Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
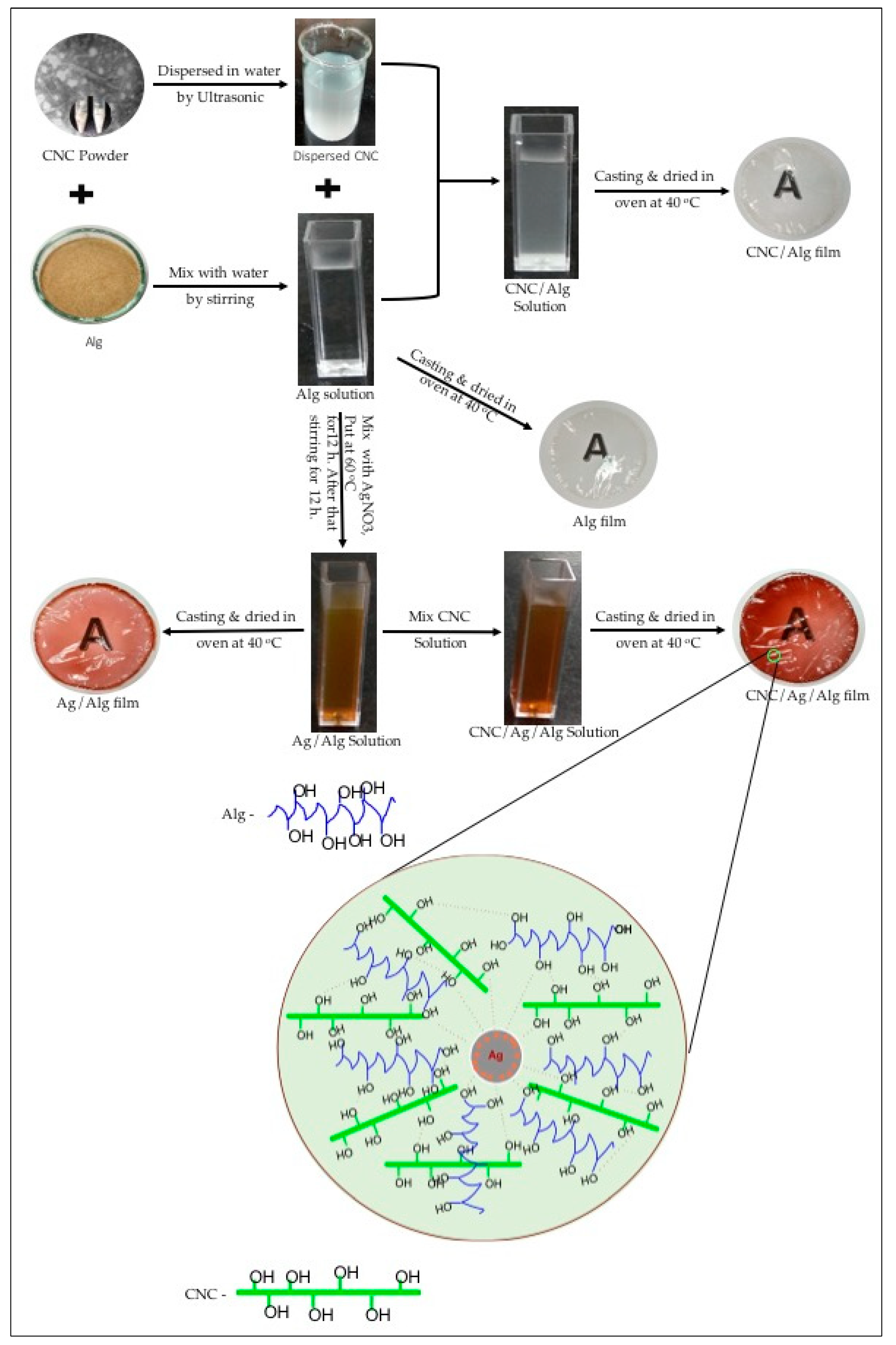
2.2. Fabrication of CNC, CNC/Alg, Ag/Alg, and CNC/Ag/Alg Samples
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Field Emission Scanning Electron Microscopy (FESEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. Optical Microscopy (OM)
2.7. X-ray Diffraction (XRD)
2.8. Thermalgravimetric Analysis (TGA)
2.9. Opacity and UV Visibility
2.10. Film Water Solubility (FWS)
2.11. Moisture Absorption
2.12. Water Vapor Permeation (WVP)
2.13. Color
2.14. Tensile Properties
2.15. Biodegradability
3. Results
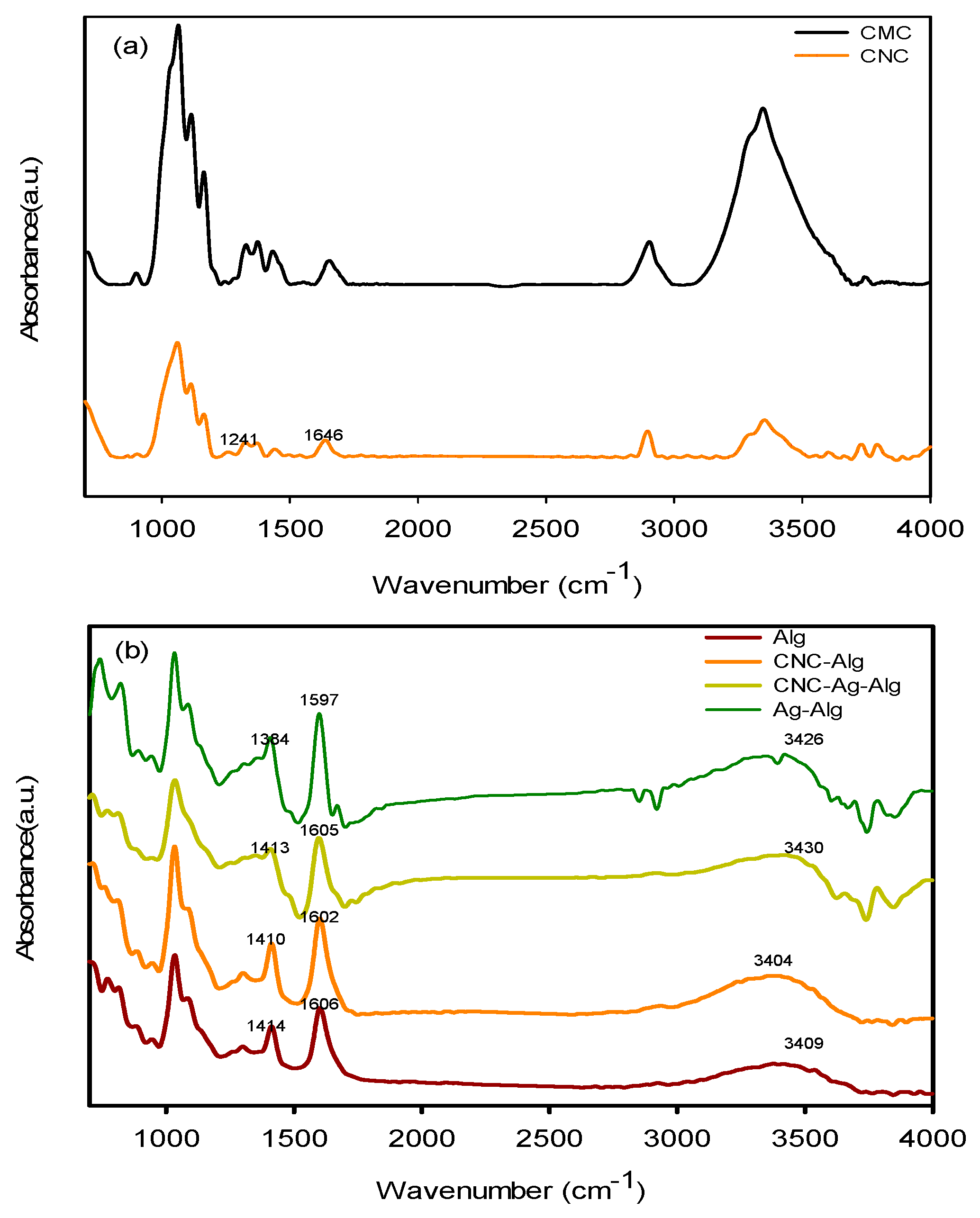
3.1. Fourier-Transform Infrared Spectroscopy
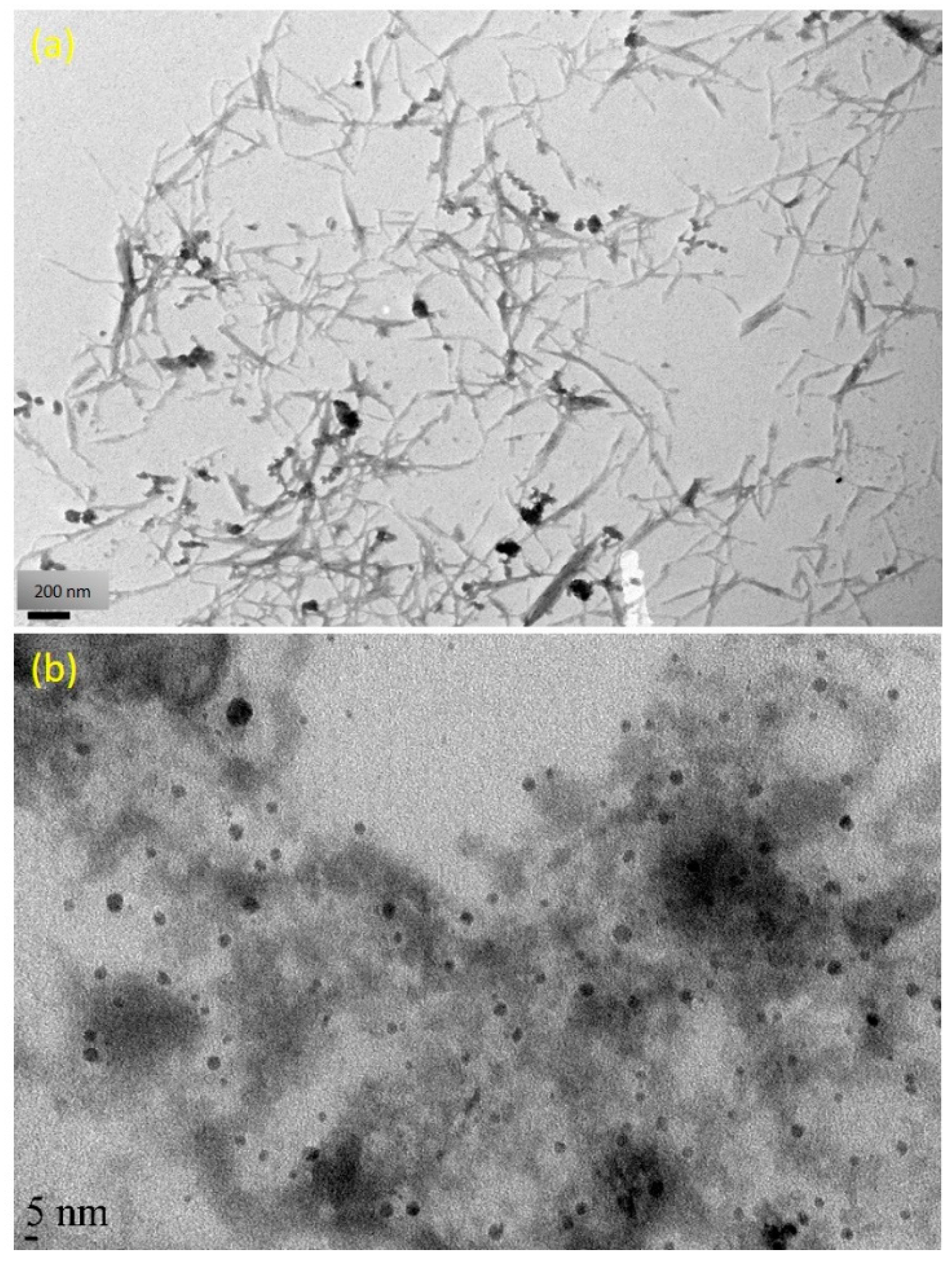
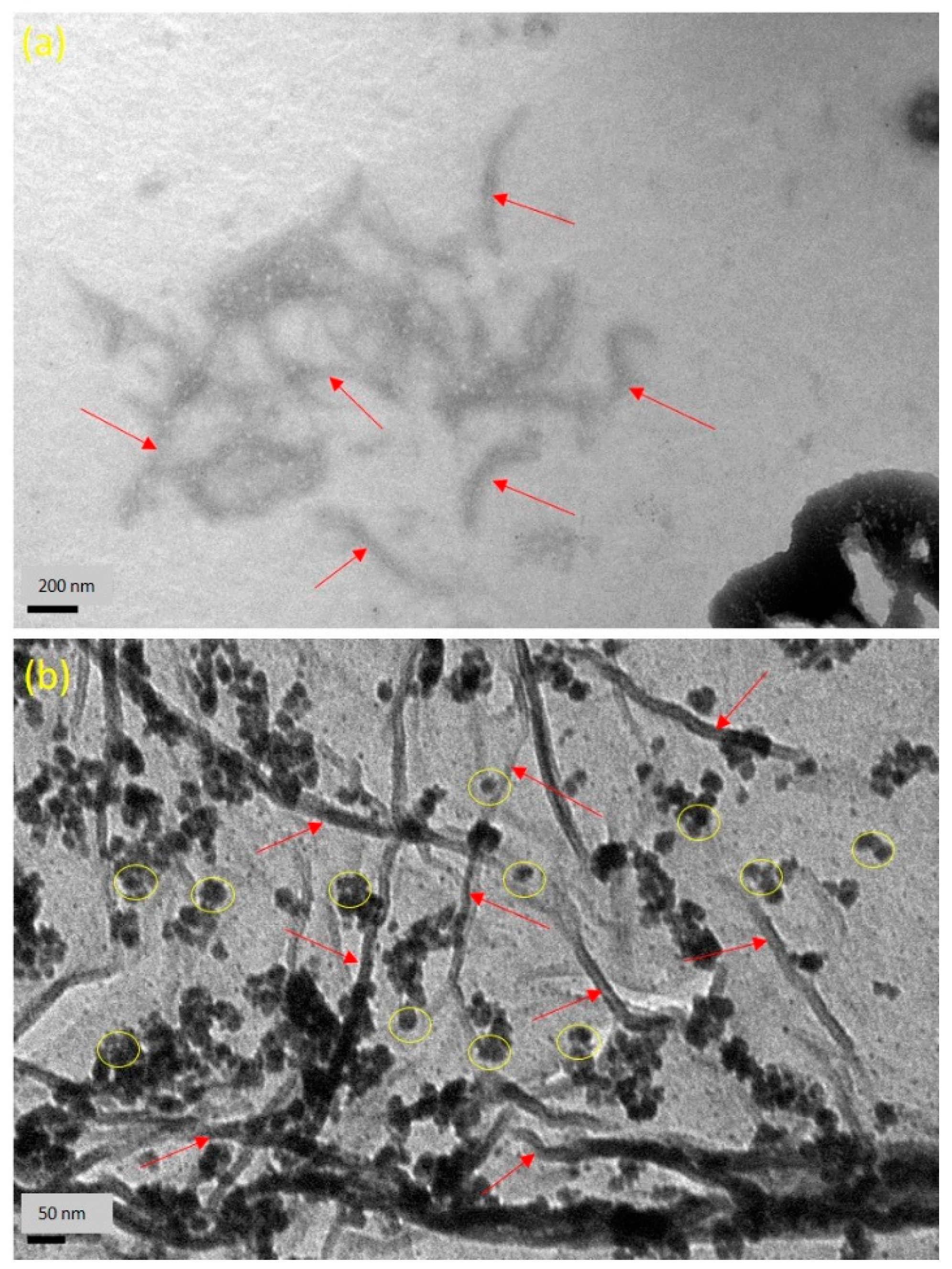
3.2. Morphology
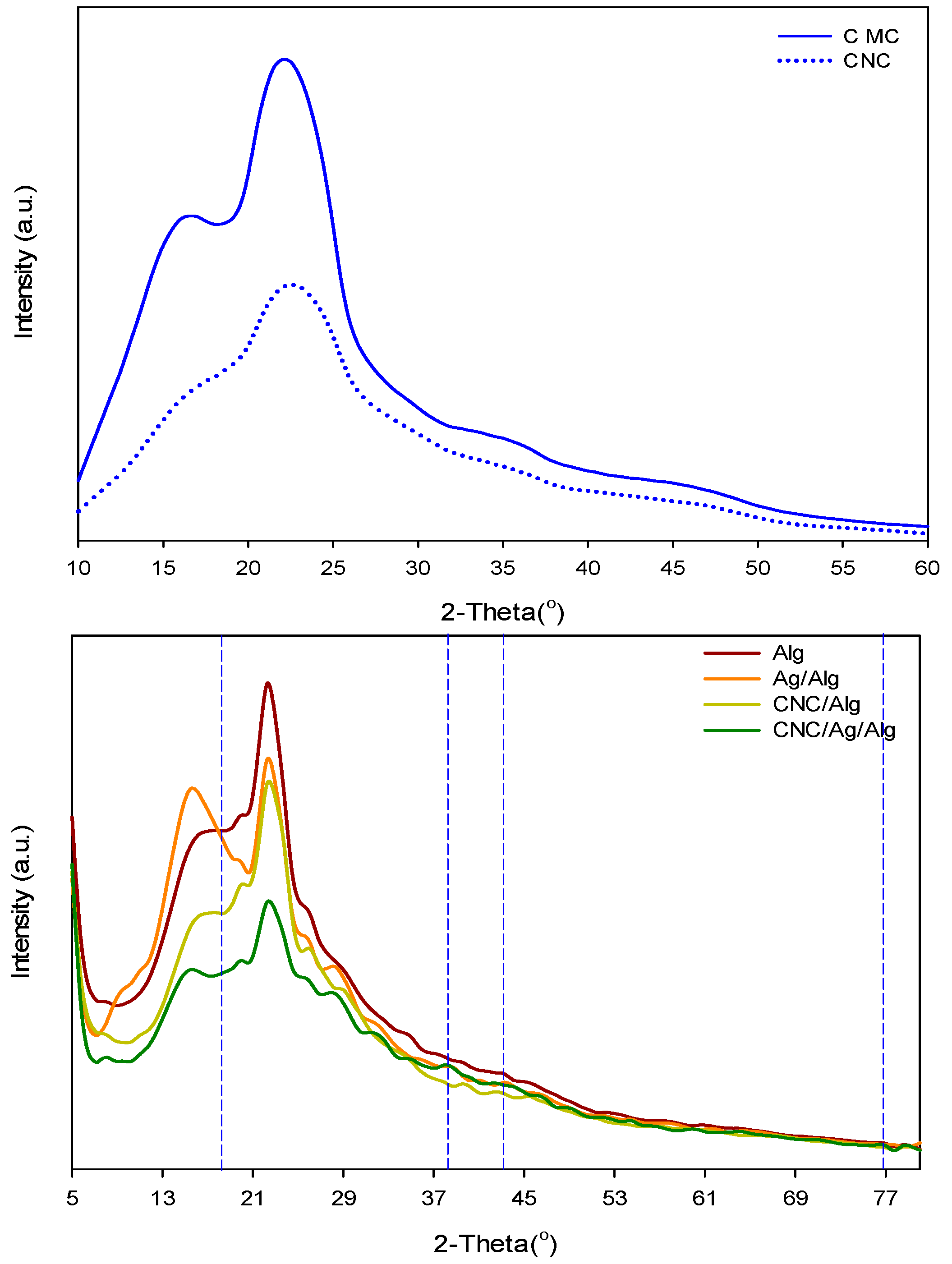
3.3. X-ray Diffraction (XRD)
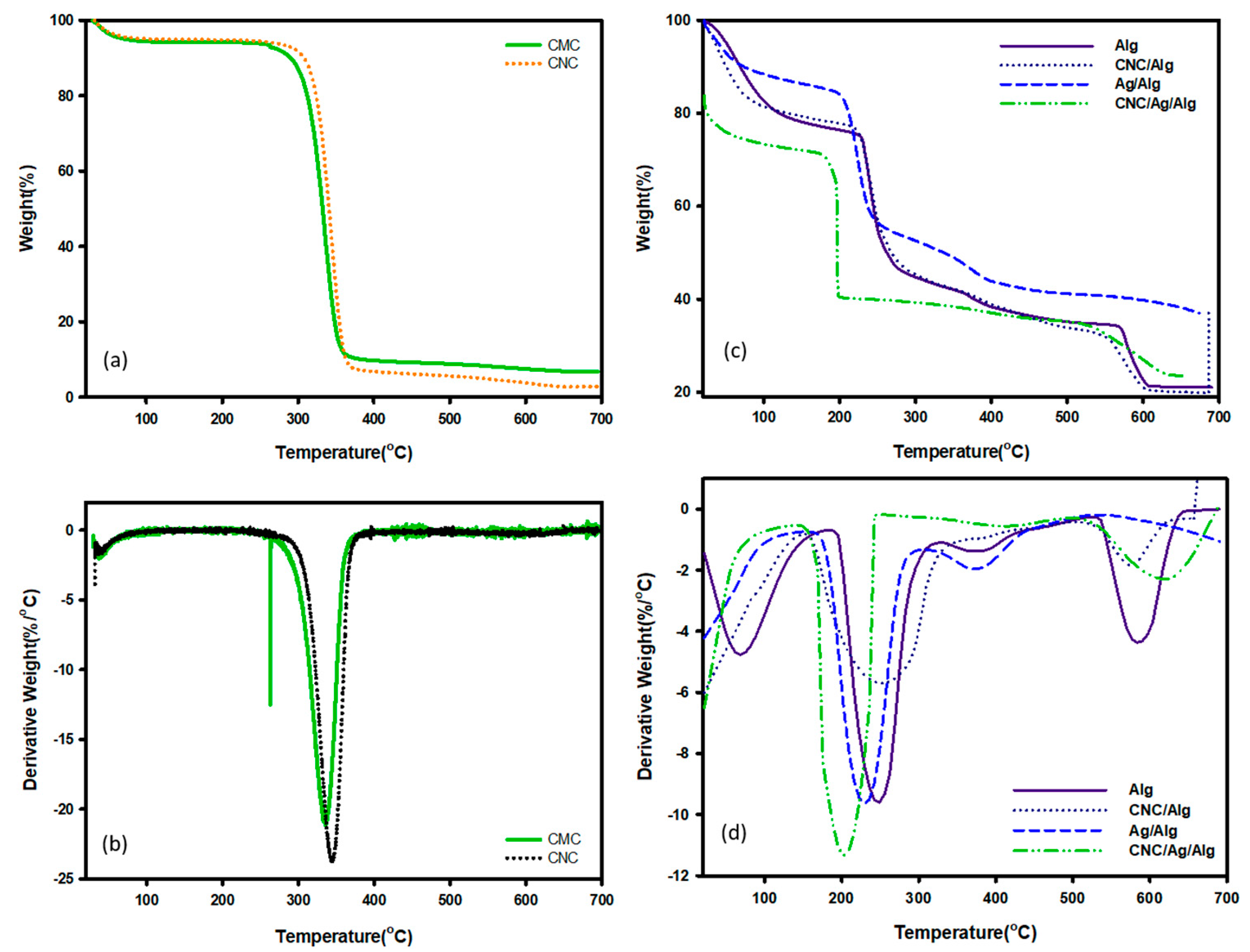
3.4. Thermal Properties
3.5. Water Barrier Properties of the Films
3.6. Thickness and Mechanical Properties of Films
3.7. Color Analysis
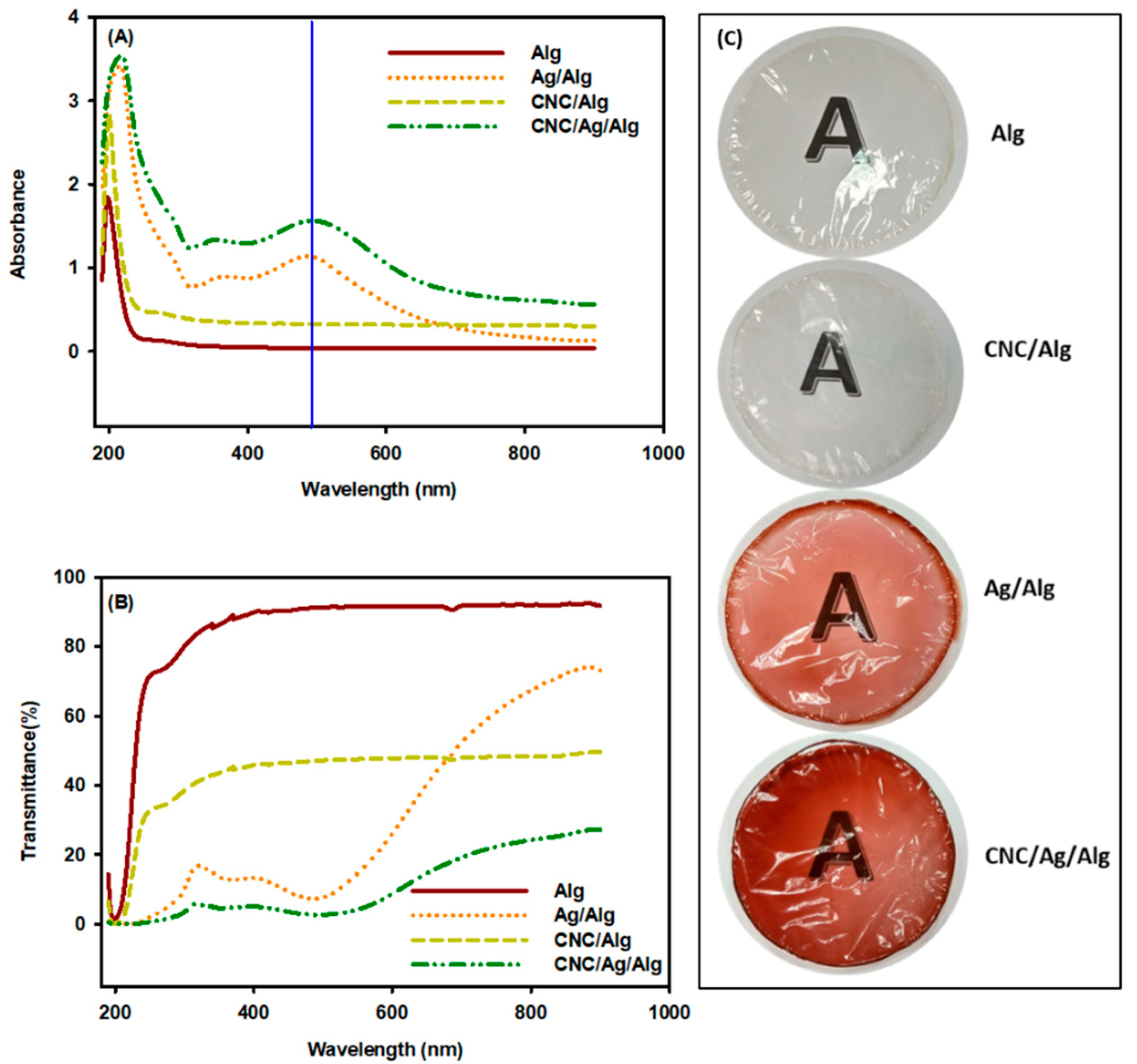
3.8. Opacity and UV Visibility of Films
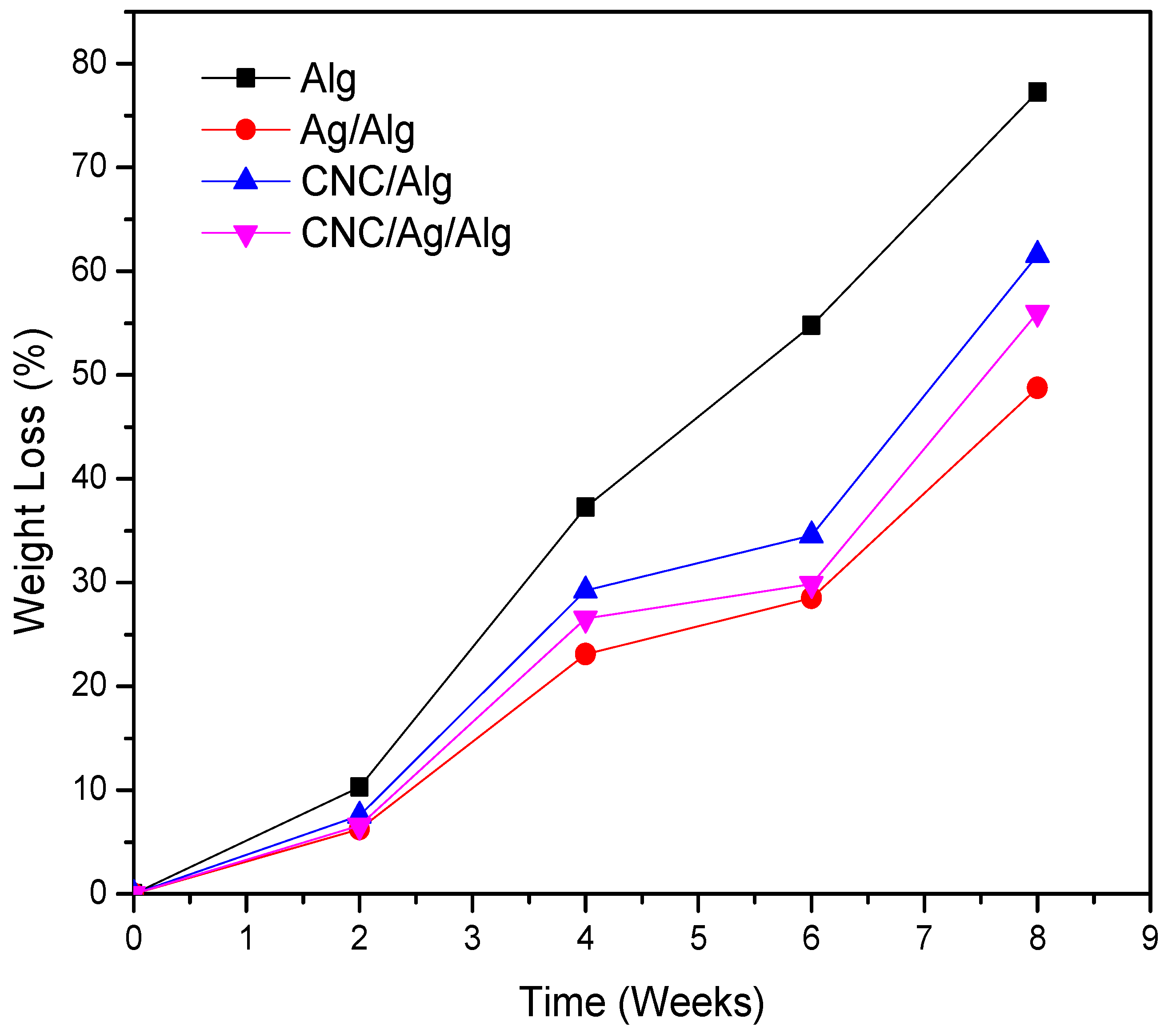
3.9. Biodegradation of Films in Soil
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Senan, C.; Wacławek, S.; Cerník, M.; Agarwal, S.; Varma, R.S. Bioplastic fibers from gum arabic for greener food wrapping applications. ACS Sustain. Chem. Eng. 2019, 7, 5900–5911. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.H.; Chiu, F.C.; Yang, J.C. Characterization of poly (lactic acid) s with reduced molecular weight fabricated through an autoclave process. Polym. Test. 2017, 60, 132–139. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Lia, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocoll. 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Ghosh, M.; Sternfeld, M.H.; Grinberg, I.; Abramovich, L.A. Injectable alginate-peptide composite hydrogel as a scaffold for bone tissue regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 4, 10887–10893. [Google Scholar] [CrossRef]
- Draget, K.I.; Braek, G.S.; Smidsrod, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Effect of alginate and -carrageenan on tensile properties and water vapor permeability of sodium caseinate-lipid based films. Carbohydr. Polym. 2008, 74, 419–426. [Google Scholar] [CrossRef]
- Shankar, S.; Kasapis, S.; Rhim, J.W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F. Preparation and characterization of carrageenan-based nanocomposite films reinforced with clay mineral and silver nanoparticles. Appl. Clay Sci. 2014, 97, 174–181. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.X.; He, L.; Huang, C.Z.; Li, Y.F. Green one-pot synthesis of silver nanoparticles/metal-organic gels hybrid and its promising SERS application. ACS Sustain. Chem. Eng. 2019, 7, 5292–5299. [Google Scholar] [CrossRef]
- Sharma, S.; Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Fabrication of antibacterial silver nanoparticle—Sodium alginate—Chitosan composite films. RSC Adv. 2012, 2, 5837–5843. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Gnoc, N.T.B.; Chin, K.B. Biosynthesis of silver nanoparticles from persimmon byproducts and incorporation in biodegradable sodium alginate thin film. J. Food Sci. 2017, 82, 2329–2336. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhong, L.; Wu, G. Preparation of high stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Han, S.S. Dual-crosslinked poly (vinyl alcohol)/sodium alginate/silver nanocomposite beads—A promising antimicrobial material. Food Chem. 2017, 234, 103–110. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf. B 2009, 69, 164–168. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Rubio, A.L.; Fabra, M.J.; Sanz, M.M. Food Packaging Based on Nanomaterials. Nanomaterials 2019, 9, 1224. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and photocatalytic antibacterial Au-TiO2/Alginate nanocomposite films for active food packaging. Nanomaterials 2018, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Romani, M.; Gigli, C.; Mannozzi Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Alkali Hydrolysis of Sulfated Cellulose Nanocrystals: Optimization of Reaction Conditions and Tailored Surface Charge. Nanomaterials 2019, 9, 1232. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Jeevananda, T. Synthesis and characterization of microcrystalline cellulose powder. Indian J. Eng. Mater. Sci. 1997, 4, 38–40. [Google Scholar]
- Thambiraj, S.; Shankaran, D.R. Preparation and physicochemical characterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Liu, H.; Liu, D.; Yao, F.; Wu, Q. Fabrication and properties of transparent polymethylmethacrylate/cellulose nanocrystals composite. Bioresour. Technol. 2010, 10, 56. [Google Scholar] [CrossRef]
- Rescignano, N.; Fortunati, E.; Montesano, S.; Emiliani, C.; Kenny, J.M.; Martino, S.; Armentano, I. PVA bio-nanocomposites: A new take-off using cellulose nanocrystals and PLGA nanoparticles. Carbohydr. Polym. 2014, 99, 47–58. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable Nanocomposite Films Based on Sodium Alginate and Cellulose Nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Kha, R.A.; Tien, C.L.; Riedl, B.; Fraschini, C.; Bouchard, J.; Calderon, J.U.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, S. Wound dressings based on chitosan-dialdehyde cellulose nanocrystals-silver nanoparticles: Mechanical strength. Polymers 2018, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yao, Y.; Wang, C. Green synthesis of silver nanoparticles impregnated bacterial cellulose-alginate composite film with improved properties. Mater. Lett. 2017, 209, 11–14. [Google Scholar] [CrossRef]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- ASHRAE. ASHRAE Handbook, Fundamentals; American Society of Heating and Refrigerating and Air conditioning, Engineers, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Pascalau, V.; Popescu, V.; Popescu, G.L.; Dudescu, M.C.; Borodi, G.; Dinescu, A.; Perhait, I.; Paul, M. The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloys Compd. 2012, 536, S418–S423. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Gum kondagogu (Cochlospermum gossypium): A template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr. Polym. 2010, 82, 670–679. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Kennedy, J.F.; Xie, B.J.; Huang, M. Characterization of konjac glucomannane gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohydr. Polym. 2007, 70, 192–197. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparations and characterization of alginate/silver composite films: Effect of types of silver particles. Carbohydr. Polym. 2016, 146, 208–216. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Li, X.; Xia, Y.; Wang, B.; Zhao, Z. Antibacterial activity and in vitro cytotoxicity evaluation of alginate-AgNP fibers. Text. Res. J. 2016, 87, 1377–1386. [Google Scholar] [CrossRef]
- Ni, X.; Wang, J.; Yue, Y.; Cheng, W.; Wang, D.; Han, G. Enhanced Antibacterial Performance and Cytocompatibility of Silver Nanoparticles Stabilized by Cellulose Nanocrystal Grafted with Chito-Oligosaccharides. Materials 2018, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.J.; Spinella, S.; Kyle, C.K.C.; Maiorana, A.; Qian, Q.; Hepworth, V.; Gross, R.A.; Singer, K.D. Optical interactions of silver nanoparticle decorated cellulose nanocrystals created from a one-pot reduction method. J. Appl. Phys. 2017, 121, 095502. [Google Scholar] [CrossRef]
- Khili, F.; Borges, J.; Almeida, P.L.; Boukherroub, R.; Omrani, A.D. Extraction of Cellulose Nanocrystals with Structure I and II and Their Applications for Reduction of Graphene Oxide and Nanocomposite Elaboration. Waste Biomass Valoriz. 2018, 10, 1913–1927. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 2007, 18, 285604. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Lopez, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, Y.; Chang, P.R.; Muir, A.D.; Falk, G. Starch-based nanocomposites reinforced with flax cellulose nanocrystals. Express Polym. Lett. 2008, 2, 502–510. [Google Scholar] [CrossRef]
- Otoni, C.G.; Bustillos, R.J.A.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating Thermal Stability of Experimental Polymers by Empirical Thermogravimetric Analysis. Anal. Chem. 1961, 33, 77. [Google Scholar] [CrossRef]
- Yadav, M.; Sand, A.; Behari, K. Synthesis and properties of a water soluble graft (chitosan-g-2-acrylamidoglycolic acid) copolymer. Int. J. Biol. Macromol. 2012, 50, 1306–1314. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; Filho, M.S.M.S.; Rosa, M.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 101, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Karim, M.R. Fabrication and characterization of poly (vinyl alcohol)/alginate blend nanofbers by electrospinning method. Colloids Surf. A 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibers. Polym. Degrad. Stab. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Paula, E.L.D.; Mano, V.; Pereira, F.V. Influence of cellulose nanowhiskers on the hydrolytic degradation behavior of poly (d,l-lactide). Polym. Degrad. Stab. 2011, 96, 1631–1638. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Noshirvani, N.; Hong, W.; Ghanbarzadeh, B.; Fasihi, H.; Montazami, R. Study of cellulose nanocrystal doped starch-polyvinyl alcohol bionanocomposite films. Int. J. Biol. Macromol. 2018, 107, 2065–2074. [Google Scholar] [CrossRef]
- Vargas, C.G.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Comparative study on the properties of films based on red rice (Oryza glaberrima) flour and starch. Food Hydrocoll. 2017, 65, 96–106. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan Film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Rhim, J.W. Effect of PLA lamination on performance characteristics of agar/κ-Carrageenan/clay bio-nanocomposite film. Food Res. Int. 2013, 51, 714–722. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, H.; Zhang, Q.; Fan, Y.; Yue, Y.; Yin, P.; Guo, L. Ca2+ Enhanced Nacre-Inspired Montmorillonite-Alginate Film with Superior Mechanical, Transparent, Fire Retardancy, and Shape Memory Properties. ACS Sustain. Chem. Eng. 2016, 8, 28816–28823. [Google Scholar] [CrossRef]
- Aroca, Á.S.; Iskandar, L.; Deb, S. Green synthetic routes to alginate-graphene oxide composite hydrogels with enhanced physical properties for bioengineering applications. Eur. Polym. J. 2018, 103, 198–206. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Lee, Y.; Hong, S.I. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: Laser ablation method. Carbohydr. Polym. 2014, 103, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Poly (vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles, characterization and antibacterial properties. Appl. Clay Sci. 2018, 161, 464–473. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Kokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Chhatre, A.; Solasa, P.; Sakle, S.; Thaokar, R.; Mehra, A. Color and surface plasmon effects in nanoparticles systems: Case of silver nanoparticles prepared by microemulsion route. Colloid Surf. A 2012, 404, 83–92. [Google Scholar] [CrossRef]
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose Nanocrystal/Silver Nanoparticle Composites as Bifunctional Nanofillers within Waterborne Polyurethane. ACS Sustain. Chem. Eng. 2012, 4, 2413–2419. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Zhang, J.; Roll, D.; Geddes, C.D.; Lakowicz, J.R. Aggregation of Silver Nanoparticle-Dextran Adducts with Concanavalin A and Competitive Complexation with Glucose. J. Phys. Chem. B 2004, 108, 12210–12214. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing origanum vulgare L: Essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Jacob, H. Preparation and characterization of agar-based nanocomposite films reinforced with bimetallic (Ag-Cu) alloy nanoparticles. Carbohydr. Polym. 2017, 155, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kang, Y.K.; Im, S.S. Biodegradability of cellulose fabrics. J. Appl. Polym. Sci. 2004, 94, 248–253. [Google Scholar] [CrossRef]
- Yabannavar, A.; Bartha, R. Biodegradability of some food packaging materials in soil. Soil Biol. Biochem. 1993, 25, 1469–1475. [Google Scholar] [CrossRef]
| Sample Code | Thickness (μm) | FWS | MA (%) | WVP (×10−11gm−1s−1Pa−1) | TS (MPa) | EB (%) |
|---|---|---|---|---|---|---|
| Alg | 10 | 99.20 | 18.87 | 6.53 | 25.60 | 17.10 |
| Ag/Alg | 11 | 89.39 | 12.33 | 4.20 | 40.30 | 13.20 |
| CNC/Alg | 11 | 61.29 | 12.22 | 5.40 | 35.50 | 14.30 |
| CNC/Ag/Alg | 12 | 56.36 | 13.24 | 4.60 | 39.40 | 14.70 |
| Sample Code | Opacity (Abs600/mm) | L | a | b | ΔE |
|---|---|---|---|---|---|
| Alg | 1.37 | 93.68 | –1.04 | 2.772 | 2.53 |
| CNC/Ag | 11.43 | 93.37 | –0.1 | 4.77 | 4.22 |
| Ag/Alg | 20.84 | 32.95 | 9.33 | 14.02 | 65.02 |
| CNC/Ag/Ag | 37.70 | 36.92 | 29.95 | 28.10 | 71.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, M.; Liu, Y.-K.; Chiu, F.-C. Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials 2019, 9, 1523. https://doi.org/10.3390/nano9111523
Yadav M, Liu Y-K, Chiu F-C. Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials. 2019; 9(11):1523. https://doi.org/10.3390/nano9111523
Chicago/Turabian StyleYadav, Mithilesh, Yu-Kuo Liu, and Fang-Chyou Chiu. 2019. "Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application" Nanomaterials 9, no. 11: 1523. https://doi.org/10.3390/nano9111523
APA StyleYadav, M., Liu, Y.-K., & Chiu, F.-C. (2019). Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials, 9(11), 1523. https://doi.org/10.3390/nano9111523






