Gold Film over SiO2 Nanospheres—New Thermally Resistant Substrates for Surface-Enhanced Raman Scattering (SERS) Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
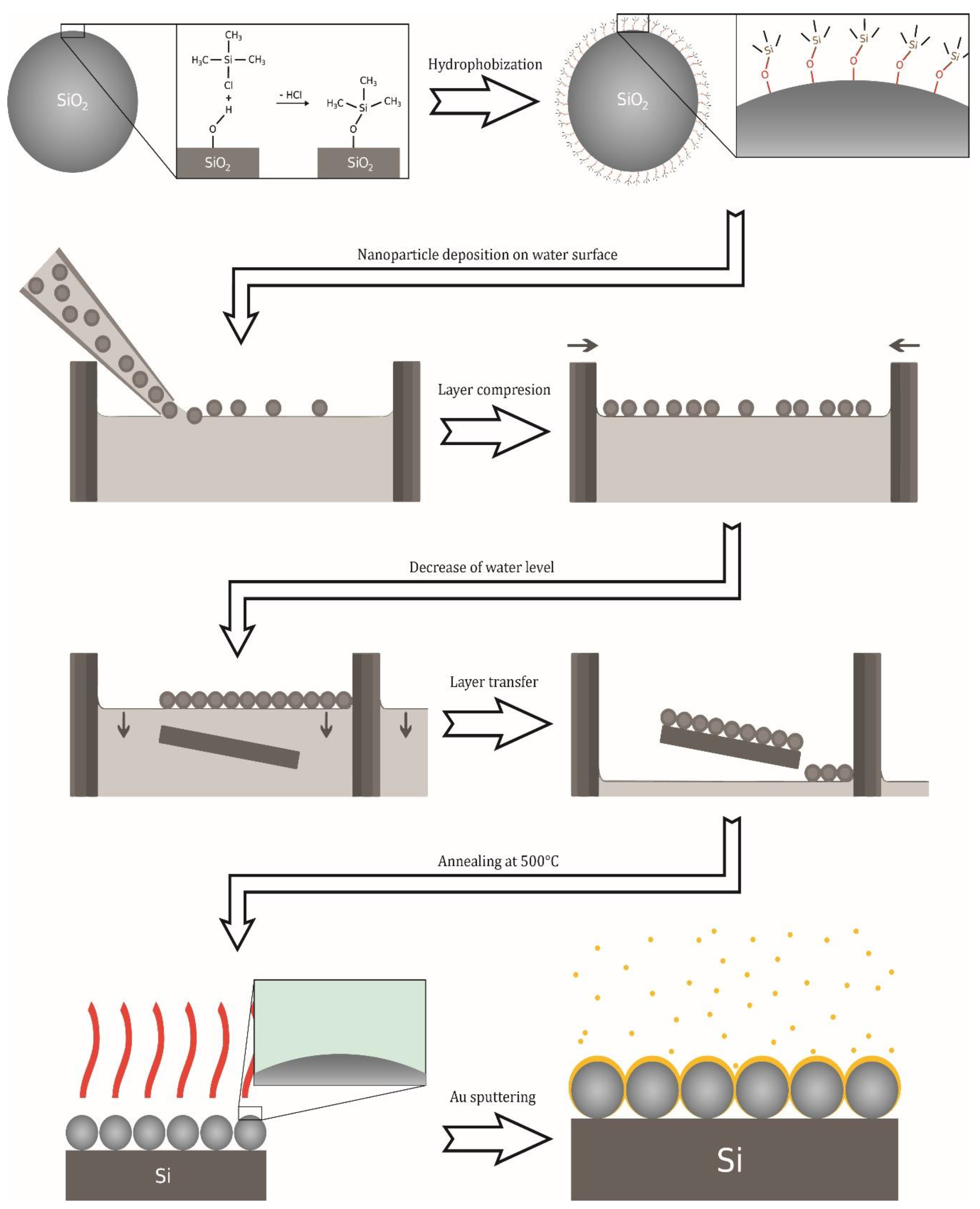
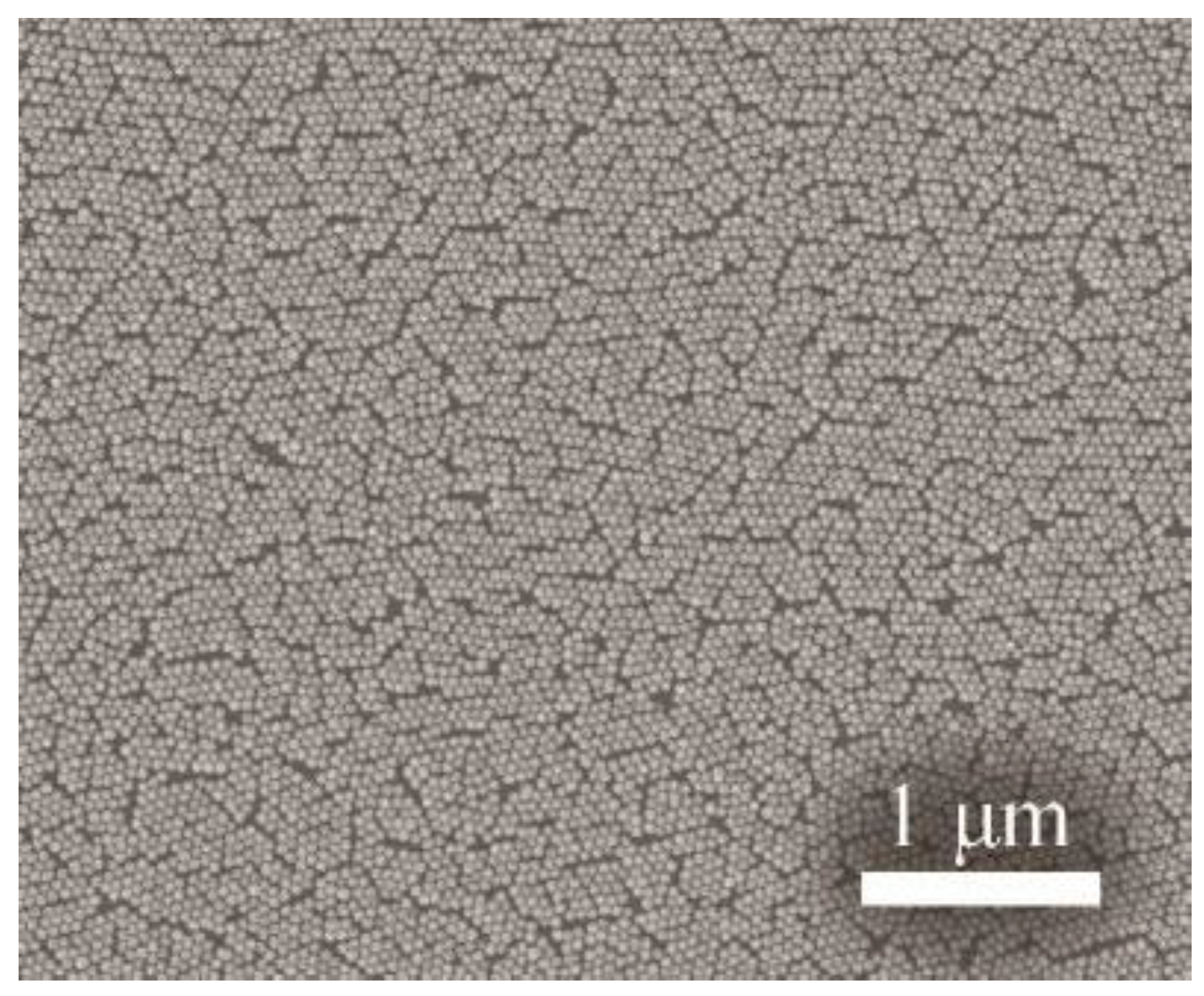
2.2. Preparation of Surface-Enhanced Raman Scattering (SERS)-Active Substrates
2.3. SERS Experiments
3. Results and Discussion
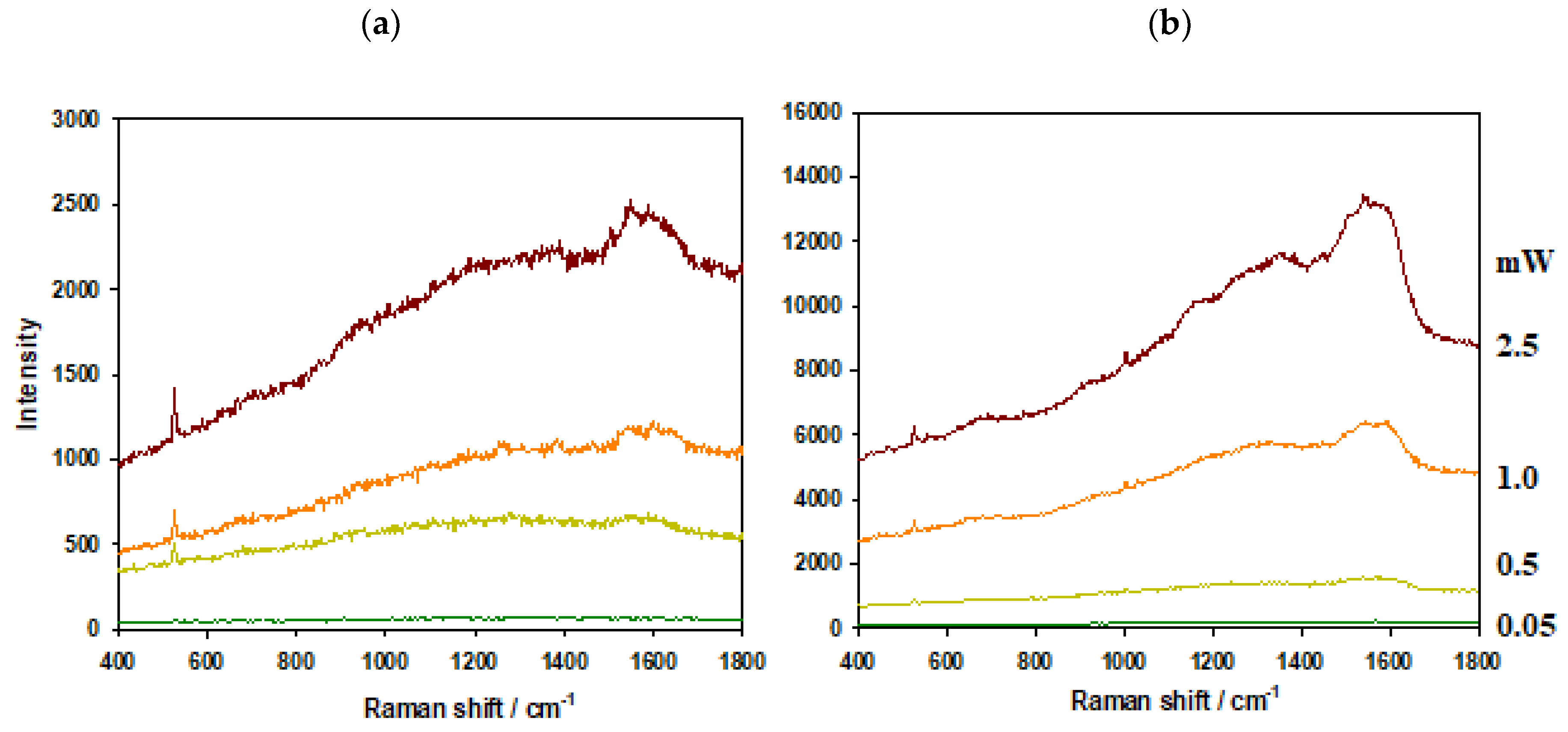
3.1. Comparison of Thermal Resistance of AuPS and AuSiO2 Substrates
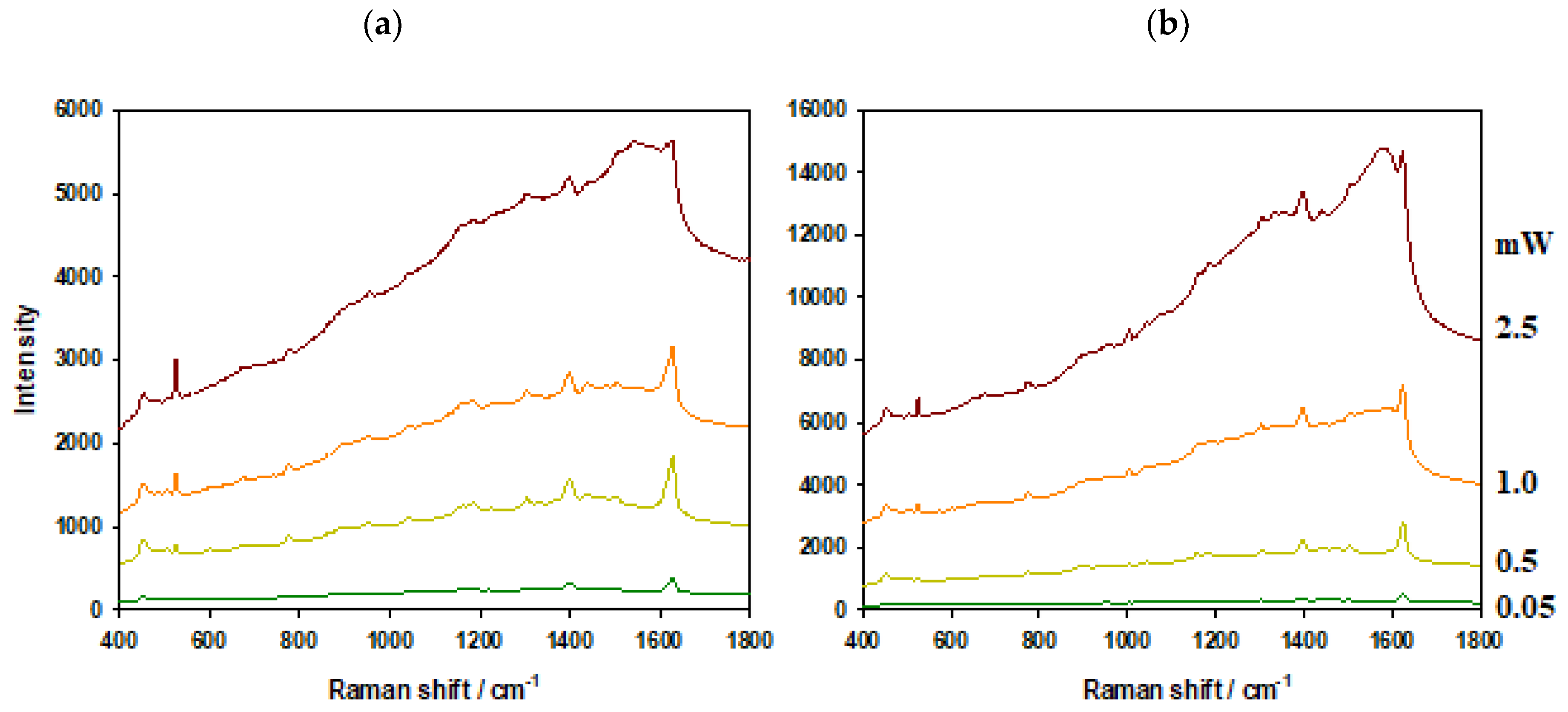
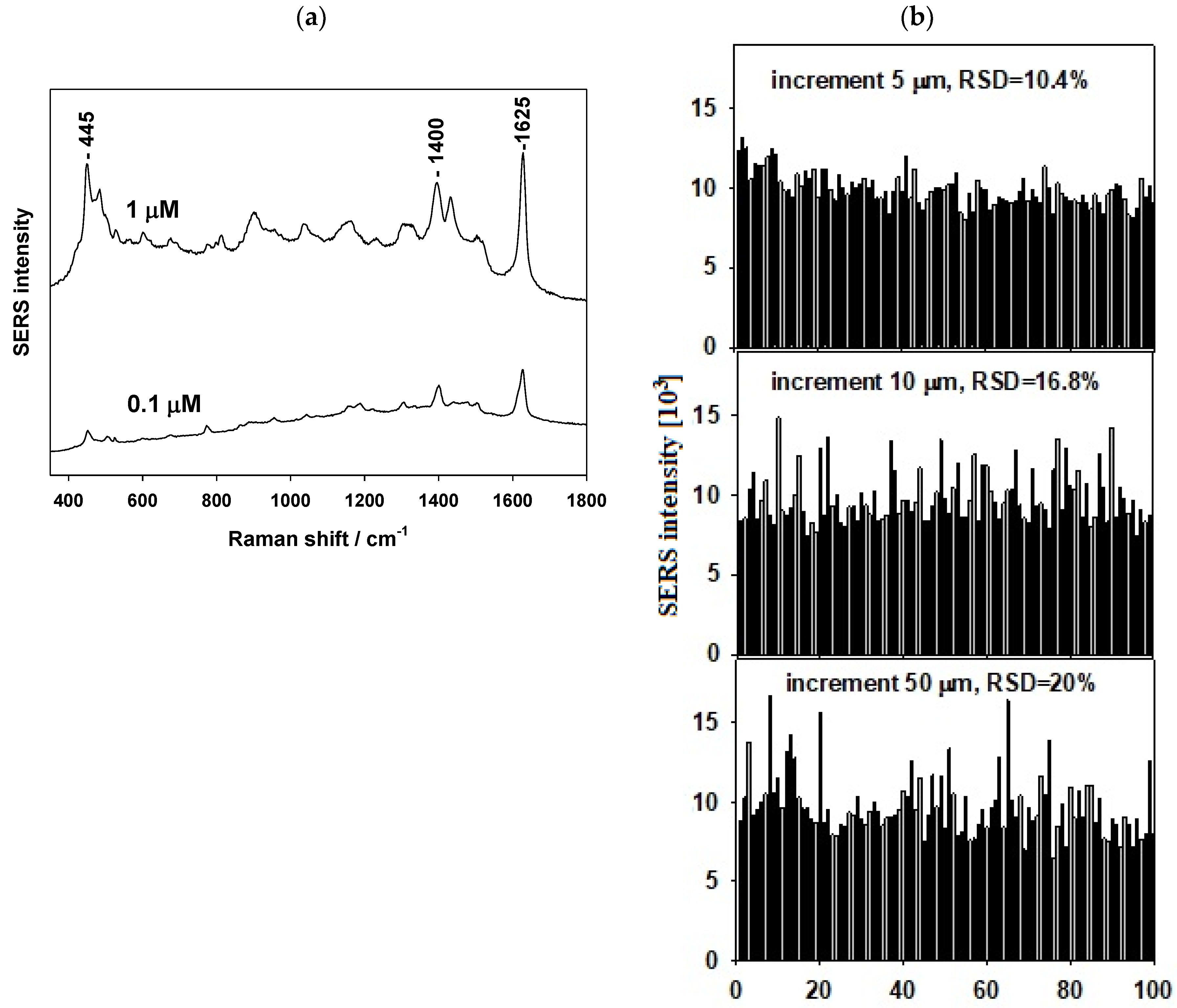
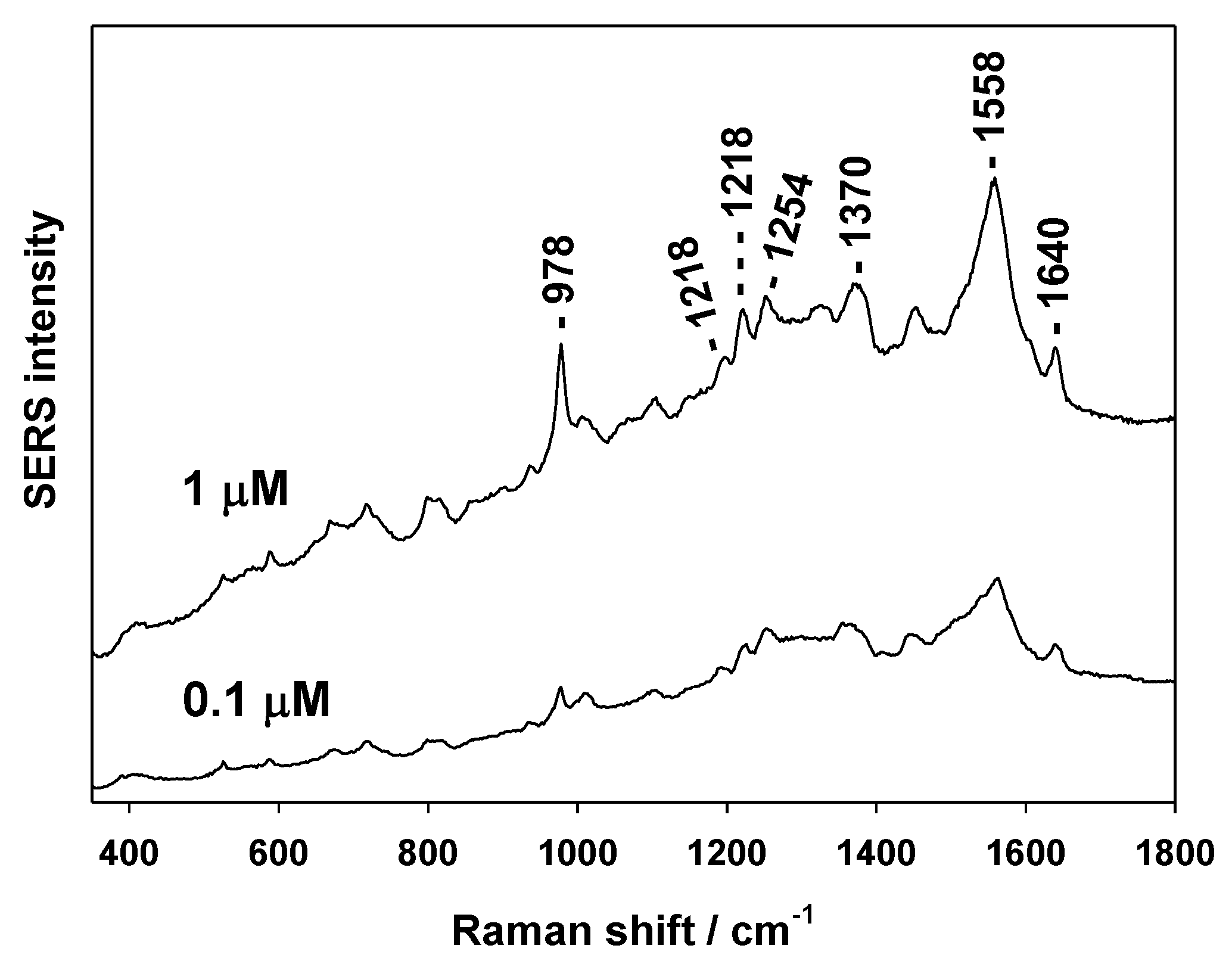
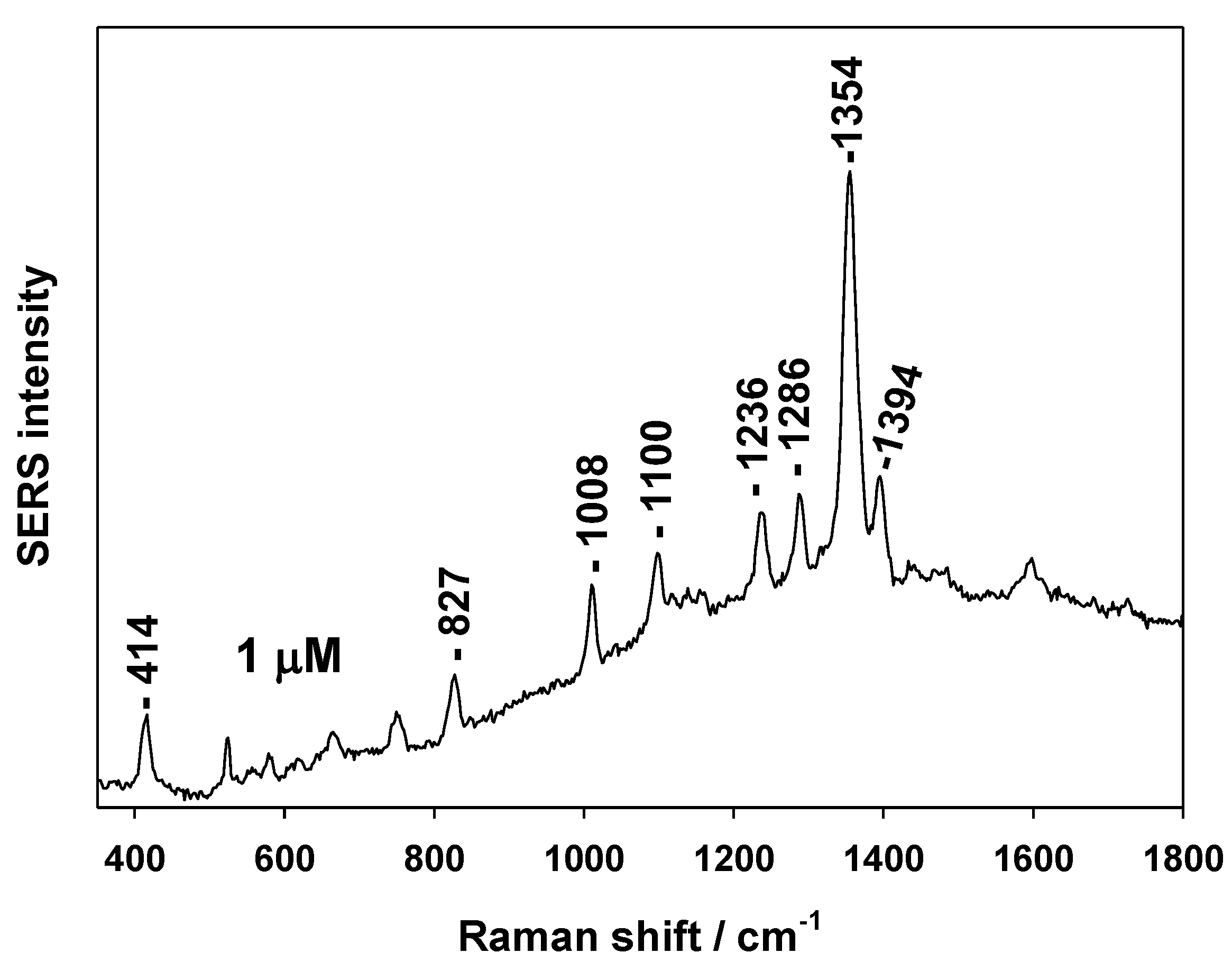
3.2. SERS Testing of AuSiO2 Substrates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlücker, S. Surface-Enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Procházka, M. Surface-Enhanced Raman Spectroscopy: Bioanalytical, Biomolecular and Medical Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy and Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Natan, M.J. Concluding remarks surface enhanced Raman scattering. Faraday Discuss. 2006, 132, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.C.; Milton, M.J.T. Nanostructures and nanostructured substrates for surface—enhanced Raman scattering (SERS). J. Raman Spectrosc. 2008, 39, 1313–1326. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Banholzer, M.J.; Millstone, J.E.; Qin, L.D.; Mirkin, C.A. Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.K.; Andrade, G.F.S.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Patze, S.; Hidi, I.J.; Knipper, R.; Radu, A.I.; Mühlig, A.; Yüksel, S.; Peksa, V.; Weber, K.; Mayerhofer, T.; et al. Plasmonic nanostructures for surface enhanced spectroscopic methods. Analyst 2016, 141, 756–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Tong, W.M.; Stickle, W.F.; Neiman, D.L.; Williams, R.S.; Hunter, L.L.; Talin, A.A.; Li, D.; Brueck, S.R.J. Plasma-Induced Formation of Ag Nanodots for Ultra-High-Enhancement Surface-Enhanced Raman Scattering Substrates. Langmuir 2007, 23, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Zhang, J.; Xie, G.Y.; Liu, Z.F.; Shao, H.B. Cicada wings: A stamp from nature for nanoimprint lithography. Small 2006, 2, 1440–1443. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Van Duyne, R.P. Nanosphere lithography: A materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Technol. A 1995, 13, 1553–1558. [Google Scholar] [CrossRef]
- Fredriksson, H.; Alaverdyan, Y.; Dmitriev, A.; Langhammer, C.; Sutherland, D.S.; Zäch, M.; Kasemo, B. Hole–mask colloidal lithography. Adv. Mat. 2007, 19, 4297–4302. [Google Scholar] [CrossRef]
- Peksa, V.; Lebrušková, P.; Šípová, H.; Štěpánek, J.; Bok, J.; Homola, J.; Procházka, M. Testing gold nanostructures fabricated by hole-mask colloidal lithography as potential substrates for SERS sensors: Sensitivity, signal variability, and the aspect of adsorbate deposition. Phys. Chem. Chem. Phys. 2016, 18, 19613–19620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zhang, X.; Yang, B. Colloidal Self-Assembly Meets Nanofabrication: From Two-Dimensional Colloidal Crystals to Nanostructure Arrays. Adv. Mat. 2010, 22, 4249–4269. [Google Scholar] [CrossRef] [PubMed]
- Dick, L.A.; McFarland, A.D.; Haynes, C.L.; Van Duyne, R.P. Metal Film over Nanosphere (MFON) Electrodes for Surface-Enhanced Raman Spectroscopy (SERS): Improvements in Surface Nanostructure Stability and Suppression of Irreversible Loss. J. Phys. Chem. B 2002, 106, 853–860. [Google Scholar] [CrossRef]
- Baia, M.; Baia, L.; Astilean, S. Gold nanostructured films deposited on polystyrene colloidal crystal templates for surface-enhanced Raman spectroscopy. Chem. Phys. Lett. 2005, 404, 3–8. [Google Scholar] [CrossRef]
- Baia, L.; Baia, M.; Popp, J.; Astilean, S. Gold films deposited over regular arrays of polystyrene nanospheres as highly effective SERS substrates from visible to NIR. J. Phys. Chem. B 2006, 110, 23982–23986. [Google Scholar] [CrossRef] [PubMed]
- Štolcová, L.; Peksa, V.; Proška, J.; Procházka, M. Gold film over very small (107 nm) spheres as efficient substrate for sensitive and reproducible surface-enhanced Raman scattering (SERS) detection of biologically important molecules. J. Raman Spectrosc. 2018, 49, 499–505. [Google Scholar] [CrossRef]
- Haynes, C.L.; Yonzon, C.R.; Zhang, X.; Van Duyne, R.P. Surface-enhanced Raman sensors: Early history and the development of sensors for quantitative biowarfare agent and glucose detection. J. Raman Spectrosc. 2005, 36, 471–484. [Google Scholar] [CrossRef]
- Peksa, V.; Jahn, M.; Štolcová, L.; Schulz, V.; Proška, J.; Procházka, M.; Weber, K.; Cialla-May, D.; Popp, J. Quantitative SERS analysis of azorubine (E 122) in sweet drinks. Anal. Chem. 2015, 87, 2840–2844. [Google Scholar] [CrossRef]
- Hugall, J.T.; Baumberg, J.J. Demonstrating photoluminescence from Au is electronic inelastic light scattering of a plasmonic metal: The origin of SERS backgrounds. Nano Lett. 2015, 15, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A. Some aspects of SERS temporal fluctuations: Analysis of the most intense spectra of hydrogenated amorphous carbon deposited on silver. J. Raman Spectrosc. 2007, 38, 1494–1499. [Google Scholar] [CrossRef]
- Scholes, F.H.; Bendavid, A.; Glenn, F.L.; Critchley, M.; Davis, T.J.; Sexton, B.A. Silica-overcoated substrates for detection of proteins by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2008, 39, 673–678. [Google Scholar] [CrossRef]
- Brosseau, C.L.; Gambardella, A.; Casadio, F.; Grzywacz, C.M.; Wouters, J.; Van Duyne, R.P. Ad-hoc Surface-Enhanced Raman Spectroscopy Methodologies for the Detection of Artist Dyestuffs: Thin Layer Chromatography-Surface Enhanced Raman Spectroscopy and in Situ On the Fiber Analysis. Anal. Chem. 2009, 81, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Lin, M.P.; Chen, S.W.; Lee, P.H.; Li, J.H.; Su, W.F. Surface-enhanced Raman scattering substrate based on a Ag coated monolayer array of SiO2 spheres for organic dye detection. RSC Adv. 2014, 4, 10043–10050. [Google Scholar] [CrossRef]
- Shishido, M.; Kitagawa, D. Preparation of ordered mono-particulate film from colloidal solutions on the surface of water and continuous transcription of film to substrate. Colloids and Surf. A 2007, 311, 32–41. [Google Scholar] [CrossRef]
- Hajduková, N.; Procházka, M.; Molnár, P.; Štěpánek, J. SERRS of free-base porphyrins on immobilized metal gold and silver nanoparticles. Vib. Spectrosc. 2008, 48, 142–147. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouba, K.; Proška, J.; Procházka, M. Gold Film over SiO2 Nanospheres—New Thermally Resistant Substrates for Surface-Enhanced Raman Scattering (SERS) Spectroscopy. Nanomaterials 2019, 9, 1426. https://doi.org/10.3390/nano9101426
Kouba K, Proška J, Procházka M. Gold Film over SiO2 Nanospheres—New Thermally Resistant Substrates for Surface-Enhanced Raman Scattering (SERS) Spectroscopy. Nanomaterials. 2019; 9(10):1426. https://doi.org/10.3390/nano9101426
Chicago/Turabian StyleKouba, Karel, Jan Proška, and Marek Procházka. 2019. "Gold Film over SiO2 Nanospheres—New Thermally Resistant Substrates for Surface-Enhanced Raman Scattering (SERS) Spectroscopy" Nanomaterials 9, no. 10: 1426. https://doi.org/10.3390/nano9101426
APA StyleKouba, K., Proška, J., & Procházka, M. (2019). Gold Film over SiO2 Nanospheres—New Thermally Resistant Substrates for Surface-Enhanced Raman Scattering (SERS) Spectroscopy. Nanomaterials, 9(10), 1426. https://doi.org/10.3390/nano9101426




