Ag Nanostructures with Spikes on Adhesive Tape as a Flexible Sers-Active Substrate for In Situ Trace Detection of Pesticides on Fruit Skin
Abstract
1. Introduction
2. Experimental Section
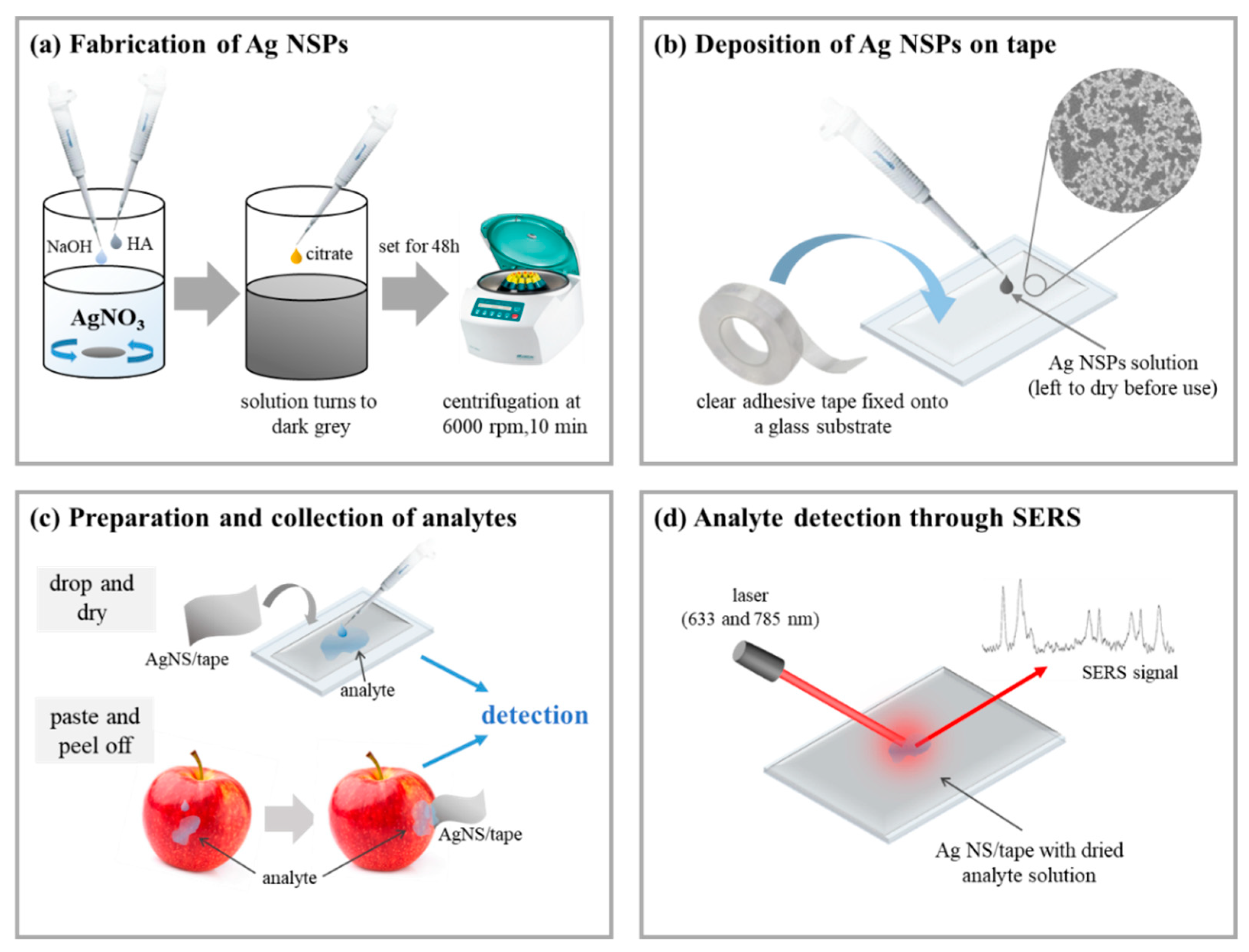
2.1. Synthesis of Surface-Enhanced Raman Scattering (SERS)-Active Silver Nanostructures with Spikes Deposited on Tape (Ag NSPs_x/Tape)
2.2. Morphological Characterization of Ag NSPs_x/Tape
2.3. Acquisition of SERS Spectra and Enhancement Factor
2.4. Application of Flexible Ag NSPs_x/Tape to Pesticide Detection
3. Results and Discussion
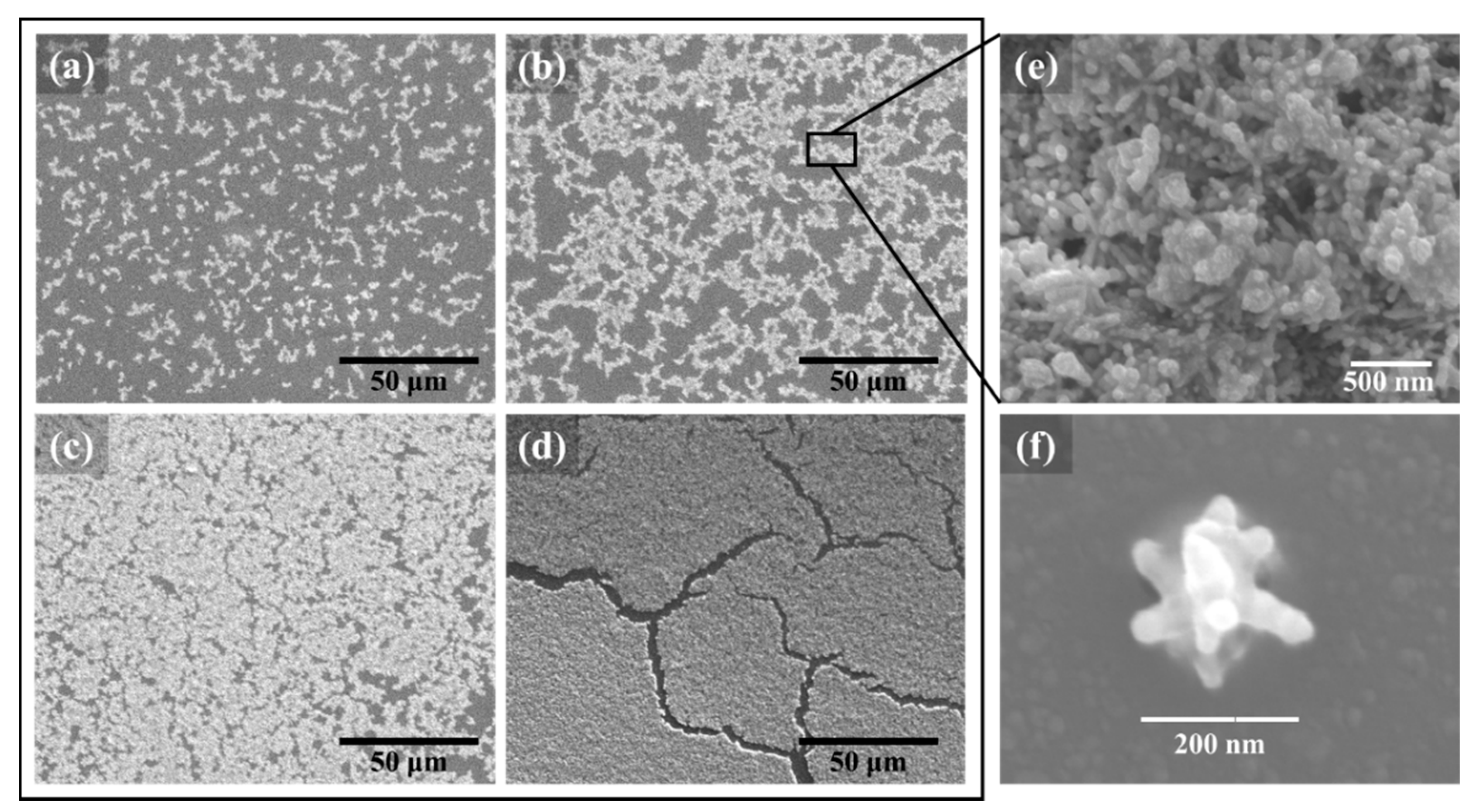
3.1. Surface Characterization and Agglomeration Behavior of Ag NSPs_x/Tape
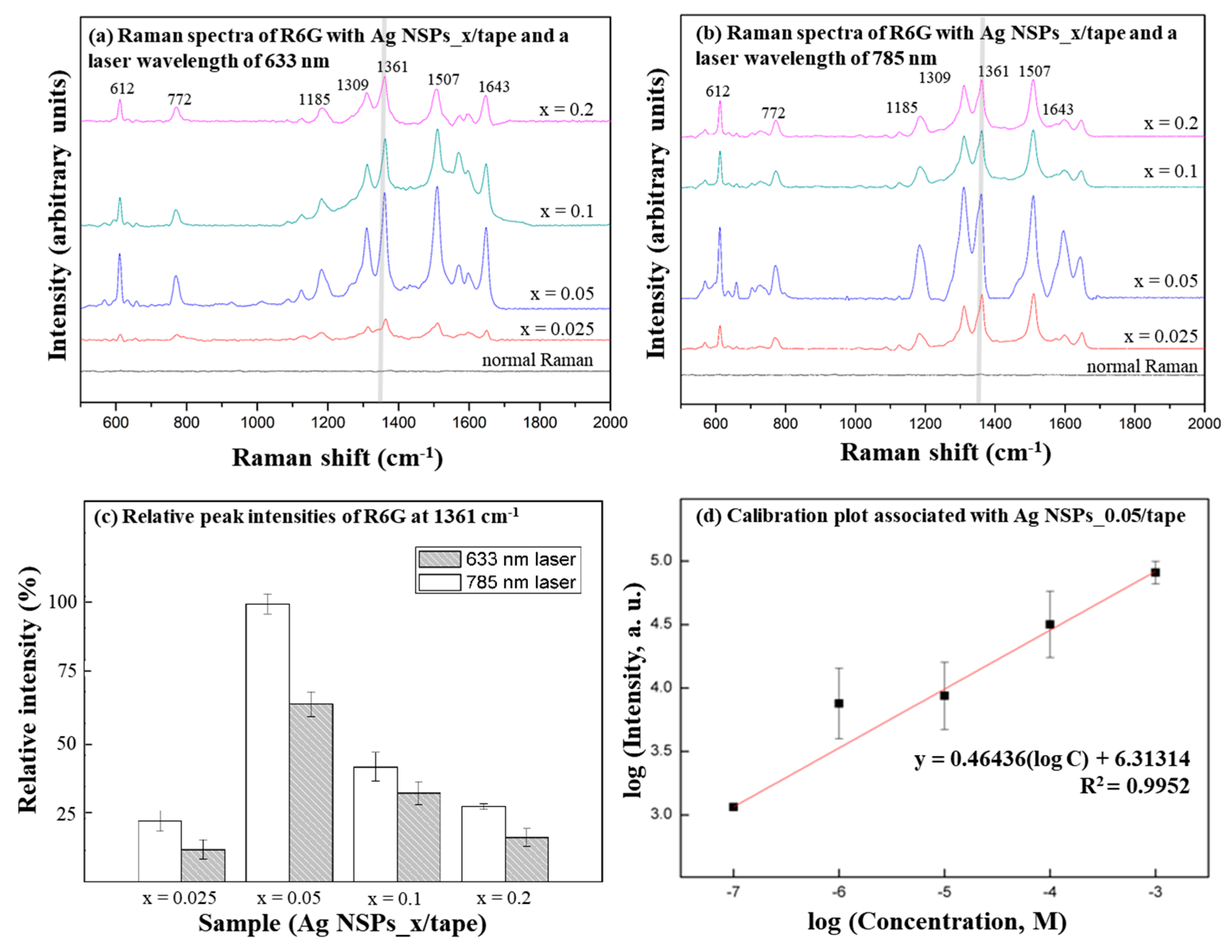
3.2. Evaluation of the SERS Effect of Ag NSPs_x/Tape
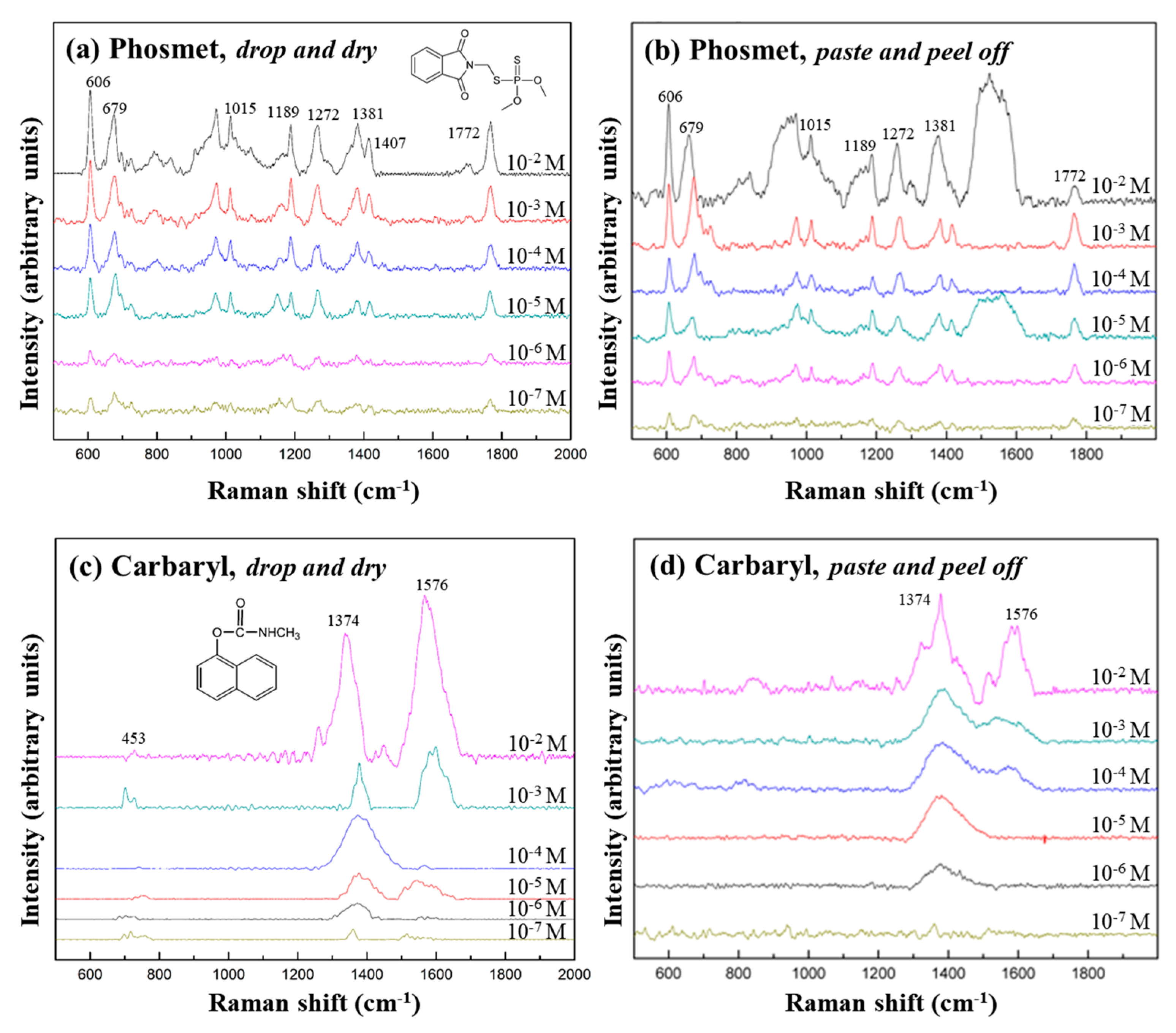
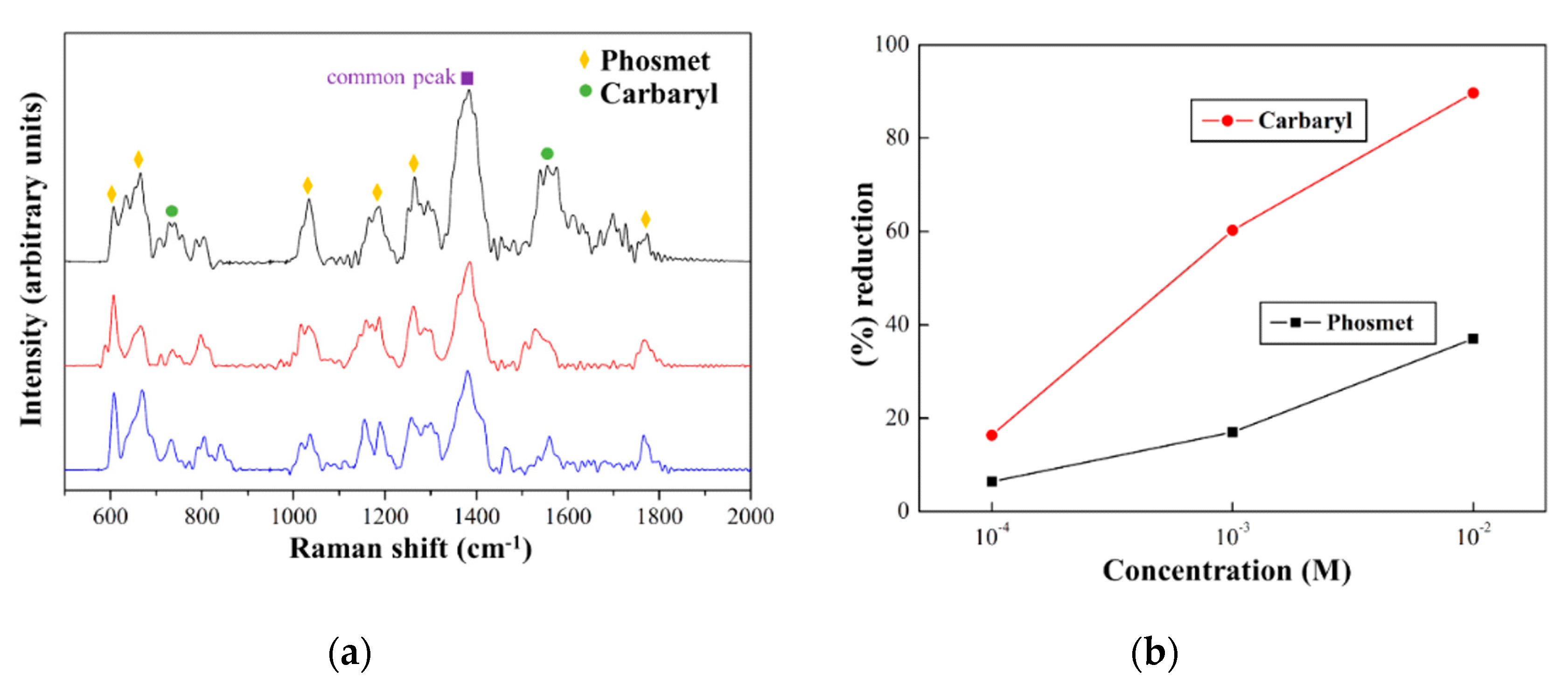
3.3. Detection of Pesticides from Apple Peel with the Flexible Ag NSPs_x/Tape
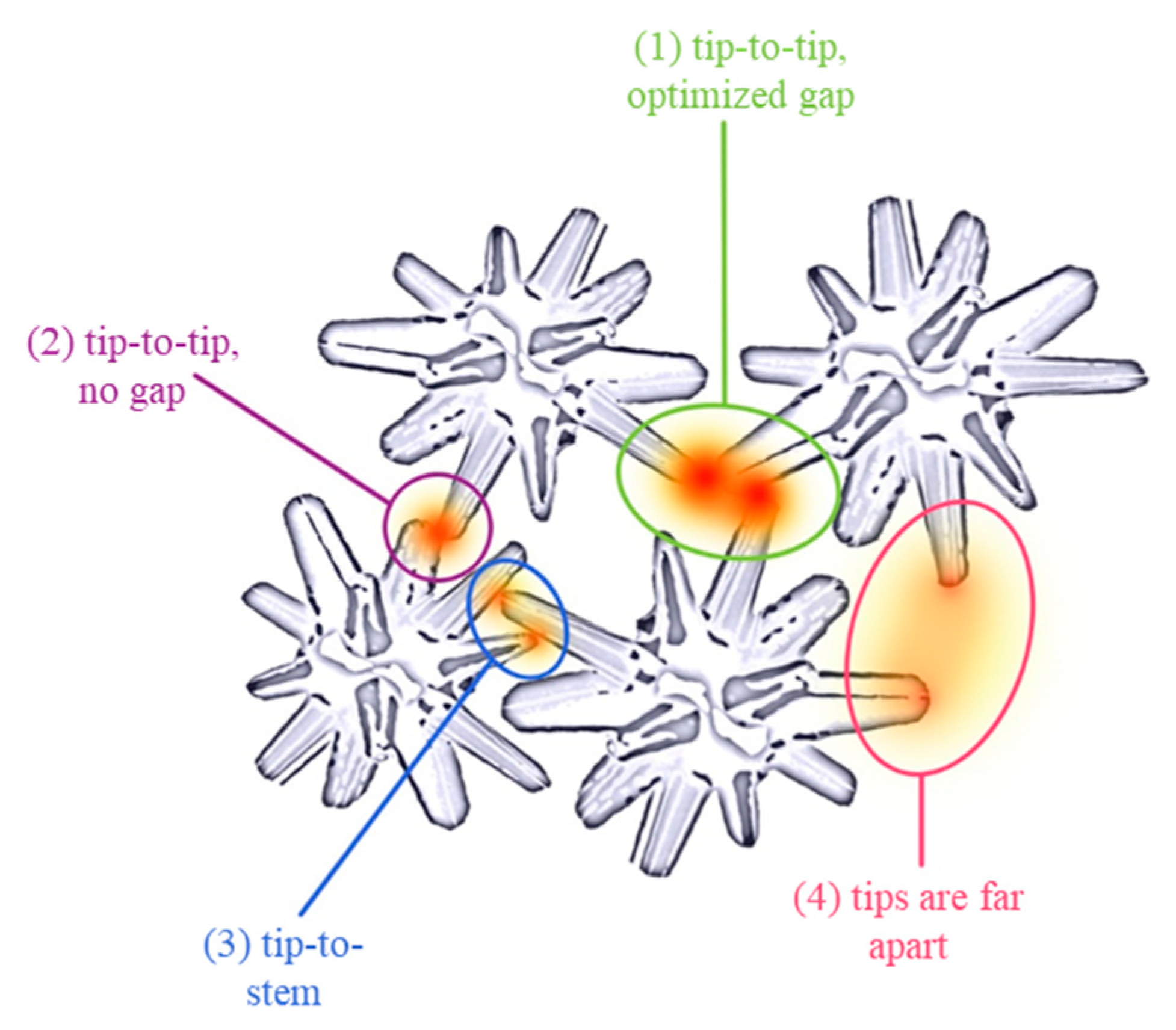
3.4. Mechanism of SERS upon Ag NSPs_x/Tape
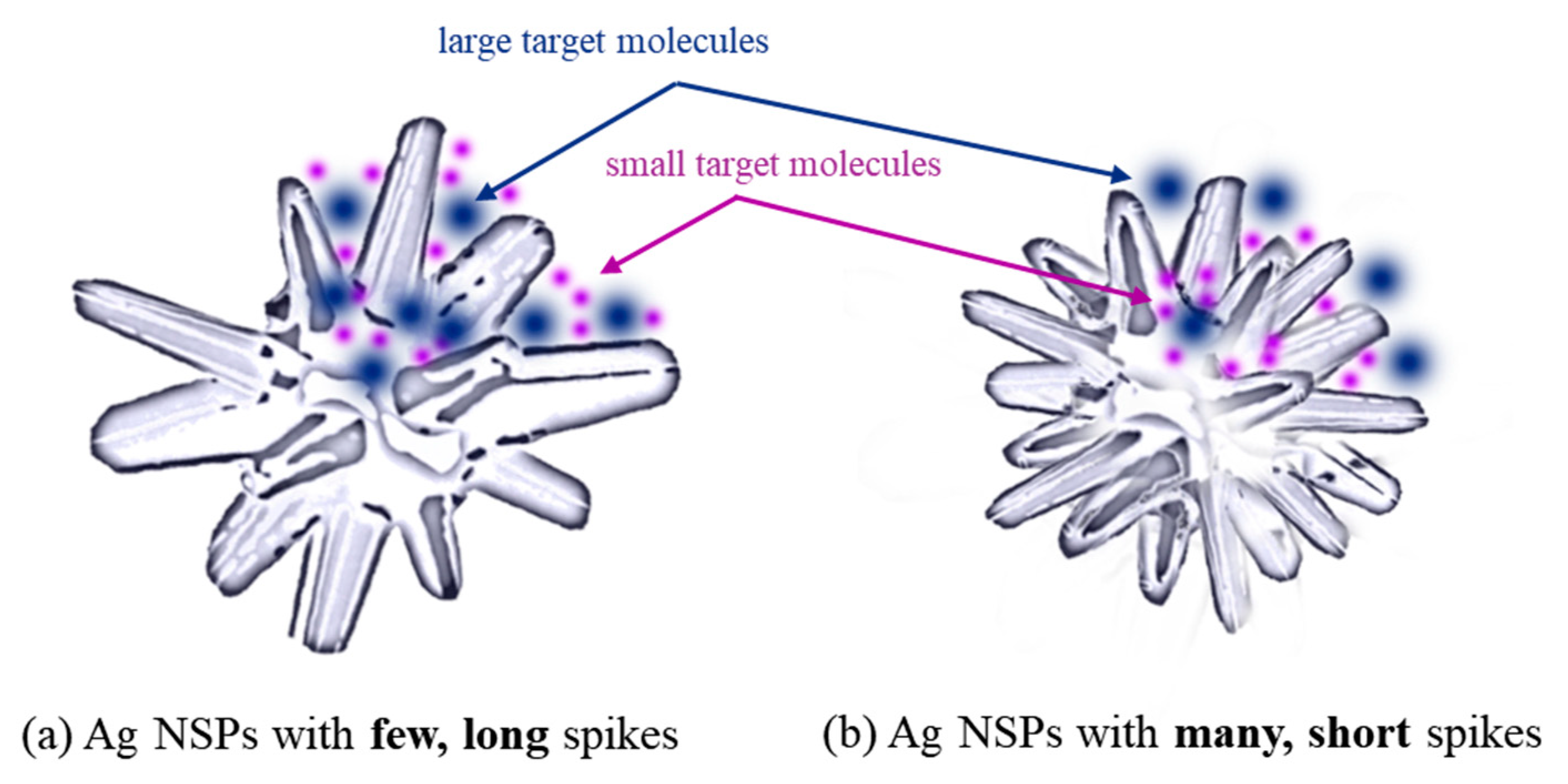
3.5. Effect of Ag NSP Spike Length on Target Detection through SERS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shiohara, A.; Langer, J.; Polavarapu, L.; Liz-Marzán, L.M. Solution processed polydimethylsiloxane/gold nanostar flexible substrates for plasmonic sensing. Nanoscale 2014, 6, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Carregal-Romero, S.; Coelho, J.P.; Guerrero-Martínez, A. Recent progress on colloidal metal nanoparticles as signal enhancers in nanosensing. Adv. Colloid Interface Sci. 2016, 233, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Fu, W.; Chen, C.-C.; Chen, G.D.; Juang, Y.D. Gold nanoparticle-coated ZrO2-nanofiber surface as a SERS-active substrate for trace detection of pesticide residue. Nanomaterials 2018, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Reznickova, A.; Slepicka, P.; Nguyenova, H.Y.; Kolska, Z.; Dendisova, M.; Svorcik, V. Copper-gold sandwich structures on PE and PET and their SERS enhancement effect. RSC Adv. 2017, 7, 23055–23064. [Google Scholar] [CrossRef]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for Surface-Enhanced Raman Spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085. [Google Scholar] [CrossRef]
- Yuan, Y.; Panwar, N.; Yap, S.H.K.; Wu, Q.; Zeng, S.; Xu, J.; Tjin, S.C.; Song, J.; Qu, J.; Yong, K.T. SERS-based ultrasensitive sensing platform: An insight into design and practical applications. Coord. Chem. Rev. 2017, 337, 1–33. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Yu, Z.; Huang, J.; Ni, D.; Shu, H.; Dong, Q. The effect of solvent environment toward optimization of SERS sensors for pesticides detection from chemical enhancement aspects. Sens. Actuators B Chem. 2018, 256, 721–728. [Google Scholar] [CrossRef]
- He, S.; Chua, J.; Tan, E.K.M.; Kah, J.C.Y. Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv. 2017, 7, 16264–16272. [Google Scholar] [CrossRef]
- Alyami, A.; Quinn, A.J.; Iacopino, D. Flexible and transparent Surface Enhanced Raman Scattering (SERS)-Active Ag NPs/PDMS composites for in-situ detection of food contaminants. Talanta 2019, 201, 58–64. [Google Scholar] [CrossRef]
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and robust SERS active substrates for conformal rapid detection of pesticide residues from fruits. Sens. Actuators B Chem. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, S.; Li, Y.; Zhao, F.; Chen, J.; Wang, S.; Wu, H.; Xu, J.; Chu, M.; Liao, J.; et al. Flexible and adhesive tape decorated with silver nanorods for in-situ analysis of pesticides residues and colorants. Microchim. Acta 2019, 186, 603. [Google Scholar] [CrossRef] [PubMed]
- Sitjar, J.; Liao, J.-D.; Lee, H.; Liu, B.H.; Fu, W. SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides. Nanomaterials 2019, 9, 664. [Google Scholar] [CrossRef]
- Sui, C.; Wang, K.; Wang, S.; Ren, J.; Bai, X.; Bai, J. SERS activity with tenfold detection limit optimization on a type of nanoporous AAO-based complex multilayer substrate. Nanoscale 2016, 8, 5920–5927. [Google Scholar] [CrossRef]
- He, S.; Kang, M.W.C.; Khan, F.J.; Tan, E.K.M.; Reyes, M.A.; Kah, J.C.Y. Optimizing gold nanostars as a colloid-based surface-enhanced Raman scattering (SERS) substrate. J. Opt. 2015, 17, 114013. [Google Scholar] [CrossRef]
- Fraire, J.C.; Pérez, L.A.; Coronado, E.A. Cluster size effects in the surface-enhanced raman scattering response of ag and Au nanoparticle aggregates: Experimental and theoretical insight. J. Phys. Chem. C 2013, 117, 23090–23107. [Google Scholar] [CrossRef]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; Gric, T., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Sivashanmugan, K.; Lee, H.; Syu, C.H.; Liu, B.H.C.; Liao, J.D. Nanoplasmonic Au/Ag/Au nanorod arrays as SERS-active substrate for the detection of pesticides residue. J. Taiwan Inst. Chem. Eng. 2017, 75, 287–291. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Lai, K.; Zhai, F.; Zhang, Y.; Wang, X.; Rasco, B.A.; Huang, Y. Application of surface enhanced Raman spectroscopy for analyses of restricted sulfa drugs. Sens. Instrum. Food Qual. Saf. 2011, 5, 91–96. [Google Scholar] [CrossRef]
- Altun, A.O.; Bond, T.; Pronk, W.; Park, H.G. Sensitive Detection of Competitive Molecular Adsorption by Surface-Enhanced Raman Spectroscopy. Langmuir 2017, 33, 6999–7006. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xing, T.; Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Tuning the surface enhanced Raman scattering performance of anisotropic Au core−Ag shell hetero-nanostructure: The effect of core geometry. J. Alloys Compd. 2019, 776, 934–947. [Google Scholar] [CrossRef]
- Mikac, L.; Ivanda, M.; Gotić, M.; Maksimović, A.; Trusso, S.; D’Andrea, C.; Foti, A.; Irrera, A.; Fazio, B.; Gucciardi, P.G. Metal nanoparticles deposited on porous silicon templates as novel substrates for SERS. Croat. Chem. Acta 2015, 88, 437–444. [Google Scholar] [CrossRef]
- Severyukhina, A.N.; Parakhonskiy, B.V.; Prikhozhdenko, E.S.; Gorin, D.A.; Sukhorukov, G.B.; Möhwald, H.; Yashchenok, A.M. Nanoplasmonic Chitosan Nanofibers as Effective SERS Substrate for Detection of Small Molecules. ACS Appl. Mater. Interfaces 2015, 7, 15466–15473. [Google Scholar] [CrossRef]
- Liu, Y.; He, B.; Zhang, Y.; Wang, H.; Ye, B. Detection of Phosmet Residues on Navel Orange Skin by Surface-enhanced Raman Spectroscopy. Intell. Autom. Soft Comput. 2015, 21, 423–432. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Thomas, R.; Fabris, L. Plasmonic properties of regiospecific core-satellite assemblies of gold nanostars and nanospheres. Phys. Chem. Chem. Phys. 2014, 17, 21133–21142. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-enhanced raman scattering in local optical fields of silver and gold nanoaggregates—From single-molecule raman spectroscopy to ultrasensitive probing in live cells. Acc. Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef]
- Li, A.; Li, S. Large-volume hot spots in gold spiky nanoparticle dimers for high-performance surface-enhanced spectroscopy. Nanoscale 2014, 6, 12921–12928. [Google Scholar] [CrossRef]
- Garcia-leis, A.; Rivera-arreba, I.; Sanchez-cortes, S. Morphological tuning of plasmonic silver nanostars by controlling the nanoparticle growth mechanism: Application in the SERS detection of the amyloid marker Congo Red. Colloids Surf. Physicochem. Eng. Asp. 2017, 535, 49–60. [Google Scholar] [CrossRef]
- Reyes Gómez, F.; Rubira, R.J.G.; Camacho, S.A.; Martin, C.S.; da Silva, R.R.; Constantino, C.J.L.; Alessio, P.; Oliveira, O.N.; Mejía-Salazar, J.R. Surface plasmon resonances in silver nanostars. Sensors 2018, 18, 3821. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Baskaran, R.; Yang, S.G.; Huh, Y.S.; Han, Y.K. Multifunctional spiky branched gold-silver nanostars with near-infrared and short-wavelength infrared localized surface plasmon resonances. J. Colloid Interface Sci. 2019, 542, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Muhammed Ajmal, C.; Baik, S.; Kim, J. Silver nanoflowers for single-particle SERS with 10 pM sensitivity. Nanotechnology 2017, 28, 465705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Xue, B.; Peng, Z.; Cao, Z.; Mu, Q.; Xuan, L. Highly effective and chemically stable surface enhanced Raman scattering substrates with flower-like 3D Ag-Au hetero-nanostructures. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitjar, J.; Liao, J.-D.; Lee, H.; Pan, L.P.; Liu, B.H.; Fu, W.-e.; Chen, G.D. Ag Nanostructures with Spikes on Adhesive Tape as a Flexible Sers-Active Substrate for In Situ Trace Detection of Pesticides on Fruit Skin. Nanomaterials 2019, 9, 1750. https://doi.org/10.3390/nano9121750
Sitjar J, Liao J-D, Lee H, Pan LP, Liu BH, Fu W-e, Chen GD. Ag Nanostructures with Spikes on Adhesive Tape as a Flexible Sers-Active Substrate for In Situ Trace Detection of Pesticides on Fruit Skin. Nanomaterials. 2019; 9(12):1750. https://doi.org/10.3390/nano9121750
Chicago/Turabian StyleSitjar, Jaya, Jiunn-Der Liao, Han Lee, Li Peng Pan, Bernard Haochih Liu, Wei-en Fu, and Guo Dung Chen. 2019. "Ag Nanostructures with Spikes on Adhesive Tape as a Flexible Sers-Active Substrate for In Situ Trace Detection of Pesticides on Fruit Skin" Nanomaterials 9, no. 12: 1750. https://doi.org/10.3390/nano9121750
APA StyleSitjar, J., Liao, J.-D., Lee, H., Pan, L. P., Liu, B. H., Fu, W.-e., & Chen, G. D. (2019). Ag Nanostructures with Spikes on Adhesive Tape as a Flexible Sers-Active Substrate for In Situ Trace Detection of Pesticides on Fruit Skin. Nanomaterials, 9(12), 1750. https://doi.org/10.3390/nano9121750






