.; Yan, Y.; Hu, B.; Che, G.; Han, D.; Yang, J.; Fan, W.; et al. Single-crystalline AgIn(MoO4)2 nanosheets grafted Ag/AgBr composites with enhanced plasmonic photocatalytic activity for degradation of tetracycline under visible light. Appl. Catal. B Environ. 2015, 164, 297–304. [Google Scholar] [CrossRef]
- David, S.; Mahadik, M.A.; Chung, H.S.; Ryu, J.H.; Jang, J.S. Facile Hydrothermally Synthesized a Novel CdS Nanoflower/Rutile-TiO2 Nanorod Heterojunction Photoanode Used for Photoelectrocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2017, 5, 7537–7548. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. Visible and direct sunlight induced H2 production from water by plasmonic Ag-TiO2 nanorods hybrid interface. Sol. Energy Mater. Sol. Cells 2017, 160, 463–469. [Google Scholar] [CrossRef]
- Zhu, Z.; Kao, C.T.; Tang, B.H.; Chang, W.C.; Wu, R.J. Efficient hydrogen production by photocatalytic water-splitting using Pt-doped TiO2 hollow spheres under visible light. Ceram. Int. 2015, 42, 6749–6754. [Google Scholar] [CrossRef]
- Hu, S.; Wang, B.; Zhu, M.; Ma, Y.; Wang, J. PlasmonicAu-TiO2/ZnO Core-Shell Nanorod Array Photoanode for Visible-Light-Driven Photoelectrochemical Water Splitting. Energy Technol. 2017, 5, 1599–1605. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Ba, N.; Gao, F.; Xie, H. Effects of NH4F quantity on N-doping level, photodegradation and photocatalytic H2 production activities of N-doped TiO2 nanotube array films. Mater. Res. Bull. 2017, 86, 268–276. [Google Scholar] [CrossRef]
- Boppella, R.; Kochuveedu, S.T.; Kim, H.; Jeong, M.J.; Marques Mota, F.; Park, J.H.; Kim, D.H. Plasmon-Sensitized Graphene/TiO2 Inverse Opal Nanostructures with Enhanced Charge Collection Efficiency for Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 7075–7083. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y.; et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Tahir, M. Synergistic effect in MMT-dispersed Au/TiO2 monolithic nanocatalyst for plasmon-absorption and metallic interband transitions dynamic CO2 photo-reduction. Appl. Catal. B Environ. 2017, 219, 329–343. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Guo, Y.; Zhao, Y.; Yuan, X.; Guo, Y. Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2014, 271, 150–159. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).