Synthesis of CaF2 Nanoparticles Coated by SiO2 for Improved Al2O3/TiC Self-Lubricating Ceramic Composites
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Processing
2.2. Synthesis of CaF2@SiO2 Powders
2.3. Preparation of the Self-Lubricating Ceramic Tool Materials
2.4. Performance Testing of Tool Materials
2.5. Characterization
3. Results and Discussion
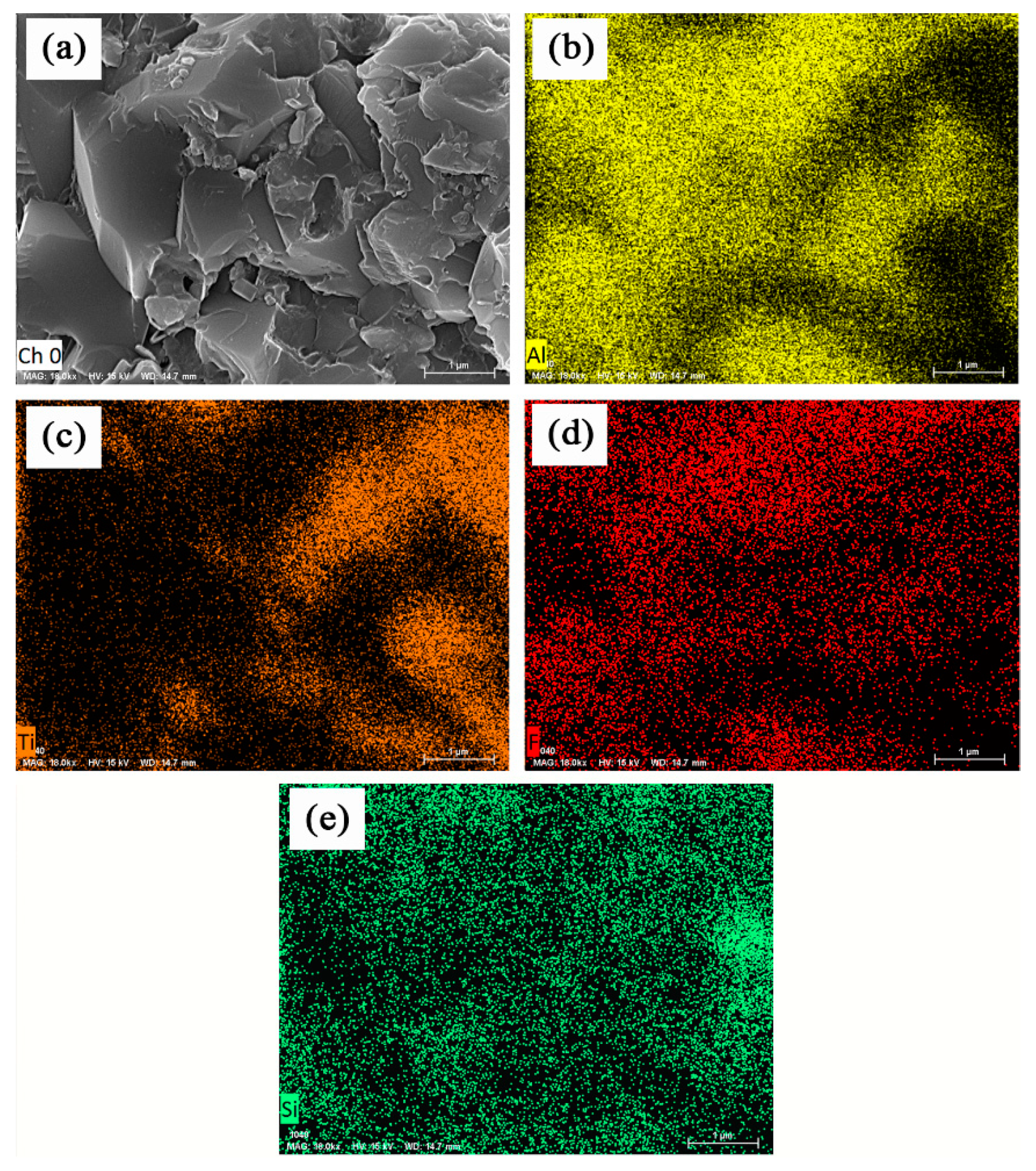
3.1. Characterization of Structure and Morphology
3.2. Effect of TESO Addition on Coating Thickness
3.3. Improvement in Mechanical Properties and Microstructurs
4. Conclusions
- SiO2 shells with different thicknesses could be obtained by changing the amount of TEOS added, and the thickness of the SiO2 shell could be controlled between 1.5 and 15 nm. However, when the TEOS amount was too large, agglomeration occurred, whereas when the TEOS amount was 3 mL, the best coating effect was obtained.
- The ceramic tool had the best mechanical properties when CaF2@SiO2-coated nanoparticles were added. The flexural strength, the fracture toughness, and the hardness were 562 ± 28 MPa, 5.51 ± 0.26 MPa·m1/2 and 15.26 ± 0.16 GPa, respectively. Compared with the ceramic tool with the CaF2 nanoparticles, the above performances were increased by 17.57%, 12.67% and 4.88%, respectively.
- Compared with the ceramic tool with CaF2 nanoparticles, the ceramic tool with CaF2@SiO2-coated nanoparticles showed a great change in its microscopic morphology. The addition of the coated powder played a role in refining the crystal grains of the ceramic tool and, at the same time, increased its transgranular fracture, improving its performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Liu, J.; Fan, X.; Peng, Q.; Guo, X.; Jiang, D.; Qian, X.; Su, L. Compact passive Q-switching of a diode-pumped Tm, Y: CaF2 laser near 2 mu m. Opt. Laser Technol. 2018, 103, 89–92. [Google Scholar] [CrossRef]
- Chen, K.; Chen, M.; Yu, Z.; Wang, Q.; Li, X.; Zhu, S.; Wang, F. Corrosion of SiO2–B2O3–Al2O3–CaF2-R2O (R = Na and K) enamels with different content of ZrO2 in H2SO4 and NaOH solutions. Ceram. Int. 2019, 45, 14958–14967. [Google Scholar] [CrossRef]
- Rezvani, M.; Farahinia, L. Structure and optical band gap study of transparent oxyfluoride glass-ceramics containing CaF2 nanocrystals. Mater. Des. 2015, 88, 252–257. [Google Scholar] [CrossRef]
- Vishwanath, S.; Liu, X.; Rouvimov, S.; Mende, P.C.; Azcatl, A.; McDonnell, S.; Wallace, R.M.; Feenstra, R.M.; Furdyna, J.K.; Jena, D.; et al. Comprehensive structural and optical characterization of MBE grown MoSe2 on graphite, CaF2 and graphene. 2D Mater. 2015, 2, 24007. [Google Scholar] [CrossRef]
- Kwon, S.; Cho, S.-H.; Lee, J.; Kang, J.; Lee, J.-H.; Cho, S.; Nersisyan, H.H. High-temperature stability of YSZ and MSZ ceramic materials in CaF2–MgF2–MgO molten salt system. J. Am. Ceram. Soc. 2017, 101, 2074–2083. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Mei, B.; Song, J.; Yi, G.; Zhou, Z.; Liu, J. Synthesis and enhanced upconversion luminescence upon two-wavelength excitation of Er3+: CaF2 transparent ceramics. J. Lumin. 2019, 213, 504–509. [Google Scholar] [CrossRef]
- Nakamura, F.; Kato, T.; Okada, G.; Kawaguchi, N.; Fukuda, K.; Yanagida, T. Scintillation and dosimeter properties of CaF2 transparent ceramic doped with Eu2+. Ceram. Int. 2017, 43, 604–609. [Google Scholar] [CrossRef]
- Nakamura, F.; Kato, T.; Okada, G.; Kawaguchi, N.; Fukuda, K.; Yanagida, T. Scintillation and dosimeter properties of CaF2 translucent ceramic produced by SPS. J. Eur. Ceram. Soc. 2017, 37, 1707–1711. [Google Scholar] [CrossRef]
- Liu, X.; Di, W.; Qin, W. Cooperative luminescence mediated near infrared photocatalysis of CaF2: Yb@BiVO4 composites. Appl. Catal. B Environ. 2017, 205, 158–164. [Google Scholar] [CrossRef]
- Mrityunjay, D.; Mahantayya, M.; Ramesh, M.R. Microstructure and tribological behavior of plasma sprayed NiCrAlY/WC-Co/cenosphere/solid lubricants composite coatings. Surf. Coat. Technol. 2018, 354, 92–100. [Google Scholar]
- Gajrani, K.K.; Suvin, P.S.; Kailas, S.V.; Mamilla, R.S. Thermal, Rheological, Wettability and Hard Machining Performance of MoS2 and CaF2 based Minimum Quantity Hybrid Nano-Green Cutting Fluids. J. Mater. Process. Technol. 2019, 266, 25–139. [Google Scholar] [CrossRef]
- Zhen, J.; Li, F.; Zhu, S.; Ma, J.; Qiao, Z.; Liu, W.; Yang, J. Friction and wear behavior of nickel-alloy-based high temperature self-lubricating composites against Si3N4 and Inconel 718. Tribol. Int. 2014, 75, 1–9. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Ji, H.; Zheng, X.; Ruan, Q.; Niu, Y.; Liu, Z.; Zeng, Y. Microstructures and tribological properties of plasma sprayed WC–Co–Cu–BaF2/CaF2 self-lubricating wear resistant coatings. Appl. Surf. Sci. 2010, 256, 4938–4944. [Google Scholar] [CrossRef]
- Cheng, J.; Zhen, J.; Zhu, S.; Yang, J.; Ma, J.; Li, W.; Liu, W. Friction and wear behavior of Ni-based solid-lubricating composites at high temperature in a vacuum environment. Mater. Des. 2017, 122, 405–413. [Google Scholar] [CrossRef]
- Fang, Y.; Fan, H.; Song, J.; Zhang, Y.; Hu, L. Surface engineering design of Al2O3/Mo self-lubricating structural ceramics—Part II: Continuous lubrication effects of a three-dimensional lubricating layer at temperatures from 25 to 800 °C. Wear 2016, 360, 97–103. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Song, P.; Wang, S.; Wang, Y.; Mao, K. Laminated structure optimization and drawing performance of Al2O3–TiC/Al2O3–TiC–CaF2 self-lubricating laminated ceramic conical die. Ceram. Int. 2015, 41, 12480–12489. [Google Scholar] [CrossRef]
- Wu, G.; Xu, C.; Xiao, G.; Yi, M.; Chen, Z. Structure design of Al2O3/TiC/CaF2 multicomponent gradient self-lubricating ceramic composite and its tribological behaviors. Ceram. Int. 2018, 44, 5550–5563. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, G.; Zhang, Y.; Fang, B. Finite element design and fabrication of Al2O3/TiC/ CaF2 gradient self-lubricating ceramic tool material. Ceram. Int. 2014, 40, 10971–10983. [Google Scholar] [CrossRef]
- Xu, C.; Wu, G.; Xiao, G.; Fang, B. Al2O3/(W,Ti)C/ CaF2 multi-component graded self-lubricating ceramic cutting tool material. Int. J. Refract. Met. Hard Mater. 2014, 45, 125–129. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Song, P.; Cheng, J.; Gu, J.; Ma, T. Dry Sliding Wear Behavior of Al2O3-TiC Ceramic Composites Added with Solid Lubricant CaF2 by Cold Pressing and Sintering. Tribol. Trans. 2014, 58, 231–239. [Google Scholar] [CrossRef]
- Kong, L.; Bi, Q.; Niu, M.; Zhu, S.; Yang, J.; Liu, W. High-temperature tribological behavior of ZrO2–MoS2–CaF2 self-lubricating composites. J. Eur. Ceram. Soc. 2013, 33, 51–59. [Google Scholar] [CrossRef]
- Chen, G.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.; Song, J.; Pandey, R.K.; Ågren, H.; Prasad, P.N.; et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef] [PubMed]
- Mahn, S.; Kemnitz, E. Modification of low-molecular polylactic acid by CaF2 nanoparticles: A new approach to change its material properties. J. Appl. Polym. Sci. 2019, 136, 47875. [Google Scholar] [CrossRef]
- Cortelletti, P.; Facciotti, C.; Cantarelli, I.X.; Canton, P.; Quintanilla, M.; Vetrone, F.; Speghinia, A.; Pedronia, M. Nd3+ activated CaF2 NPs as colloidal nanothermometers in the biological window. Opt. Mater. 2017, 68, 29–34. [Google Scholar] [CrossRef]
- Cui, X.; Hu, T.; Wang, J.; Zhong, X.; Chen, Y.; Zhang, J.; Li, X.; Yang, J.; Gao, C. Effect of Tb-doped Concentration Variation on the Electrical and Dielectric Properties of CaF2 Nanoparticles. Nanomaterial 2018, 8, 532. [Google Scholar] [CrossRef]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322–1326. [Google Scholar] [CrossRef]
- Yang, H.; Ye, F.; Liu, Q.; Liu, S.; Gao, Y.; Liu, L. A novel silica aerogel/porous Si3N4 composite prepared by freeze casting and sol-gel impregnation with high-performance thermal insulation and wave-transparent. Mater. Lett. 2015, 138, 135–138. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, G.; Zhang, J.; Yi, M.; Chen, Z.; Zhang, W.; Xu, C. Al2O3/WB2 composite ceramic tool material reinforced with graphene oxide. Int. J. Refract. Met. Hard Mater. 2019, 81, 173–182. [Google Scholar] [CrossRef]
- Singh, S.; Meena, V.K.; Sharma, M.; Singh, H. Preparation and coating of nano-ceramic on orthopaedic implant material using electrostatic spray deposition. Mater. Des. 2015, 88, 278–286. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Q. Fabricating a Novel Intragranular Microstructure for Al2O3/GdAlO3 Ceramic Composites. Material 2018, 11, 1879. [Google Scholar] [CrossRef]
- Dubey, M.K.; Bijwe, J.; Ramakumar, S.S.V. Effect of dispersant on nano-PTFE based lubricants on tribo-performance in fretting wear mode. RSC Adv. 2016, 6, 22604–22614. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Mirjavadi, S.S.; Alipour, M.; Emamian, S.; Kord, S.; Hamouda, A.M.S.; Koppad, P.G.; Keshavamurthy, R. Influence of TiO2 nanoparticles incorporation to friction stir welded 5083 aluminum alloy on the microstructure, mechanical properties and wear resistance. J. Alloy. Compd. 2017, 712, 795–803. [Google Scholar] [CrossRef]
- Dambatta, Y.S.; Sayuti, M.; Sarhan, A.A.D.; Hamdi, M. Comparative study on the performance of the MQL nanolubricant and conventional flood lubrication techniques during grinding of Si3N4 ceramic. Int. J. Adv. Manuf. Technol. 2018, 96, 3959–3976. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vadivel, S. Fiber optic ethanol gas sensor based WO3 and WO3/gC3N4 nanocomposites by a novel microwave technique. Opt. Laser Technol. 2019, 118, 44–51. [Google Scholar] [CrossRef]
- Wang, H.; Ma, R.; Nienhaus, K.; Nienhaus, G.U. Formation of a Monolayer Protein Corona around Polystyrene Nanoparticles and Implications for Nanoparticle Agglomeration. Small 2019, 15, e1900974. [Google Scholar] [CrossRef]
- Chen, H.; Xu, C.; Xiao, G.; Chen, Z.; Ma, J.; Wu, G. Synthesis of (h-BN)/SiO2 core–shell powder for improved self-lubricating ceramic composites. Ceram. Int. 2016, 42, 5504–5511. [Google Scholar] [CrossRef]
- Song, C.; Chen, J.; Abell, J.L.; Cui, Y.; Zhao, Y. Ag-SiO2 Core-Shell Nanorod Arrays: Morphological, Optical, SERS, and Wetting Properties. Langmuir 2012, 28, 1488–1495. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, C.; Xiao, G.; Yi, M.; Chen, Z.; Zhang, J. Electrostatic self-assembly preparation of reduced graphene oxide-encapsulated alumina nanoparticles with enhanced mechanical properties of alumina nanocomposites. J. Eur. Ceram. Soc. 2018, 38, 5122–5133. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Ligmajer, F.; Chan, C.F.; Bao, Z.; Wong, K.L.; Chen, X.; Hao, J.; Dai, J.; Yu, S.F.; et al. Plasmonic enhancement and polarization dependence of nonlinear upconversion emissions from single gold nanorod@SiO2@CaF2:Yb3+, Er3+ hybrid core-shell-satellite nanostructures. Light Sci. Appl. 2017, 6, e16217. [Google Scholar] [CrossRef]
- Hu, D.; Zhao, F.; Zhang, Z.; Miao, L.; Ma, R.; Zhao, W.; Ren, L.; Zhang, G.; Zhai, L.; Wang, D.; et al. Synthesis and magnetic properties of monodisperse CoFe2O4 nanoparticles coated by SiO2. Ceram. Int. 2018, 44, 22462–22466. [Google Scholar] [CrossRef]
- Wu, G.; Xu, C.; Xiao, G.; Yi, M.; Chen, Z.; Chen, H. An advanced self-lubricating ceramic composite with the addition of core-shell structured h-BN@Ni powders. Int. J. Refract. Met. Hard Mater. 2018, 72, 276–285. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Peng, S.; Guo, D.; Wen, S.; Luo, J.; Xie, G. Core-shell nanospheres to achieve ultralow friction polymer nanocomposites with superior mechanical properties. Nanoscale 2019, 11, 8237–8246. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Dai, Y.; Yang, Y.; Chew, J.W. Nickel-cobalt catalyst supported on TiO2-coated SiO2 spheres for CO2 methanation in a fluidized bed. Int. J. Hydrog. Energy 2019, 44, 13443–13455. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, N.; Ji, L.; Guo, R. An advanced self-lubricating ceramic composite with the addition of core-shell structured CaF2@Al2O3 powders. Int. J. Appl. Ceram. Technol. 2019, 16, 753–760. [Google Scholar] [CrossRef]
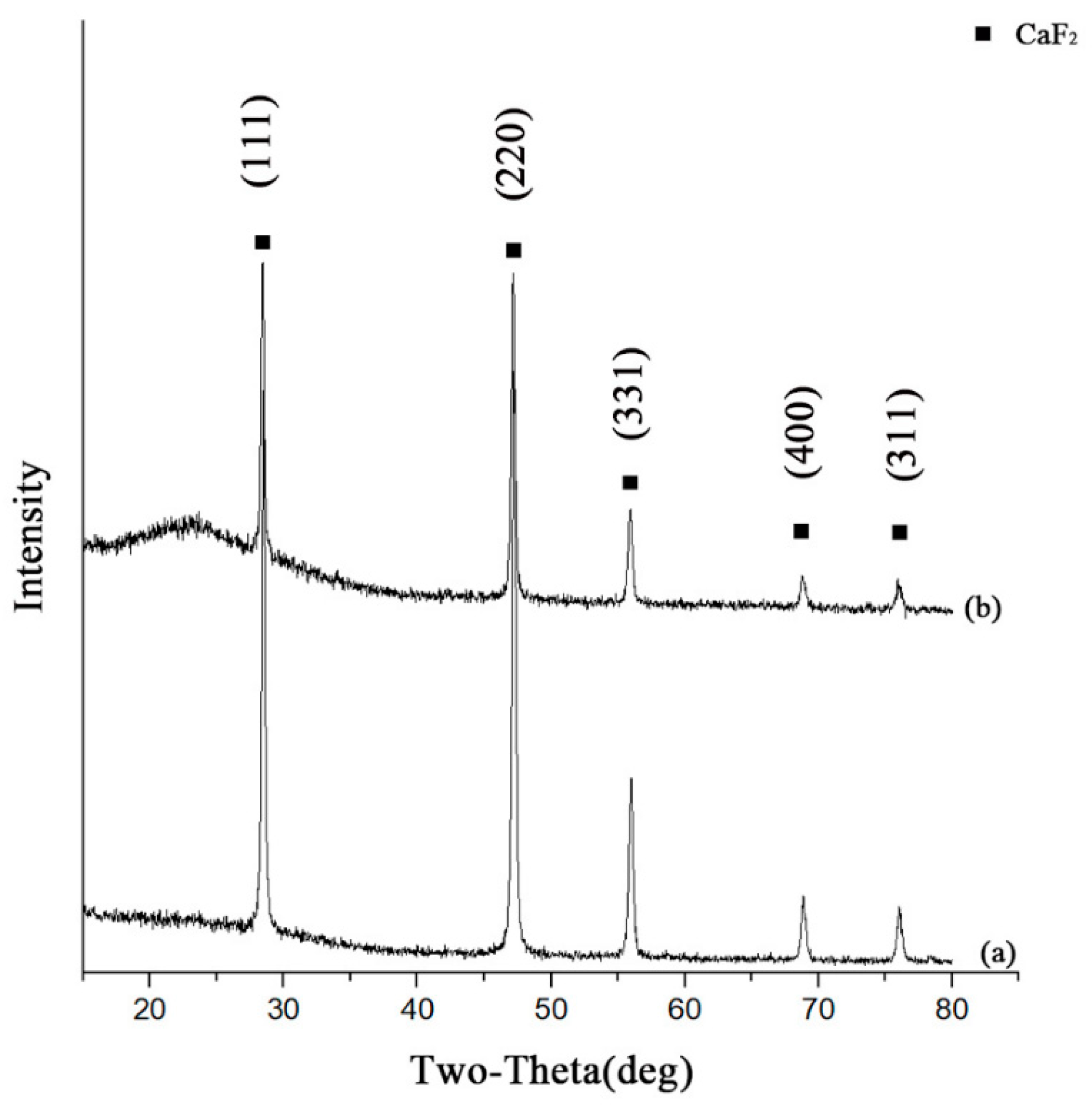
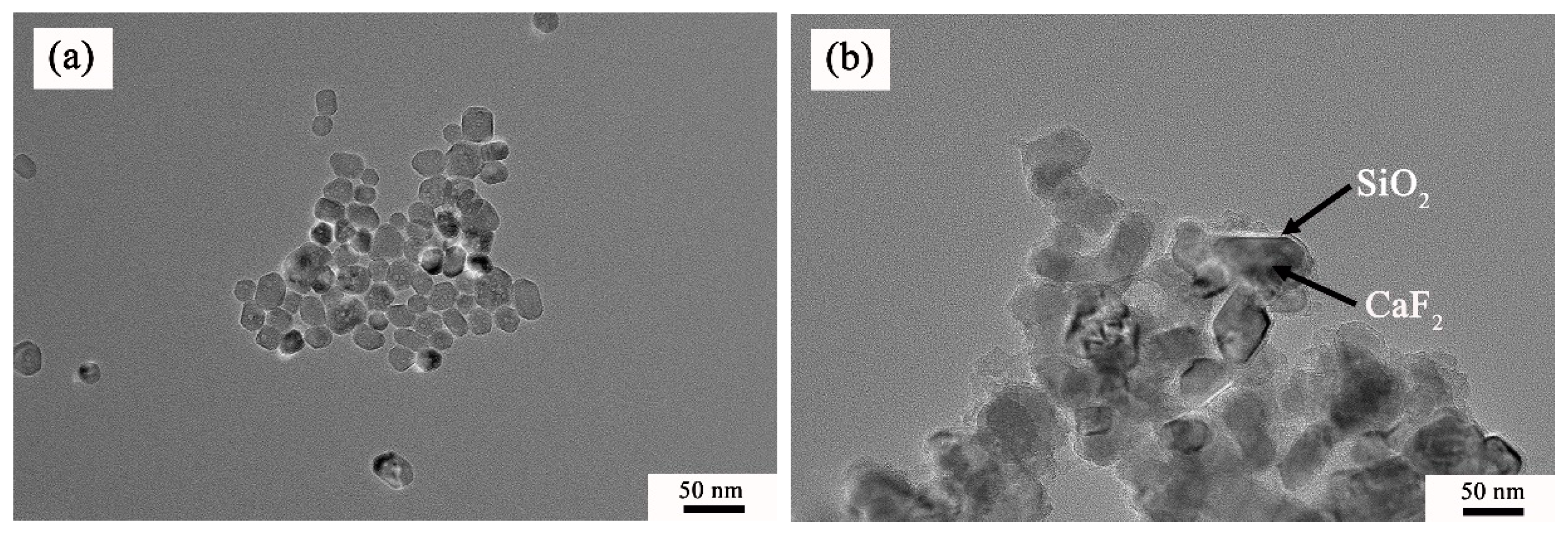
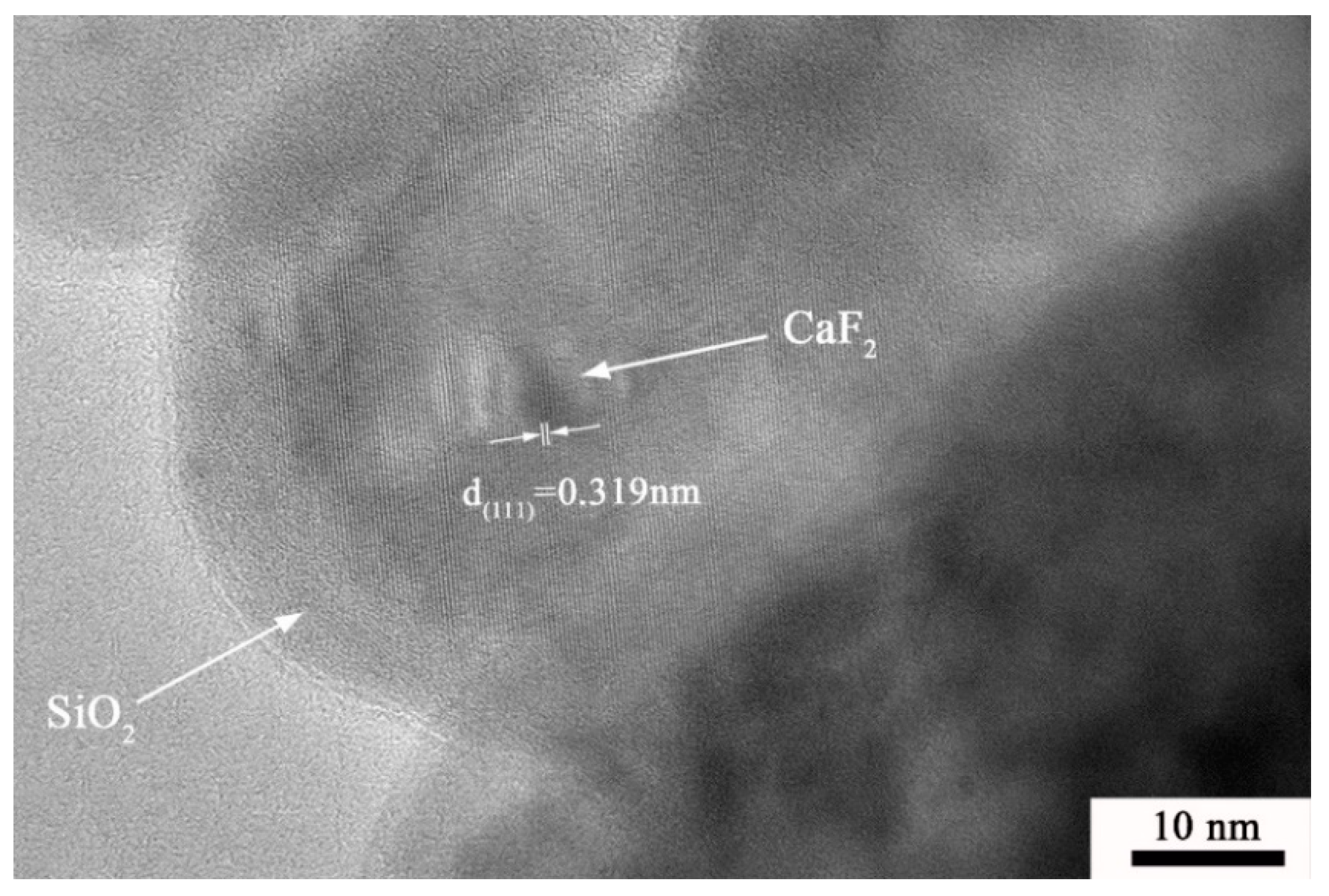

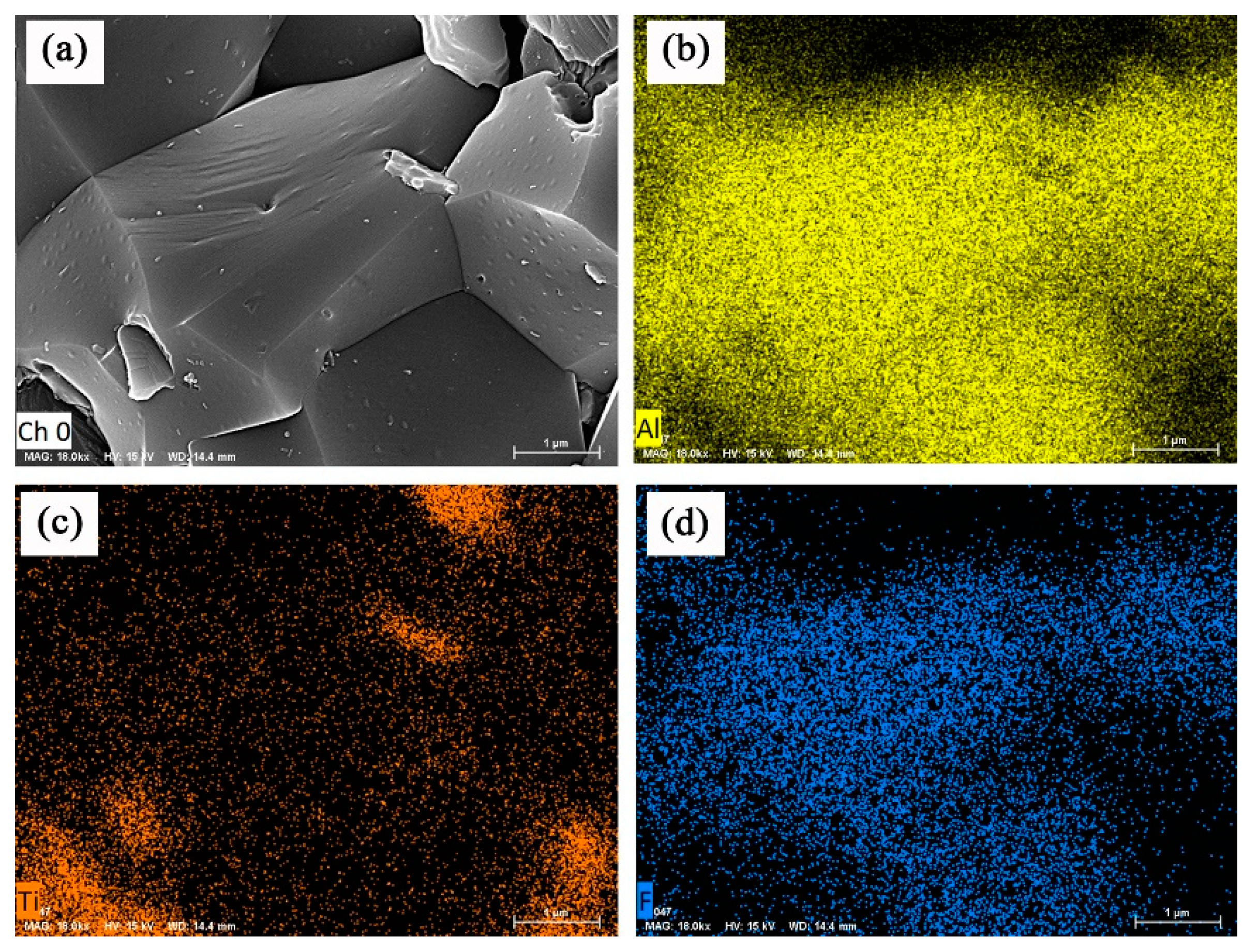
| Material | Flexural Strength/MPa | Fracture Toughness/MPa·m1/2 | Hardness/GPa |
|---|---|---|---|
| ATC | 478 ± 21 | 4.89 ± 0.13 | 14.55 ± 0.19 |
| ATCS | 562 ± 23 | 5.51 ± 0.21 | 15.26 ± 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Guo, N.; Ji, L.; Xu, C. Synthesis of CaF2 Nanoparticles Coated by SiO2 for Improved Al2O3/TiC Self-Lubricating Ceramic Composites. Nanomaterials 2019, 9, 1522. https://doi.org/10.3390/nano9111522
Chen Z, Guo N, Ji L, Xu C. Synthesis of CaF2 Nanoparticles Coated by SiO2 for Improved Al2O3/TiC Self-Lubricating Ceramic Composites. Nanomaterials. 2019; 9(11):1522. https://doi.org/10.3390/nano9111522
Chicago/Turabian StyleChen, Zhaoqiang, Niansheng Guo, Lianggang Ji, and Chonghai Xu. 2019. "Synthesis of CaF2 Nanoparticles Coated by SiO2 for Improved Al2O3/TiC Self-Lubricating Ceramic Composites" Nanomaterials 9, no. 11: 1522. https://doi.org/10.3390/nano9111522
APA StyleChen, Z., Guo, N., Ji, L., & Xu, C. (2019). Synthesis of CaF2 Nanoparticles Coated by SiO2 for Improved Al2O3/TiC Self-Lubricating Ceramic Composites. Nanomaterials, 9(11), 1522. https://doi.org/10.3390/nano9111522





