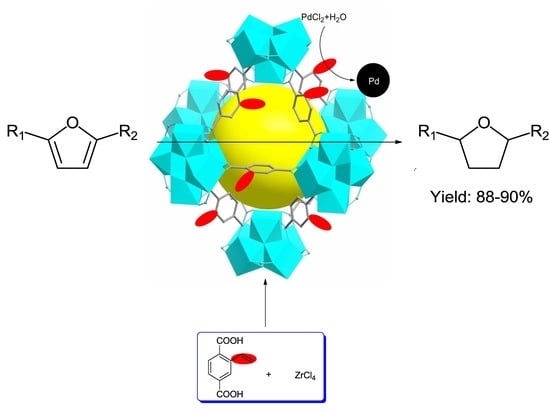
Facile Preparation of Pd/UiO-66-v for the Conversion of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol under Mild Conditions in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Characterization of Catalysts
2.4. Catalytic Reactions
2.5. Product Analysis
3. Results and Discussion
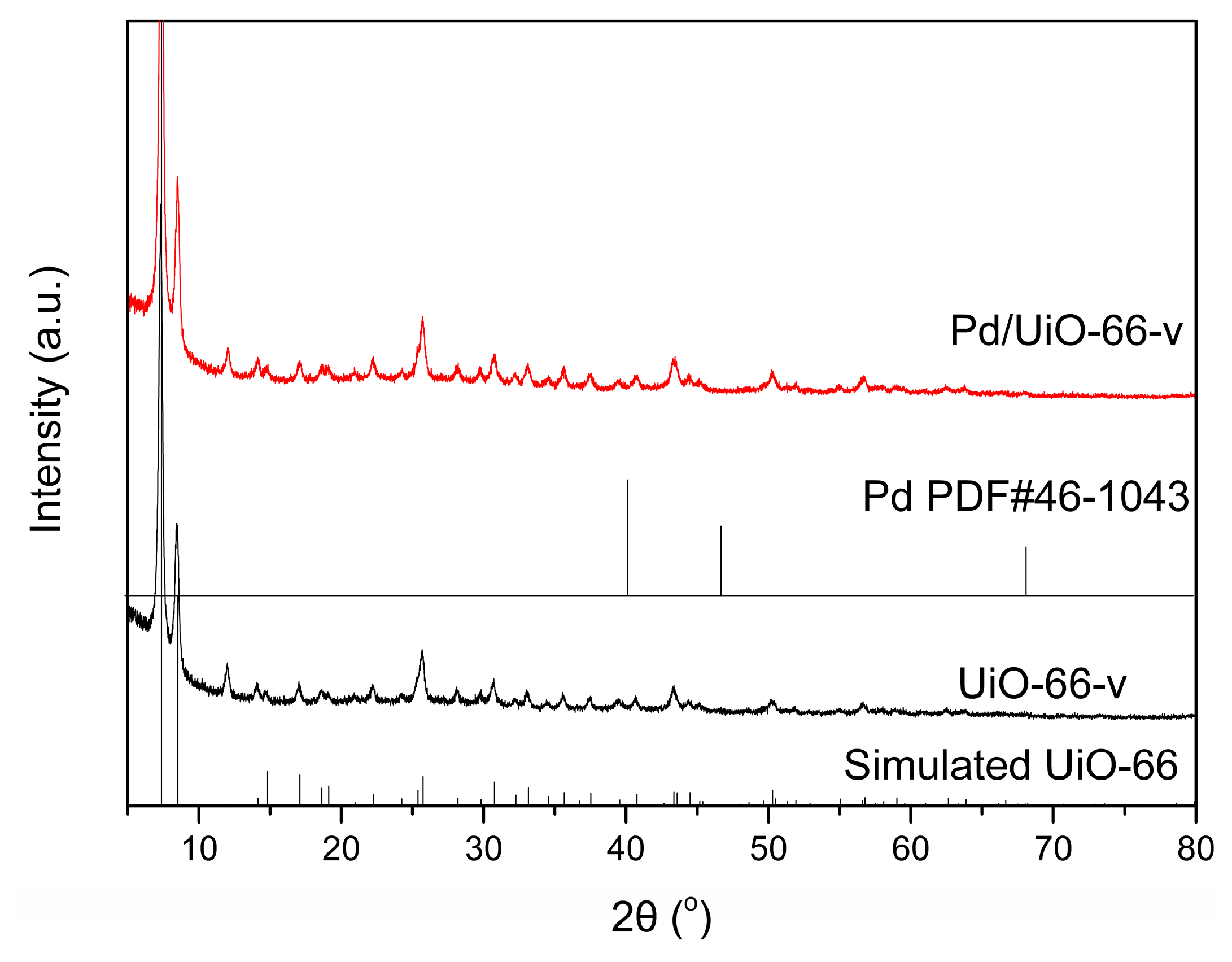
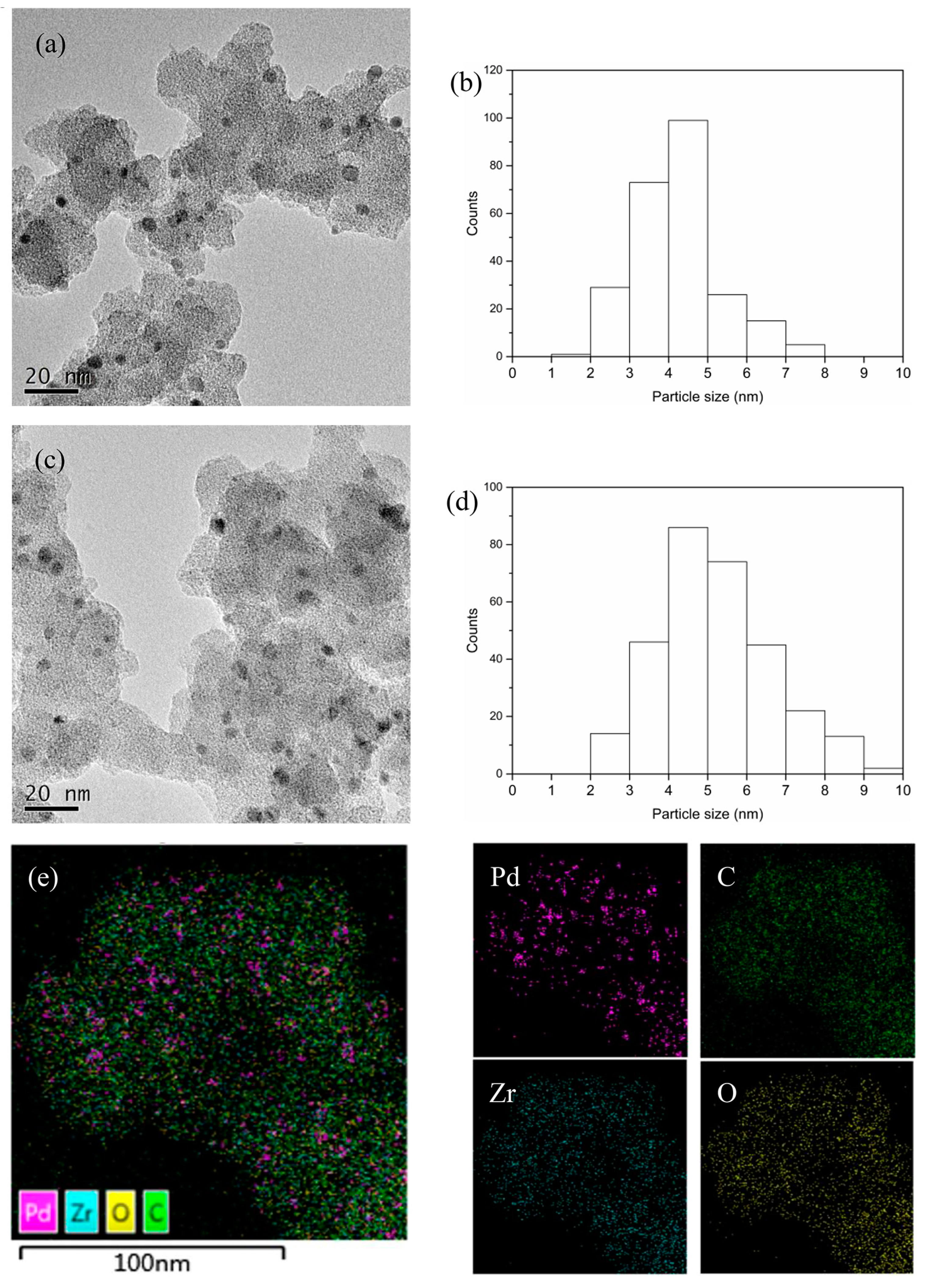
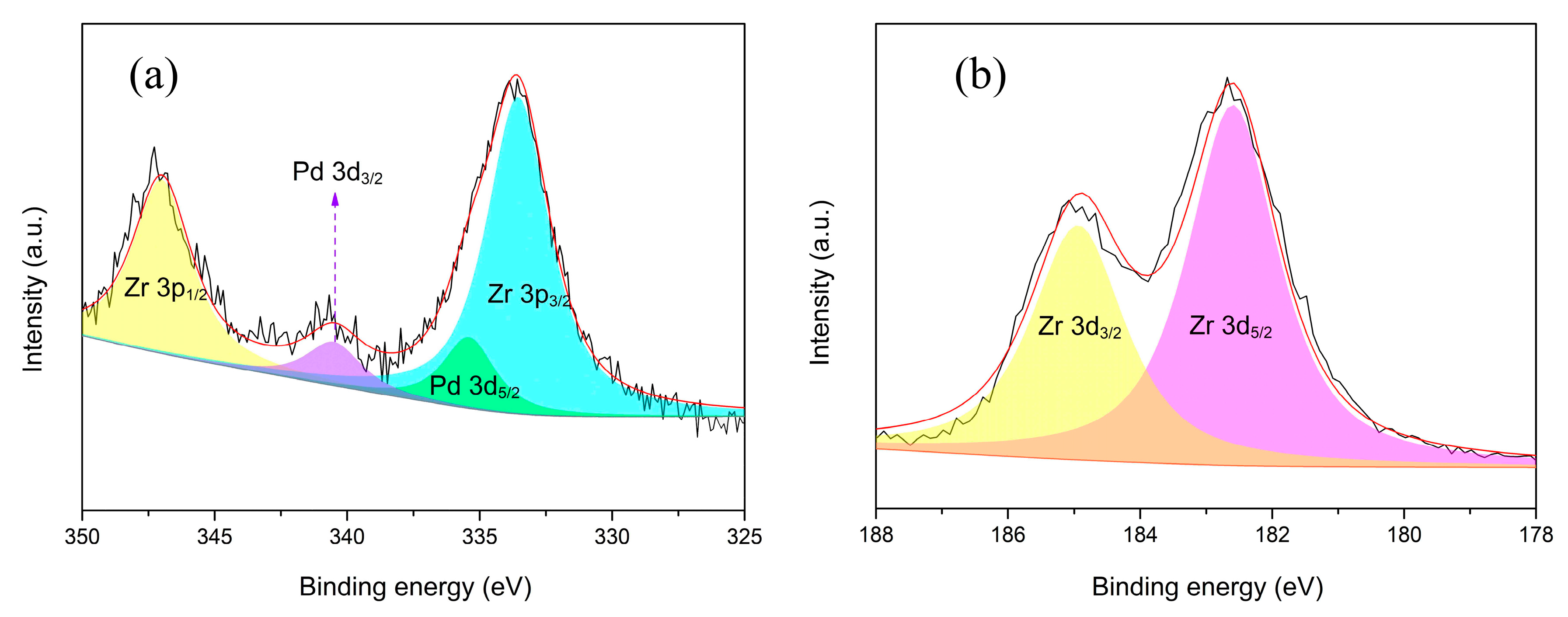
3.1. Catalyst Characterization
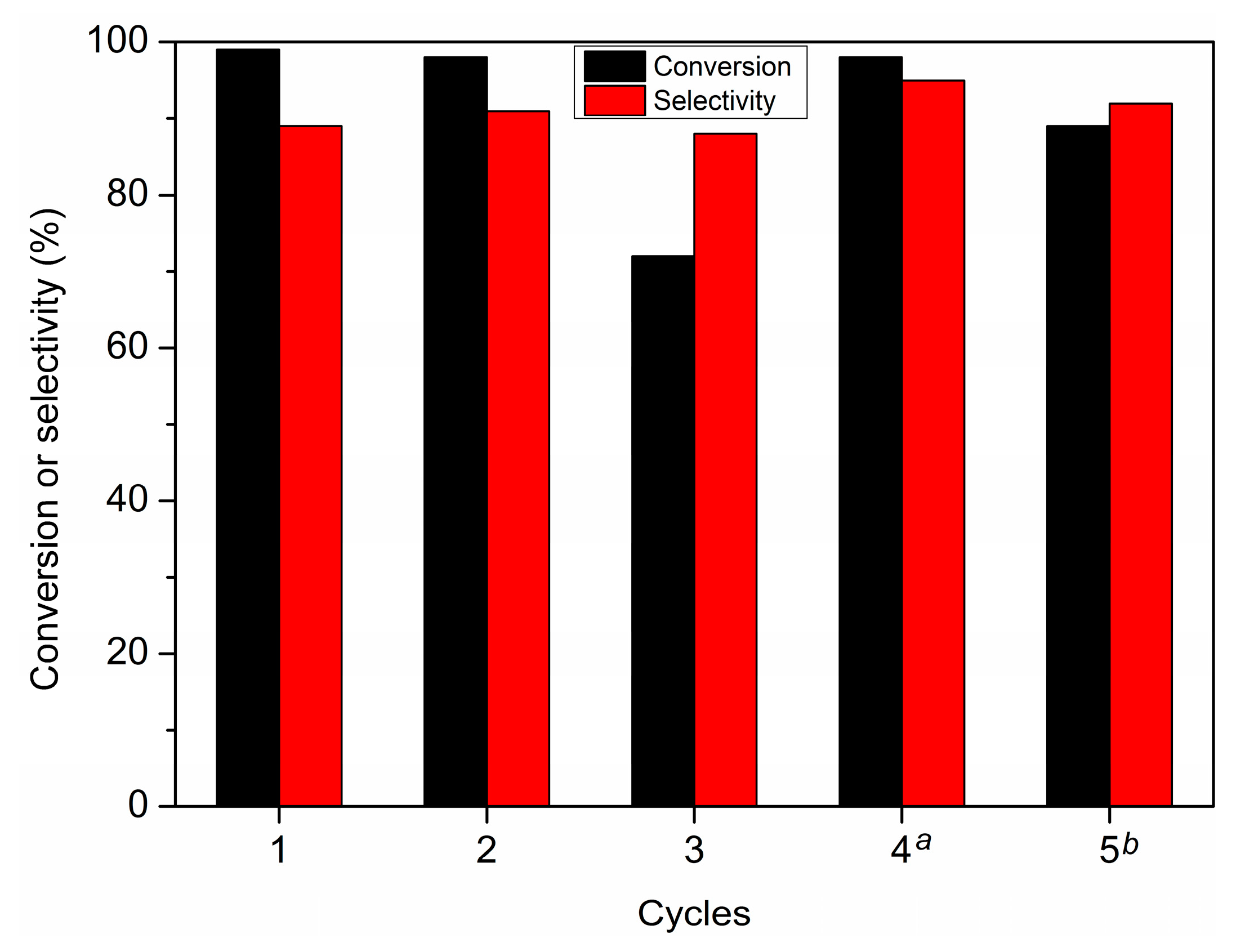
3.2. Hydrogenation of Furfuryl Alcohol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Win, D.T. Furfural-gold from garbage. AU J. Technol. 2005, 8, 185–190. [Google Scholar]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy. Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent advances in catalytic hydrogenation of furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal catalysts for the efficient transformation of biomass-derived HMF and furfural to value added chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using Co-Ni catalyst in water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef]
- Zhang, G.-S.; Zhu, M.-M.; Zhang, Q.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Li, X.-L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.-G.; Xu, H.-J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu–Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Ruan, L.; Zhang, H.; Zhou, M.; Zhu, L.; Pei, A.; Wang, J.; Yang, K.; Zhang, C.; Xiao, S.; Chen, B.H. A highly selective and efficient Pd/Ni/Ni(OH)2/C catalyst for furfural hydrogenation at low temperatures. Mol. Catal. 2020, 480, 110639. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; Haandel, L.V.; Ye, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Nanao, H.; Murakami, Y.; Sato, O.; Yamaguchi, A.; Hiyoshi, N.; Shirai, M. Furfuryl alcohol and furfural hydrogenation over activated carbon–supported palladium catalyst in presence of water and carbon dioxide. ChemistrySelect 2017, 2, 2471–2475. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Gao, L.; Wu, Y.; Yang, X.; Sheng, P.; Xiao, G. Short channeled Ni-Co/SBA-15 catalysts for highly selective hydrogenation of biomass-derived furfural to tetrahydrofurfuryl alcohol. Microporous Mesoporous Mater. 2018, 262, 154–165. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Takada, K.; Tamura, M.; Tomishige, K. Total hydrogenation of furfural and 5-hydroxymethylfurfural over supported Pd–Ir alloy catalyst. ACS Catal. 2014, 4, 2718–2726. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total hydrogenation of furfural over a silica-supported nickel catalyst prepared by the reduction of a nickel nitrate precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Huang, R.; Cui, Q.; Yuan, Q.; Wu, H.; Guan, Y.; Wu, P. Total hydrogenation of furfural over Pd/Al2O3 and Ru/ZrO2 mixture under mild conditions: Essential role of tetrahydrofurfural as an intermediate and support effect. ACS Sustain. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Yin, D.; Ren, H.; Li, C.; Liu, J.; Liang, C. Highly selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over MIL-101(Cr)-NH2 supported Pd catalyst at low temperature. Chin. J. Catal. 2018, 39, 319–326. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Zhu, W.; Xiao, G. Selective hydrogenation of furfuryl alcohol to tetrahydrofurfuryl alcohol over Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2016, 43, 1179–1195. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic CuNi/CNTs catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of biomass-derived furfural to tetrahydrofurfuryl alcohol over hydroxyapatite-supported Pd catalyst under mild conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Lan, X.; Wang, T. Supported ultrafine NiCo bimetallic alloy nanoparticles derived from bimetal–organic frameworks: A highly active catalyst for furfuryl alcohol hydrogenation. ACS Catal. 2018, 2121–2128. [Google Scholar] [CrossRef]
- Parikh, J.; Srivastava, S.; Jadeja, G.C. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol using supported nickel–cobalt catalysts. Ind. Eng. Chem. Res. 2019, 58, 16138–16152. [Google Scholar] [CrossRef]
- Albilali, R.; Douthwaite, M.; He, Q.; Taylor, S.H. The selective hydrogenation of furfural over supported palladium nanoparticle catalysts prepared by sol-immobilisation: Effect of catalyst support and reaction conditions. Catal. Sci. Technol. 2018, 8, 252–267. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Jia, X.; Du, Z.; Duan, Y.; Xu, J. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol on alkaline earth metal modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Liu, K.; Zhang, Q.; Chen, K.-J. Biowaste-derived bimetallic Ru–MoOx catalyst for the direct hydrogenation of furfural to tetrahydrofurfuryl alcohol. ACS Sustain. Chem. Eng. 2019, 7, 12858–12866. [Google Scholar] [CrossRef]
- Pendem, S.; Bolla, S.R.; Morgan, D.J.; Shinde, D.B.; Lai, Z.; Nakka, L.; Mondal, J. Metal-organic-framework derived Co-Pd bond is preferred over Fe-Pd for reductive upgrading of furfural to tetrahydrofurfuryl alcohol. Dalton Trans. 2019, 48, 8791–8802. [Google Scholar] [CrossRef]
- Ma, R.; Wu, X.-P.; Tong, T.; Shao, Z.-J.; Wang, Y.; Liu, X.; Xia, Q.; Gong, X.-Q. The critical role of water in the ring opening of furfural alcohol to 1,2-pentanediol. ACS Catal. 2016, 7, 333–337. [Google Scholar] [CrossRef]
- Gao, F.; Liu, H.; Hu, X.; Chen, J.; Huang, Z.; Xia, C. Selective hydrogenolysis of furfuryl alcohol to 1,5- and 1,2-pentanediol over Cu-LaCoO3 catalysts with balanced Cu0-CoO sites. Chin. J. Catal. 2018, 39, 1711–1723. [Google Scholar] [CrossRef]
- Tong, T.; Liu, X.; Guo, Y.; Norouzi Banis, M.; Hu, Y.; Wang, Y. The critical role of CeO2 crystal-plane in controlling Pt chemical states on the hydrogenolysis of furfuryl alcohol to 1,2-pentanediol. J. Catal. 2018, 365, 420–428. [Google Scholar] [CrossRef]
- Date, N.S.; Chikate, R.C.; Roh, H.-S.; Rode, C.V. Bifunctional role of Pd/MMT-K 10 catalyst in direct transformation of furfural to 1,2-pentanediol. Catal. Today 2018, 309, 195–201. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase. Green Chem. 2012, 14, 3402. [Google Scholar] [CrossRef]
- Sitthisa, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar] [CrossRef]
- Wang, C.T.; Liu, Z.Q.; Wang, L.; Dong, X.; Zhang, J.; Wang, G.X.; Han, S.C.; Meng, X.J.; Zheng, A.M.; Xiao, F.S. Importance of zeolite wettability for selective hydrogenation of furfural over Pd@Zeolite catalysts. ACS Catal. 2018, 8, 474–481. [Google Scholar] [CrossRef]
- Iqbal, S.; Liu, X.; Aldosari, O.F.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Akram, A.; King, G.M.; Davies, T.E.; Morgan, D.J.; et al. Conversion of furfuryl alcohol into 2-methylfuran at room temperature using Pd/TiO2 catalyst. Catal. Sci. Technol. 2014, 4, 2280–2286. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, S.X.; Feng, Y.Q.; Zhang, B.; Xu, Z. A continuous process for the preparation of pyridine from tetrahydrofurfuryl alcohol with (MoO3-NiO)/Al2O3 as Catalyst. Adv. Mater. Res. 2012, 581–582, 27–32. [Google Scholar]
- Li, L.; Barnett, K.J.; McClelland, D.J.; Zhao, D.; Liu, G.; Huber, G.W. Gas-phase dehydration of tetrahydrofurfuryl alcohol to dihydropyran over γ-Al2O3. Appl. Catal. B Environ. 2019, 245, 62–70. [Google Scholar] [CrossRef]
- Soghrati, E.; Choong, C.; Poh, C.K.; Kawi, S.; Borgna, A. Single-pot conversion of tetrahydrofurfuryl alcohol into tetrahydropyran over a Ni/HZSM-5 catalyst under aqueous-phase conditions. ChemCatChem 2017, 9, 1402–1408. [Google Scholar] [CrossRef]
- Brentzel, Z.J.; Barnett, K.J.; Huang, K.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Chemicals from biomass: Combining ring-opening tautomerization and hydrogenation reactions to produce 1,5-pentanediol from furfural. ChemSusChem 2017, 10, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lee, J.D.; Ji, Y.; Onn, T.M.; Luo, J.; Murray, C.B.; Gorte, R.J. A study of tetrahydrofurfuryl alcohol to 1,5-pentanediol over Pt–WOx/C. Catal. Lett. 2018, 148, 1047–1054. [Google Scholar] [CrossRef]
- Soghrati, E.; Kok Poh, C.; Du, Y.; Gao, F.; Kawi, S.; Borgna, A. C−O hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol over bi-functional nickel-tungsten catalysts. ChemCatChem 2018, 10, 4652–4664. [Google Scholar] [CrossRef]
- Liu, S.; Okuyama, Y.; Tamura, M.; Nakagawa, Y.; Imai, A.; Tomishige, K. Production of renewable hexanols from mechanocatalytically depolymerized cellulose by using Ir-ReOx/SiO2 catalyst. ChemSusChem 2015, 8, 628–635. [Google Scholar] [CrossRef]
- Huang, K.; Brentzel, Z.J.; Barnett, K.J.; Dumesic, J.A.; Huber, G.W.; Maravelias, C.T. Conversion of furfural to 1,5-pentanediol: Process synthesis and analysis. ACS Sustain. Chem. Eng. 2017, 5, 4699–4706. [Google Scholar] [CrossRef]
- Yuan, Q.; Ye, F.; Xue, T.; Guan, Y. Room temperature hydrogenation of furfuryl alcohol by Pd/titanate nanotube. Appl. Catal. A Gen. 2015, 507, 26–33. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef]
- Li, G.; Zhao, S.; Zhang, Y.; Tang, Z. Metal-organic frameworks encapsulating active nanoparticles as emerging composites for catalysis: Recent progress and perspectives. Adv. Mater. 2018, 30, 1800702. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the art and prospects in metal—Organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zheng, M.; Li, D.; Deng, D.; Wu, C.; Yang, Y. Synthesis of Pd/SBA-15 catalyst employing surface-bonded vinyl as a reductant and its application in the hydrogenation of nitroarenes. RSC Adv. 2017, 7, 3443–3449. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, M.; Li, D.; Deng, D.; Ma, L.-F.; Yang, Y. Conversion of HMF to methyl cyclopentenolone by the Pd/Nb2O5 and Ca-Al catalysts via two-steps procedure. Green Chem. 2017, 19, 5103–5113. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Deng, D.; Li, D.; Zheng, M.; Duan, Y. Highly selective conversion of HMF to 1-hydroxy- 2,5-hexanedione on Pd/MIL-101(Cr). ChemistrySelect 2019, 4, 11165–11171. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Zhang, J.; Li, D.; Deng, D.; Duan, Y. Fabrication of Pd/SiO2 with controllable wettability for enhanced catalytic hydrogenation activity at ambient H2 pressure. ChemCatChem 2019, 11, 5430–5434. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colon, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Shen, L.; Wu, W.; Liang, R.; Lin, R.; Wu, L. Highly dispersed palladium nanoparticles anchored on UiO-66(NH2) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale 2013, 5, 9374. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, B.; Chai, X.; Liu, J.; Gu, J.; Ning, P. Comparison of Pd-UiO-66 and Pd-UiO-66-NH2 catalysts performance for phenol hydrogenation in aqueous medium. Fuel 2017, 205, 130–141. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and Auger parameter of Pd and PdO. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
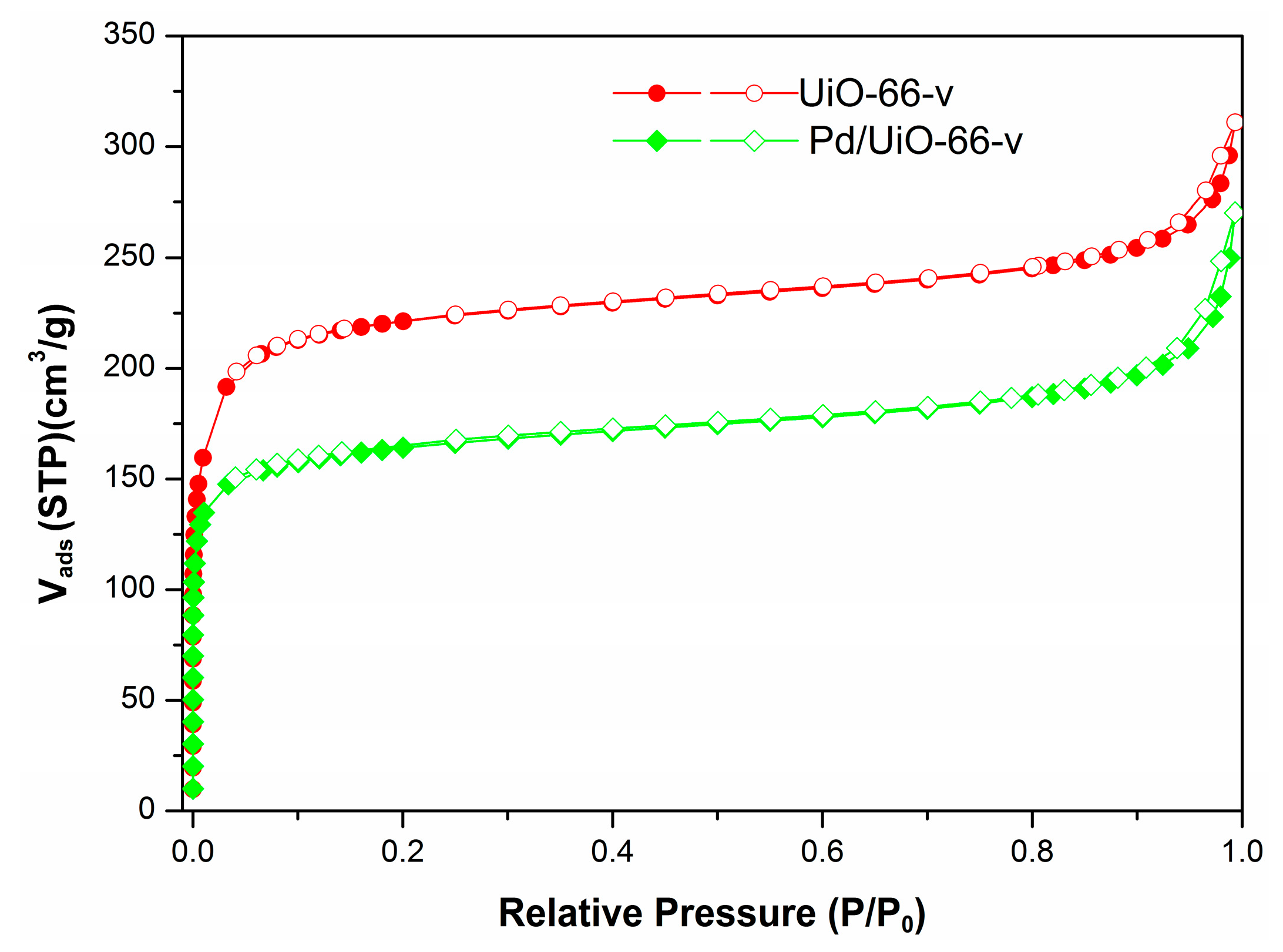
| Entry | Sample | SBET (m2·g−1) | St-plot (m2·g−1) | PV 1 (cm3·g−1) | PV 2 (cm3·g−1) | Pd Content 3 (wt.%) |
|---|---|---|---|---|---|---|
| 1 | UiO-66-v | 862 | 726 | 0.42 | 0.28 | - |
| 2 | Pd/UiO-66-v | 630 | 514 | 0.41 | 0.20 | 2.42 (2.35) 4 |
| Element | Pd 3d5/2 | Pd 3d3/2 | Zr 3p3/2 | Zr 3p1/2 | Zr 3d5/2 | Zr 3d3/2 |
|---|---|---|---|---|---|---|
| B.E. (eV) | 335.4 | 340.6 | 333.5 | 347.0 | 182.6 | 185.0 |
| Entry | Catalysts | Solvent | P (MPa) | Time (h) | Conversion (%) | Selectivity (%) | Carbon Balance |
|---|---|---|---|---|---|---|---|
| 1 | None | H2O | 4 | 2 | <1 | n.d. | >99 |
| 2 | UiO-66-v | H2O | 4 | 2 | 2 | n.d. | 98 |
| 3 | Pd/UiO-66-v | H2O | 0.5 | 2 | 28 | 92 | 98 |
| 4 | Pd/UiO-66-v | H2O | 1 | 2 | 52 | 91 | 95 |
| 5 | Pd/UiO-66-v | H2O | 2 | 2 | 79 | 90 | 92 |
| 6 | Pd/UiO-66-v | H2O | 3 | 2 | 88 | 90 | 91 |
| 7 | Pd/UiO-66-v | H2O | 4 | 2 | 92 | 91 | 92 |
| 8 | Pd/UiO-66-v | H2O | 0.5 | 12 | 99 | 90 | 90 |
| 9 | Pd/UiO-66-v | methanol | 4 | 2 | 14 | 94 | 99 |
| 10 | Pd/UiO-66-v | ethanol | 4 | 2 | 38 | 93 | 97 |
| 11 | Pd/UiO-66-v | isopropanol | 4 | 2 | 2 | 96 | >99 |
| Entry | C (mol·L−1) | Time (h) | TOF (h−1) | Conversion (%) | Selectivity (%) | Carbon Balance |
|---|---|---|---|---|---|---|
| 1 | 0.5 | 2 | 202 | 92 | 91 | 92 |
| 2 | 1.0 | 2 | 286 | 65 | 90 | 94 |
| 3 | 1.0 | 4 | 211 | 96 | 86 | 87 |
| 4 | 1.5 | 2 | 257 | 39 | 88 | 95 |
| 5 | 1.5 | 6 | 193 | 88 | 85 | 87 |
| 6 | 2.0 | 2 | 255 | 29 | 89 | 97 |
| 7 | 2.0 | 8 | 161 | 73 | 83 | 88 |
| 8 | 2.5 | 2 | 187 | 17 | 91 | 98 |
| 9 | 2.5 | 10 | 128 | 58 | 83 | 90 |
| Entry | Substrate | Solvent | Conversion (%) 2 | Selectivity (%) 2 | Carbon Balance | |
|---|---|---|---|---|---|---|
| 1 | 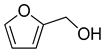 | H2O | 99 | 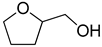 | 89 | 89 |
| 2 | 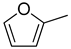 | ethanol | 99 | 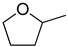 | 90 | 90 |
| 3 | 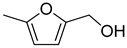 | ethanol | 44 (97) | 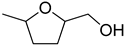 | 93 (91) | 97 (91) |
| 4 | 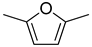 | ethanol | 11 (98) | 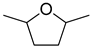 | 90 (90) | 99 (90) |
| 5 | 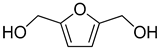 | H2O | 18 (99) | 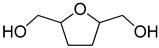 | 95 (92) | 99 (92) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Deng, D.; Sui, D.; Xie, Y.; Li, D.; Duan, Y. Facile Preparation of Pd/UiO-66-v for the Conversion of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol under Mild Conditions in Water. Nanomaterials 2019, 9, 1698. https://doi.org/10.3390/nano9121698
Yang Y, Deng D, Sui D, Xie Y, Li D, Duan Y. Facile Preparation of Pd/UiO-66-v for the Conversion of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol under Mild Conditions in Water. Nanomaterials. 2019; 9(12):1698. https://doi.org/10.3390/nano9121698
Chicago/Turabian StyleYang, Yanliang, Dongsheng Deng, Dong Sui, Yanfu Xie, Dongmi Li, and Ying Duan. 2019. "Facile Preparation of Pd/UiO-66-v for the Conversion of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol under Mild Conditions in Water" Nanomaterials 9, no. 12: 1698. https://doi.org/10.3390/nano9121698
APA StyleYang, Y., Deng, D., Sui, D., Xie, Y., Li, D., & Duan, Y. (2019). Facile Preparation of Pd/UiO-66-v for the Conversion of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol under Mild Conditions in Water. Nanomaterials, 9(12), 1698. https://doi.org/10.3390/nano9121698