A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hybrid P(AAm-AAc)-AuNP Microgels
2.3. Characterization Methods
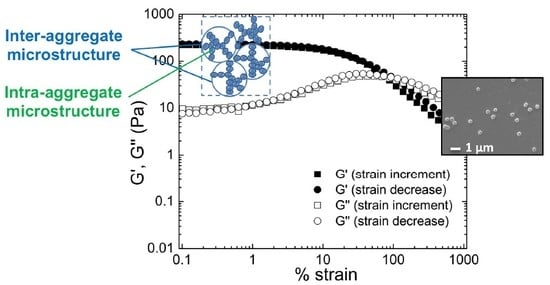
3. Results and Discussion
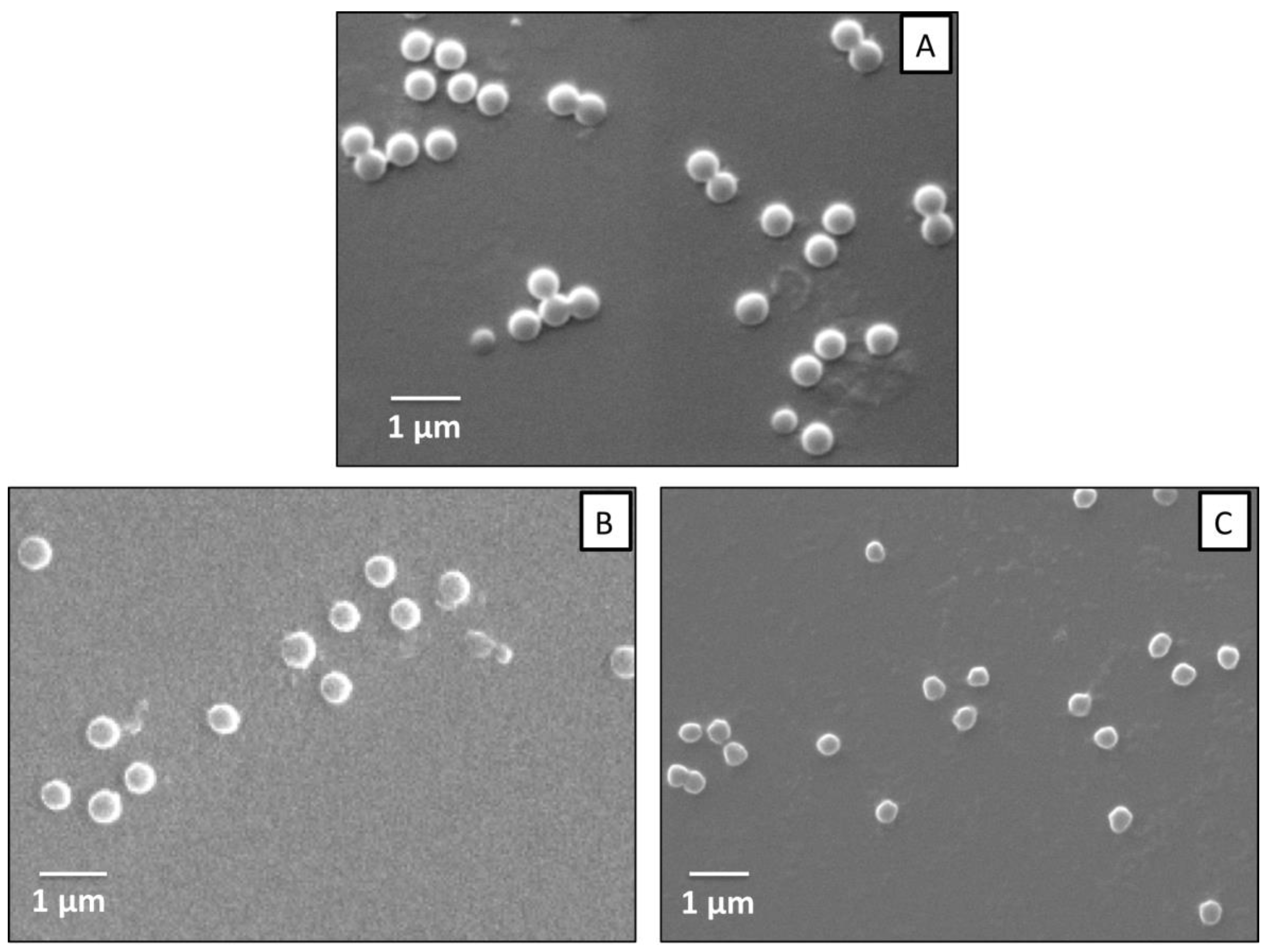
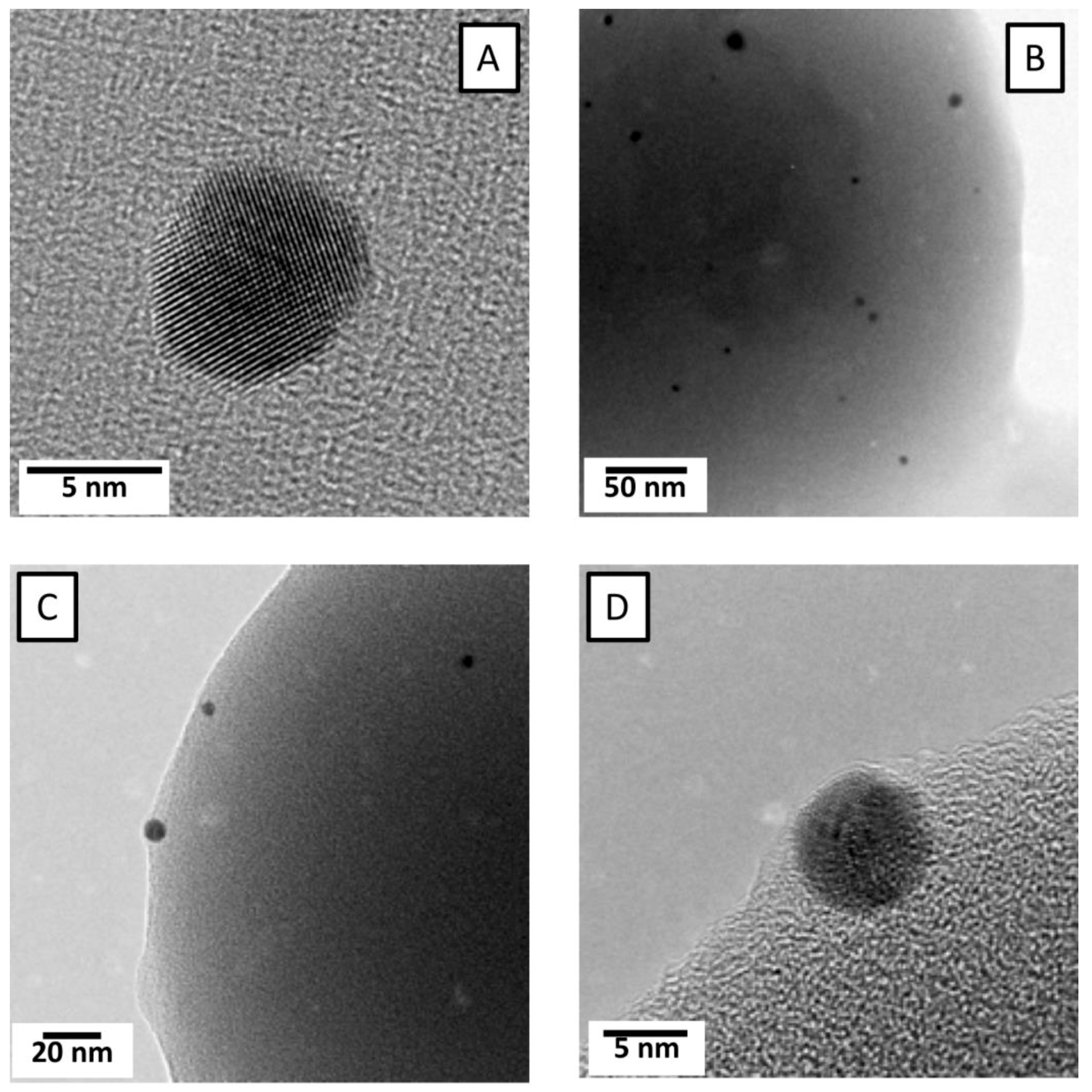
3.1. Morphology
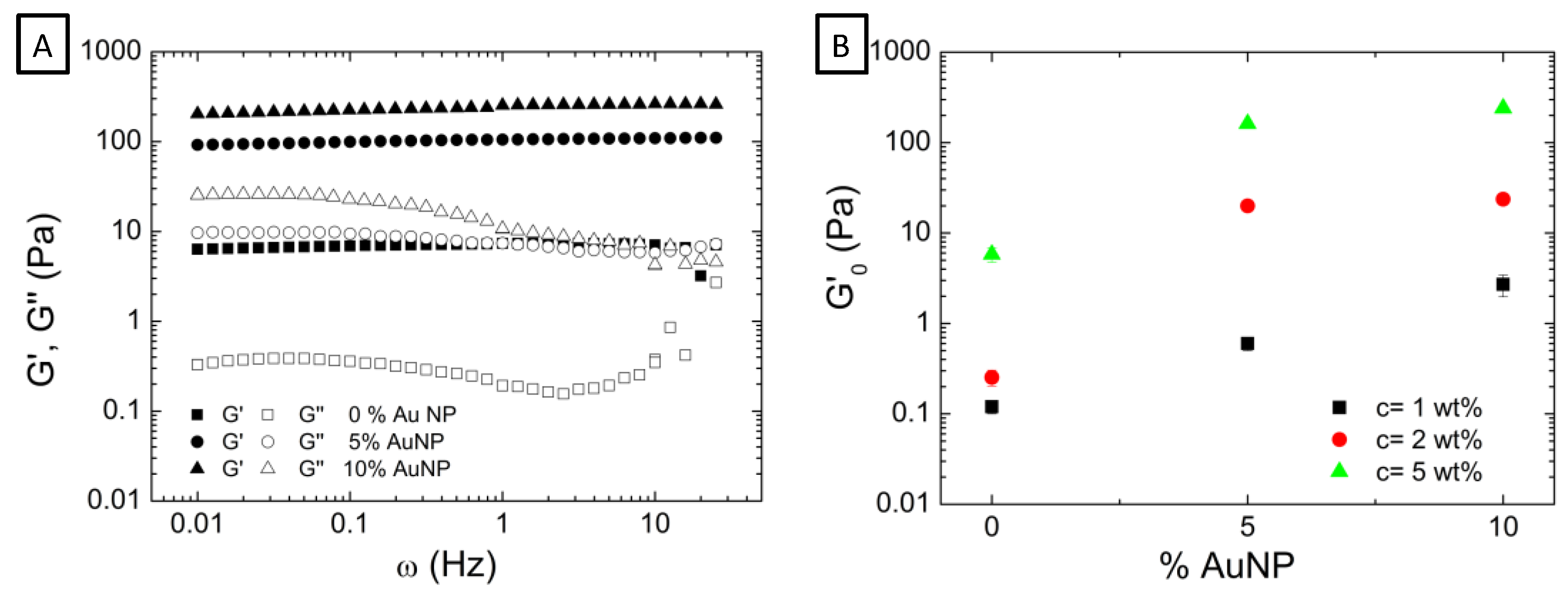
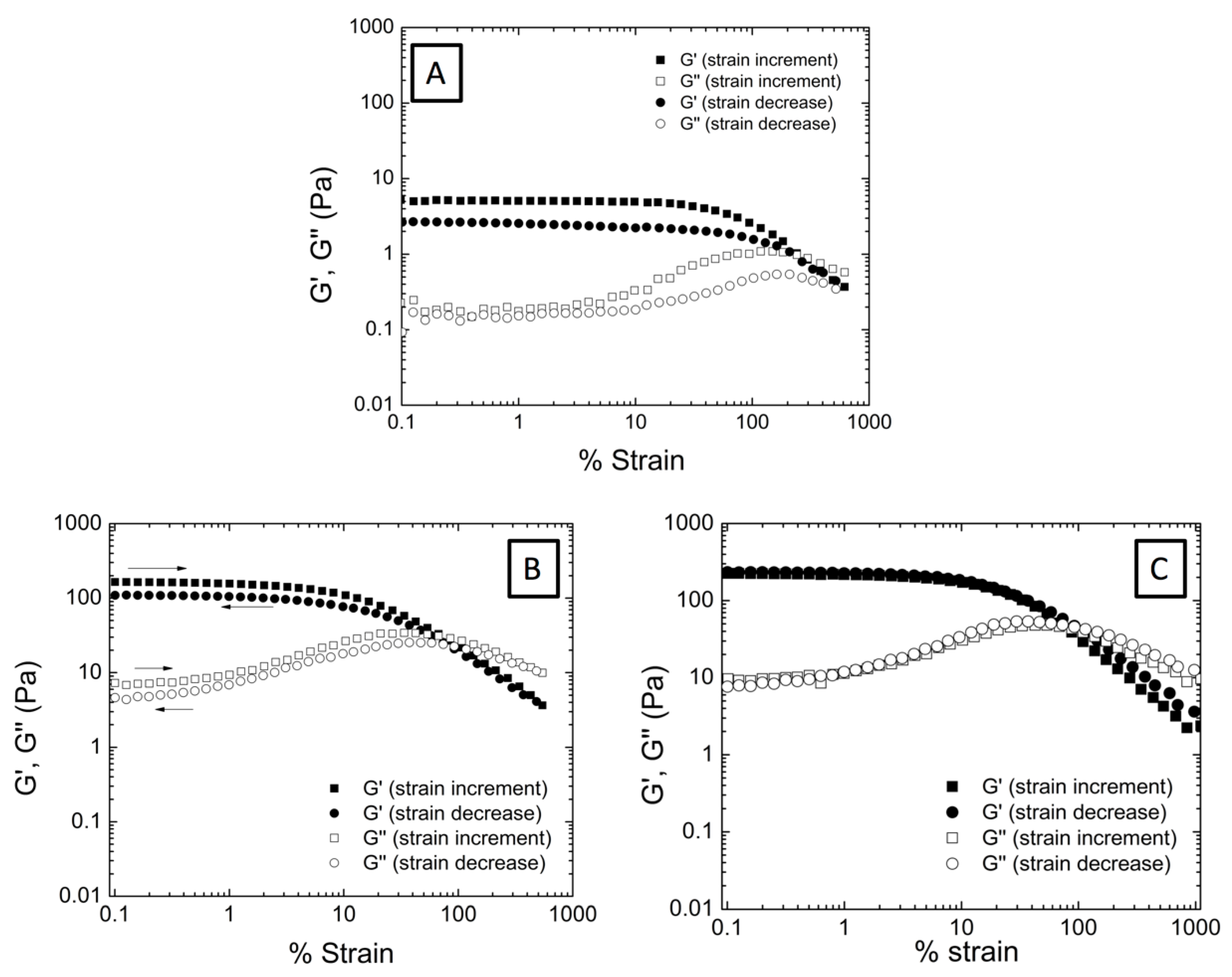
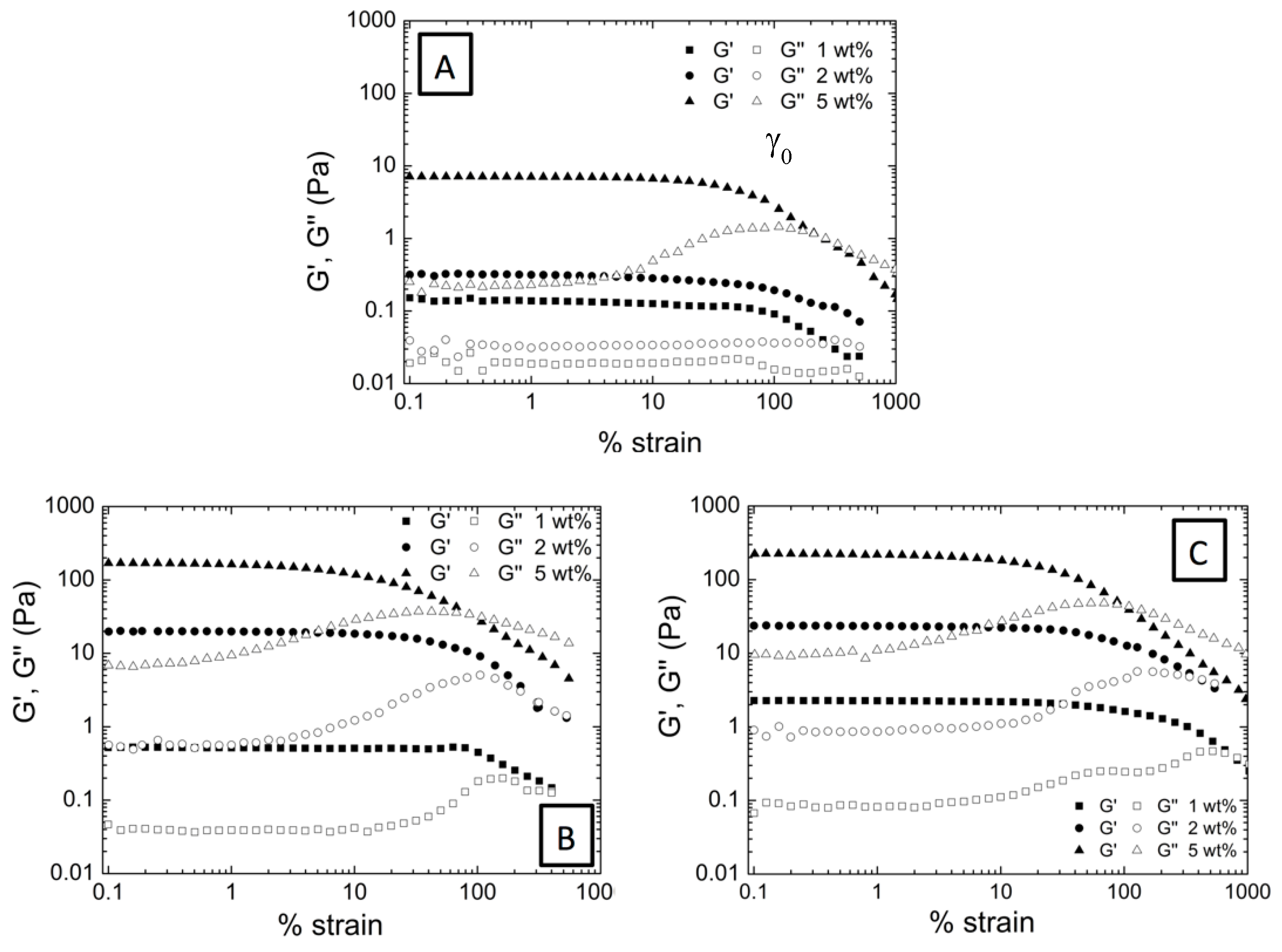
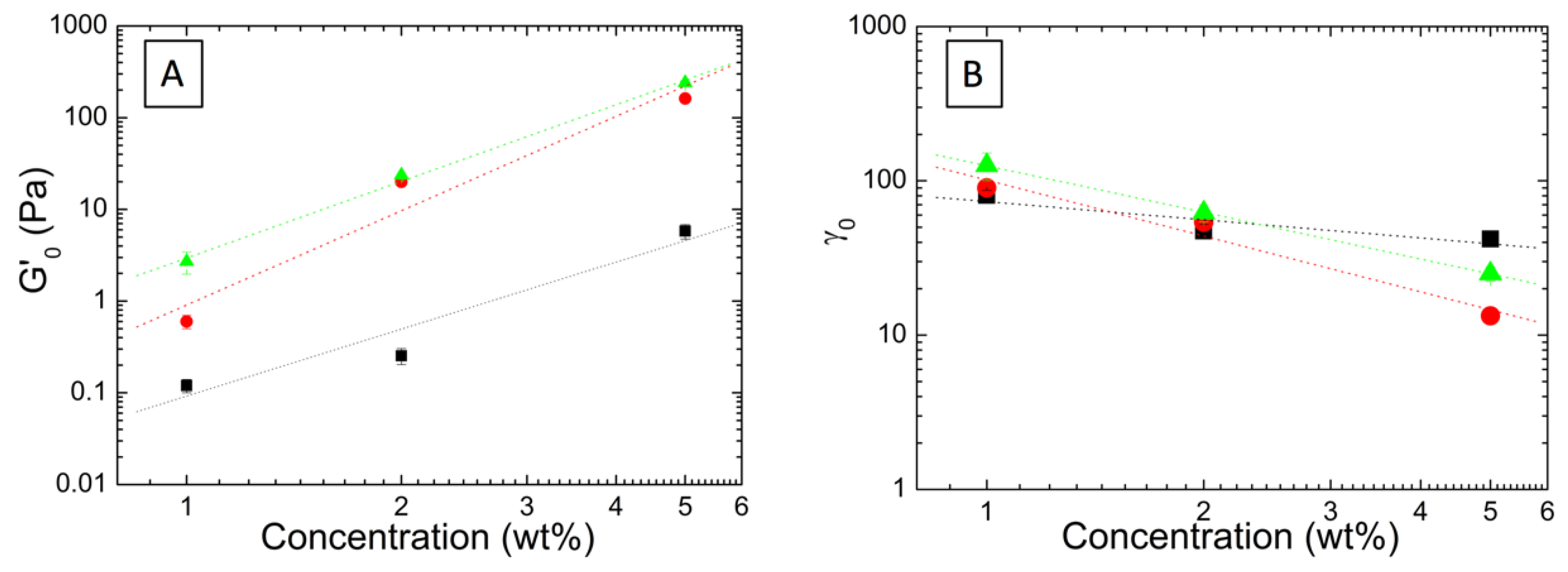
3.2. Effect of the Incorporation of AuNP in the Viscoelastic Properties of Aqueous Hybrid Microgel Dispersions
3.3. Fractal Analysis of Aqueous Hybrid Microgel Dispersions by Means of Shi et al. and Wu and Morbidelli Scaling Theory: Effect of AuNP in the Microgel Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gennes, P.-G. Nobel Lecture—Soft Matter; 4930838533; Angewandte Chemie International Edition: Paris, France, 1991. [Google Scholar]
- De Gennes, P.G. Ultradivided matter. Nature 2001, 412, 385. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Muratalin, M.; Luckham, P.F.; Esimova, A.; Aidarova, S.; Mutaliyeva, B.; Madybekova, G.; Sharipova, A.; Issayeva, A. Study of N-isopropylacrylamide-based microgel particles as a potential drug delivery agents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 8–17. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Sharma, S.N.; Campbell, K.T.; Stilhano, R.S.; Gijsbers, R.; Silva, E.A. Microgels produced using microfluidic on-chip polymer blending for controlled released of VEGF encoding lentivectors. Acta Biomater. 2018, 69, 265–276. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Li, N.; Wang, X.; Zhou, F.; Liu, W. Hairy Polyelectrolyte Brushes-Grafted Thermosensitive Microgels as Artificial Synovial Fluid for Simultaneous Biomimetic Lubrication and Arthritis Treatment. ACS Appl. Mater. Interfaces 2014, 6, 20452–20463. [Google Scholar] [CrossRef]
- Joshi, A.; Nandi, S.; Chester, D.; Brown, A.C.; Muller, M. Study of Poly(N-isopropylacrylamide-co-acrylic acid) (pNIPAM) Microgel Particle Induced Deformations of Tissue-Mimicking Phantom by Ultrasound Stimulation. Langmuir 2018, 34, 1457–1465. [Google Scholar] [CrossRef]
- Gan, T.; Zhang, Y.; Guan, Y. In Situ Gelation of P(NIPAM-HEMA) Microgel Dispersion and Its Applications as Injectable 3D Cell Scaffold. Biomacromolecules 2009, 10, 1410–1415. [Google Scholar] [CrossRef]
- Gelissen, A.P.; Schmid, A.J.; Plamper, F.A.; Pergushov, D.V.; Richtering, W. Quaternized microgels as soft templates for polyelectrolyte layer-by-layer assemblies. Polymer 2014, 55, 1991–1999. [Google Scholar] [CrossRef]
- Jia, S.; Tang, Z.; Guan, Y.; Zhang, Y. Order–Disorder Transition in Doped Microgel Colloidal Crystals and Its Application for Optical Sensing. ACS Appl. Mater. Interfaces 2018, 10, 14254–14258. [Google Scholar] [CrossRef]
- Park, C.W.; South, A.B.; Hu, X.; Verdes, C.; Kim, J.-D.; Lyon, L.A. Gold nanoparticles reinforce self-healing microgel multilayers. Colloid Polym. Sci. 2010, 289, 583–590. [Google Scholar] [CrossRef]
- Krüger, A.J.D.; Köhler, J.; Cichosz, S.; Rose, J.C.; Gehlen, D.B.; Haraszti, T.; Möller, M.; De Laporte, L.; Krüger, A.J.D.; Koehler, J. A catalyst-free, temperature controlled gelation system for in-mold fabrication of microgels. Chem. Commun. 2018, 54, 6943–6946. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Echeverria, C.; Borges, J.P.; Godinho, M.H.; Soares, P.I.P. Towards the development of multifunctional hybrid fibrillary gels: Production and optimization by colloidal electrospinning. RSC Adv. 2017, 7, 48972–48979. [Google Scholar] [CrossRef]
- Marques, S.C.S.; Soares, P.I.P.; Zabala, C.E.; Godinho, M.H.; Borges, J.P. Confinement of thermoresponsive microgels into fibres via colloidal electrospinning: Experimental and statistical analysis. RSC Adv. 2016, 6, 76370–76380. [Google Scholar] [CrossRef]
- Diaz, J.E.; Barrero, A.; Marquez, M.; Fernandez-Nieves, A.; Loscertales, I.G. Absorption properties of microgel-pvp composite nanofibers made by electrospinning. Macromol. Rapid Commun. 2010, 31, 183–189. [Google Scholar] [PubMed]
- Leon, A.M.; Aguilera, J.M.; Park, D.J. Mechanical, rheological and structural properties of fiber-containing microgels based on whey protein and alginate. Carbohydr. Polym. 2019, 207, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; López, D.; Mijangos, C.; Zabala, C.E. UCST Responsive Microgels of Poly(acrylamide−acrylic acid) Copolymers: Structure and Viscoelastic Properties. Macromolecules 2009, 42, 9118–9123. [Google Scholar] [CrossRef]
- Zabala, C.E.; Peppas, N.A.; Mijangos, C. Novel strategy for the determination of UCST-like microgels network structure: Effect on swelling behavior and rheology. Soft Matter 2012, 8, 337–346. [Google Scholar]
- Antonietti, M.; Gröhn, F.; Hartmann, J.; Bronstein, L. Nonclassical Shapes of Noble-Metal Colloids by Synthesis in Microgel Nanoreactors. Angew. Chem. Int. Ed. 1997, 36, 2080–2083. [Google Scholar] [CrossRef]
- Yoshida, A.; Kitayama, Y.; Kiguchi, K.; Yamada, T.; Akasaka, H.; Sasaki, R.; Takeuchi, T. Gold Nanoparticle-Incorporated Molecularly Imprinted Microgels as Radiation Sensitizers in Pancreatic Cancer. ACS Appl. Bio Mater. 2019, 2, 1177–1183. [Google Scholar] [CrossRef]
- Peng, J.; Tang, D.; Jia, S.; Zhang, Y.; Sun, Z.; Yang, X.; Zou, H.; Lv, H. In situ thermal synthesis of molybdenum oxide nanocrystals in thermoresponsive microgels. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 130–140. [Google Scholar] [CrossRef]
- Brändel, T.; Sabadasch, V.; Hannappel, Y.; Hellweg, T. Improved Smart Microgel Carriers for Catalytic Silver Nanoparticles. ACS Omega 2019, 4, 4636–4649. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions†. Langmuir 2007, 23, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, S.; Kumacheva, E. Polymer Microgels: Reactors for Semiconductor, Metal, and Magnetic Nanoparticles. J. Am. Chem. Soc. 2004, 126, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Mijangos, C.; Zabala, C.E. UCST-Like Hybrid PAAm-AA/Fe3O4 Microgels. Effect of Fe3O4 Nanoparticles on Morphology, Thermosensitivity and Elasticity. Langmuir 2011, 27, 8027–8035. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Soares, P.; Robalo, A.; Pereira, L.; Novo, C.M.; Ferreira, I.; Borges, J.P. One-pot synthesis of dual-stimuli responsive hybrid PNIPAAm-chitosan microgels. Mater. Des. 2015, 86, 745–751. [Google Scholar] [CrossRef]
- Echeverria, C.; Mijangos, C. Effect of gold nanoparticles on the thermosensitivity, morphology, and optical properties of poly(acrylamide-acrylic acid) microgels. Macromol. Rapid Commun. 2010, 31, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, L.; Schumacher, M.; De Aberasturi, D.J.; Merkl, J.-P.; Henriksen-Lacey, M.; De Oliveira, T.M.; Janschel, M.; Schmidtke, C.; Bals, S.; Weller, H.; et al. Encapsulation of Noble Metal Nanoparticles through Seeded Emulsion Polymerization as Highly Stable Plasmonic Systems. Adv. Funct. Mater. 2019, 29, 29. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Multifunctionality in metal@microgel colloidal nanocomposites. J. Mater. Chem. A 2013, 1, 20–26. [Google Scholar] [CrossRef]
- Strozyk, M.S.; Carregal-Romero, S.; Henriksen-Lacey, M.; Brust, M.; Liz-Marzán, L.M. Biocompatible, Multiresponsive Nanogel Composites for Codelivery of Antiangiogenic and Chemotherapeutic Agents. Chem. Mater. 2017, 29, 2303–2313. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A. Scaling behavior of the elastic properties of colloidal gels. Phys. Rev. A 1990, 42, 4772–4779. [Google Scholar] [CrossRef]
- Wu, H.; Morbidelli, M. A Model Relating Structure of Colloidal Gels to Their Elastic Properties. Langmuir 2001, 17, 1030–1036. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Sprakel, J.; Lindström, S.B.; Kodger, T.E.; Weitz, D.A. Stress enhancement in the delayed yielding of colloidal gels. Phys. Rev. Lett. 2011, 106, 248303. [Google Scholar] [CrossRef]
- Stokes, J.R.; Frith, W.J. Rheology of gelling and yielding soft matter systems. Soft Matter 2008, 4, 1133. [Google Scholar] [CrossRef]
- Diba, M.; Wang, H.; Kodger, T.E.; Parsa, S.; Leeuwenburgh, S.C.G. Highly Elastic and Self-Healing Composite Colloidal Gels. Adv. Mater. 2017, 29, 1604672. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Y.; Guan, Y.; Zhu, X.X. Gelation Kinetics of Thermosensitive PNIPAM Microgel Dispersions. Macromol. Chem. Phys. 2011, 212, 2052–2060. [Google Scholar] [CrossRef]
- Gan, T.; Guan, Y.; Zhang, Y. Thermogelable PNIPAM microgel dispersion as 3D cell scaffold: Effect of syneresis. J. Mater. Chem. 2010, 20, 5937. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, Y.; Guan, Y.; Zhang, Y. Tuning properties of injectable hydrogel scaffold by PEG blending. Polymer 2012, 53, 5124–5131. [Google Scholar] [CrossRef]
- Wang, T.; Jin, L.; Song, Y.; Li, J.; Gao, Y.; Shi, S. Rheological study on the thermoinduced gelation behavior of poly(N -isopropylacrylamide-co -acrylic acid) microgel suspensions. J. Appl. Polym. Sci. 2017, 134, 45259. [Google Scholar] [CrossRef]
- Fraylich, M.R.; Liu, R.; Richardson, S.M.; Baird, P.; Hoyland, J.; Freemont, A.J.; Alexander, C.; Shakesheff, K.; Cellesi, F.; Saunders, B.R. Thermally-triggered gelation of PLGA dispersions: Towards an injectable colloidal cell delivery system. J. Colloid Interface Sci. 2010, 344, 61–69. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Y.; Guan, Y.; Zhu, X.X. Fractal Structures of the Hydrogels Formed in Situ from Poly(N-isopropylacrylamide) Microgel Dispersions. Langmuir 2012, 28, 10873–10880. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Aragón-Gutiérrez, A.; Fernández-García, M.; Muñoz-Bonilla, A.; López, D. Thermoresponsive Poly(N-Isopropylacrylamide-co-Dimethylaminoethyl Methacrylate) Microgel Aqueous Dispersions with Potential Antimicrobial Properties. Polymers 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.D.; Ball, R.C. Computer simulation of chemically limited aggregation. J. Phys. A Math. Gen. 1985, 18, L517–L521. [Google Scholar] [CrossRef]
- Vermant, J.; Ceccia, S.; Dolgovskij, M.K.; Maffettone, P.L.; Macosko, C.W. Quantifying dispersion of layered nanocomposites via melt rheology. J. Rheol. 2007, 51, 429. [Google Scholar] [CrossRef]
- Kolb, M.; Botet, R.; Jullien, R. Scaling of Kinetically Growing Clusters. Phys. Rev. Lett. 1983, 51, 1123–1126. [Google Scholar] [CrossRef]
- Meakin, P. Formation of Fractal Clusters and Networks by Irreversible Diffusion-Limited Aggregation. Phys. Rev. Lett. 1983, 51, 1119–1122. [Google Scholar] [CrossRef]
- Witten, T.A.; Sander, L.M. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Yan, C.; Mackay, M.E.; Czymmek, K.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable Solid Peptide Hydrogel as a Cell Carrier: Effects of Shear Flow on Hydrogels and Cell Payload. Langmuir 2012, 28, 6076–6087. [Google Scholar] [CrossRef]
| AQUEOUS PHASE | ORGANIC PHASE | |||||
|---|---|---|---|---|---|---|
| Acrylamide | Acrylic Acid | MBA | AuNP | Dodecane | Span 80 | |
| (Mol) | (Mol) | (Mol) | (%wt) | (mL) | (g) | |
| P(AAm-co-AAc) | 0.056 | 2.77 × 10−4 | 1.29 × 10−3 | 0 | 30 | 0.5056 |
| P(AAm-co-AAc)–5% AuNP | 0.056 | 2.77 × 10−4 | 1.29 × 10−3 | 5 | 30 | 0.5056 |
| P(AAm-co-AAc)–10% AuNP | 0.056 | 2.77 × 10−4 | 1.29 × 10−3 | 10 | 30 | 0.5056 |
| Samples | Slopes (Figure 6A,B) | Shih et al. | Wu and Morbidelli | Regime | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | Df | x | Df | β | α | ||
| P(AAm-co-AAc) | 2.56 ± 0.16 | −0.22 ± 0.17 | --- | <0 | 2.14 ± 0.28 | 2.2 ± 0.5 | 0.63 ± 0.17 | Transition (weak) |
| P(AAm-co-AAc)-5% AuNP | 3.82 ± 0.78 | −0.62 ± 0.22 | --- | <0 | 2.3 ± 0.7 | 2.3 ± 1.2 | 0.57 ± 0.38 | Transition (weak) |
| P(AAm-co-AAc)-10% AuNP | 2.29 ± 0.06 | −1.18 ± 0.13 | 1.19 | 1.13 | 1.2 ± 0.4 | 4.14 ± 0.1 | 0.04 ± 0.03 | Strong-link |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría, C.; Mijangos, C. A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies. Nanomaterials 2019, 9, 1499. https://doi.org/10.3390/nano9101499
Echeverría C, Mijangos C. A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies. Nanomaterials. 2019; 9(10):1499. https://doi.org/10.3390/nano9101499
Chicago/Turabian StyleEcheverría, Coro, and Carmen Mijangos. 2019. "A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies" Nanomaterials 9, no. 10: 1499. https://doi.org/10.3390/nano9101499
APA StyleEcheverría, C., & Mijangos, C. (2019). A Way to Predict Gold Nanoparticles/Polymer Hybrid Microgel Agglomeration Based on Rheological Studies. Nanomaterials, 9(10), 1499. https://doi.org/10.3390/nano9101499