From Ethanolamine Precursor Towards ZnO—How N Is Released from the Experimental and Theoretical Points of View
Abstract
1. Introduction
2. Experimental and Theoretical Methods
2.1. Materials and Procedures
2.2. Quantum-Mechanical Calculations
3. Results and Discussion
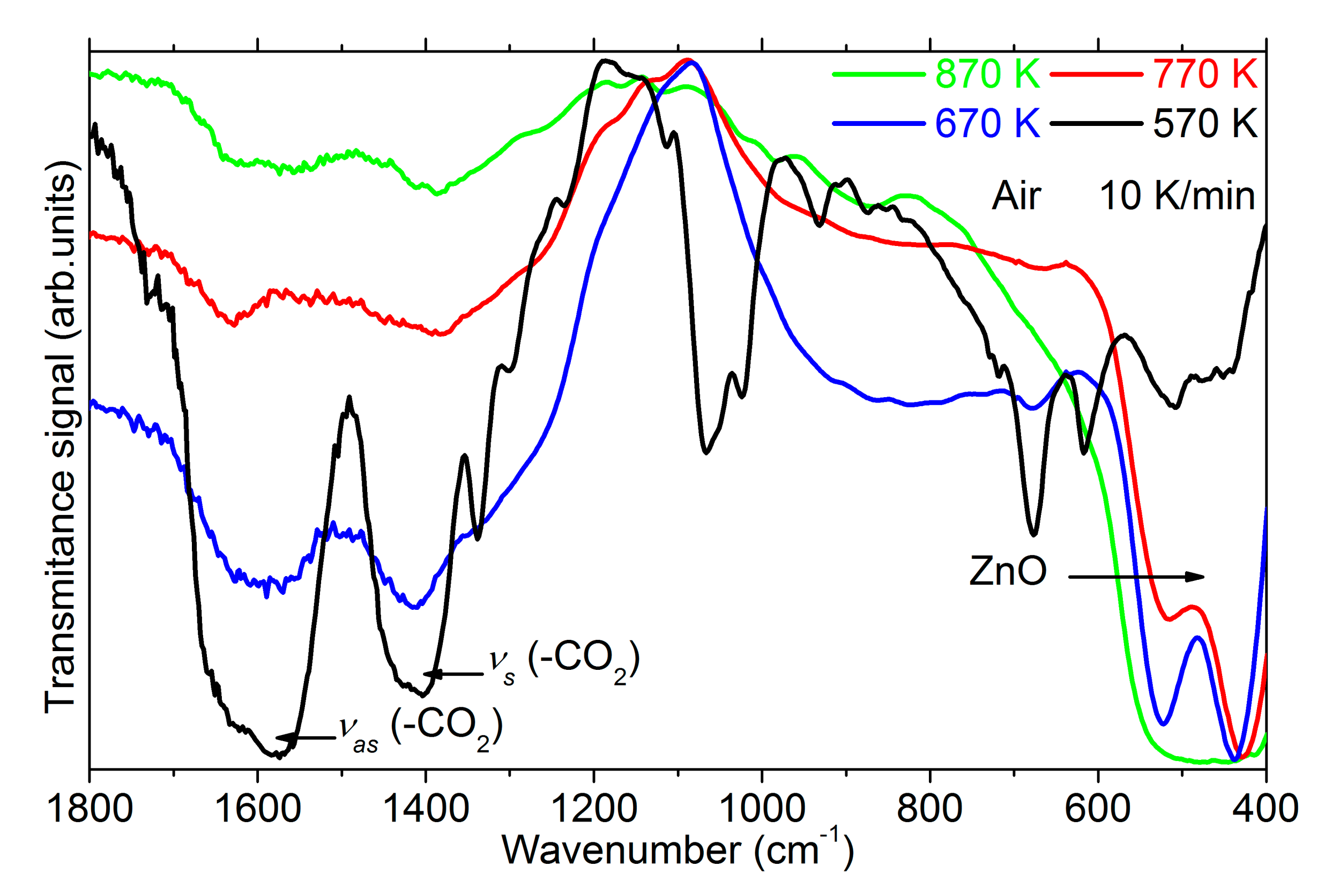
3.1. Experimental Evidence
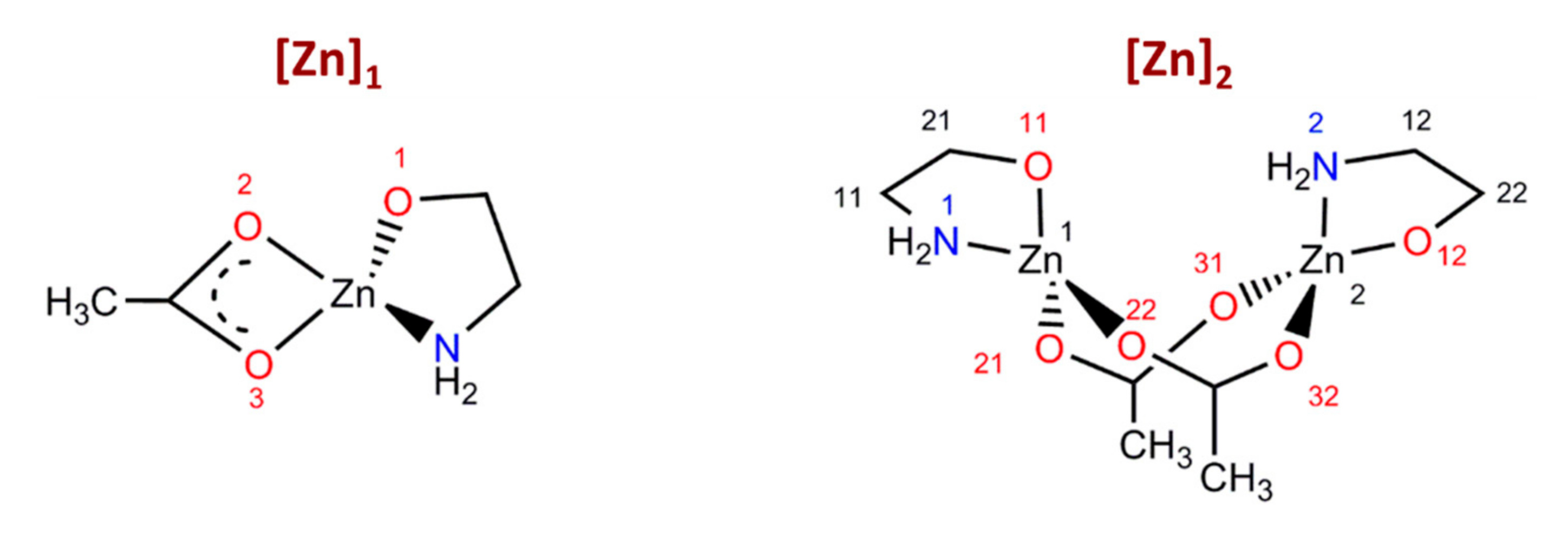
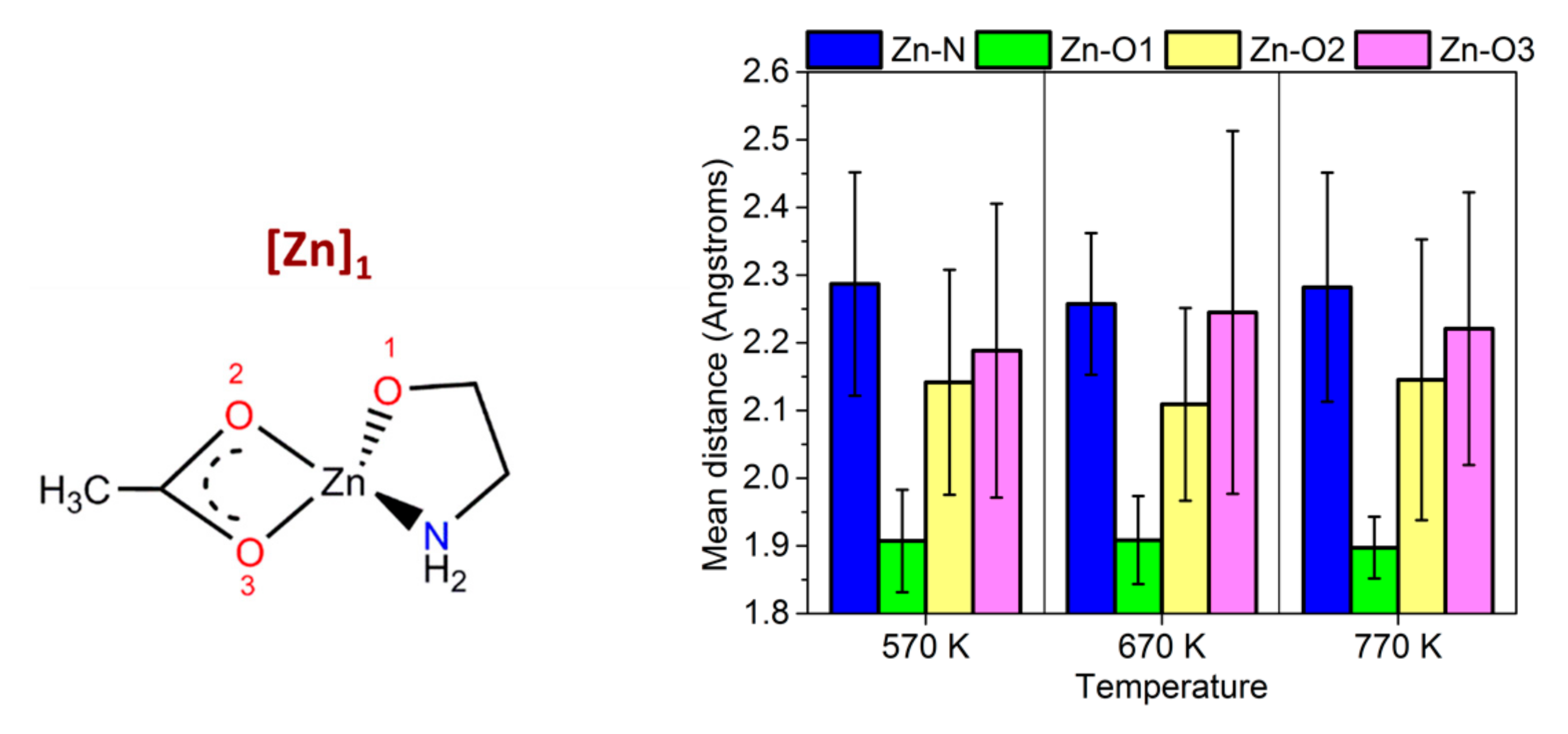
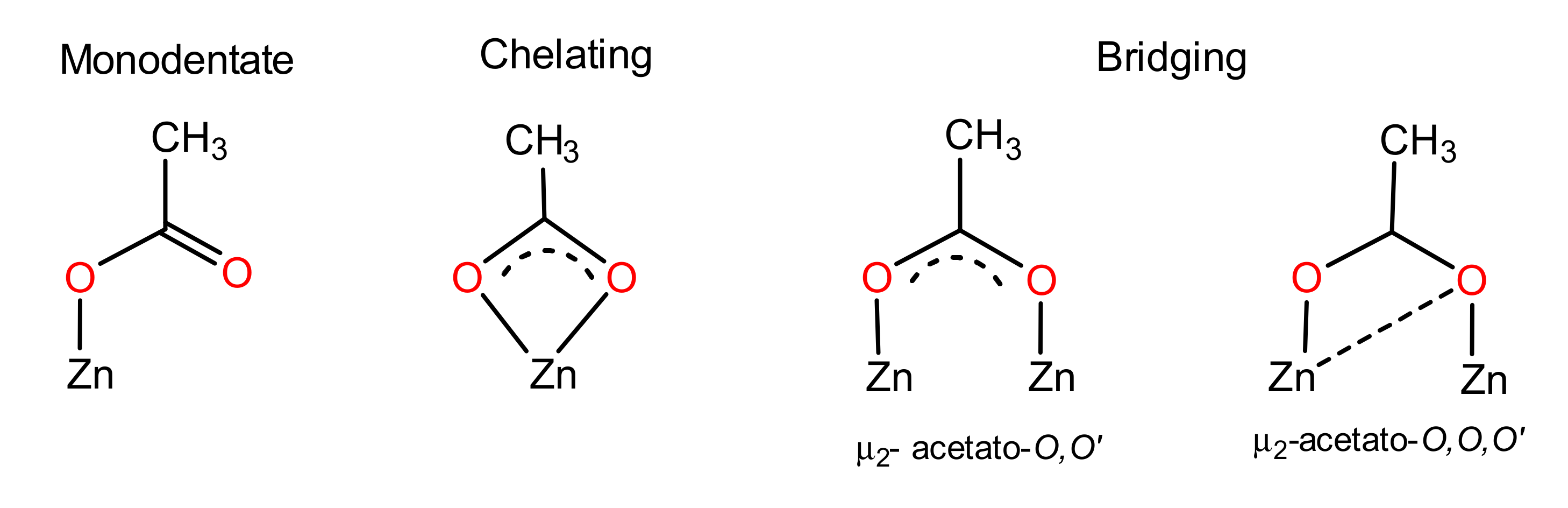
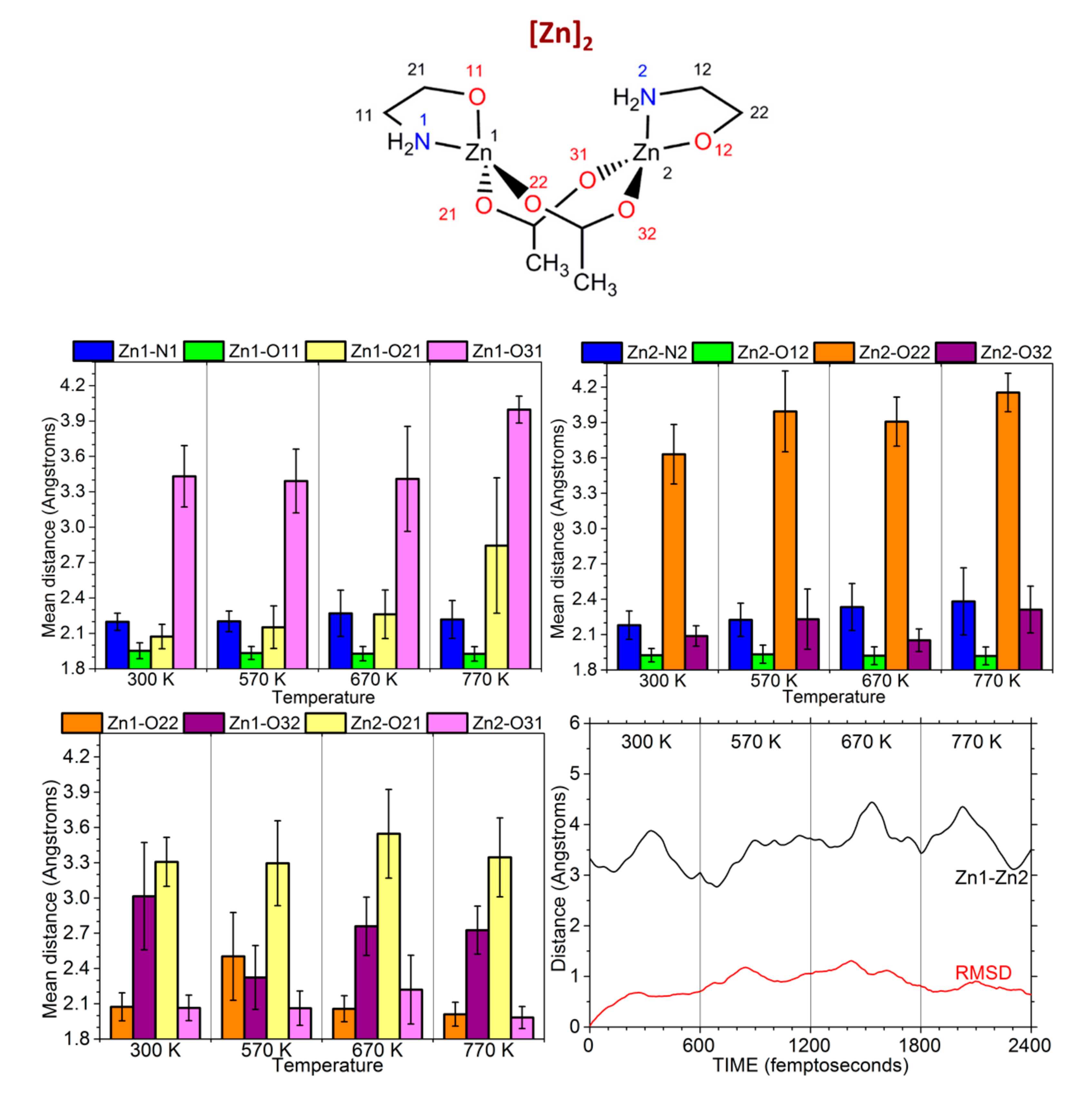
3.2. Car-Parrinello Molecular-Dynamics Simulations
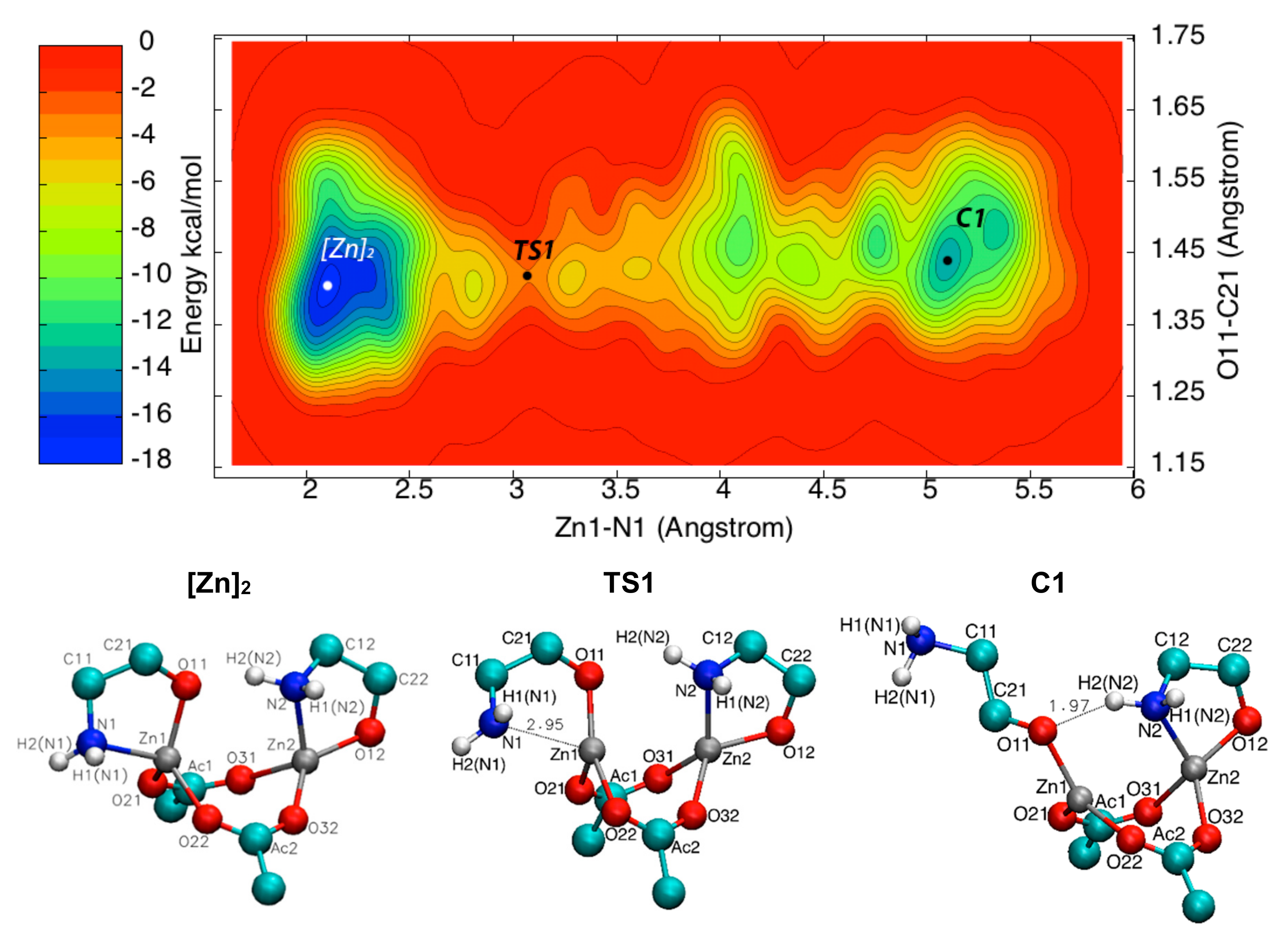
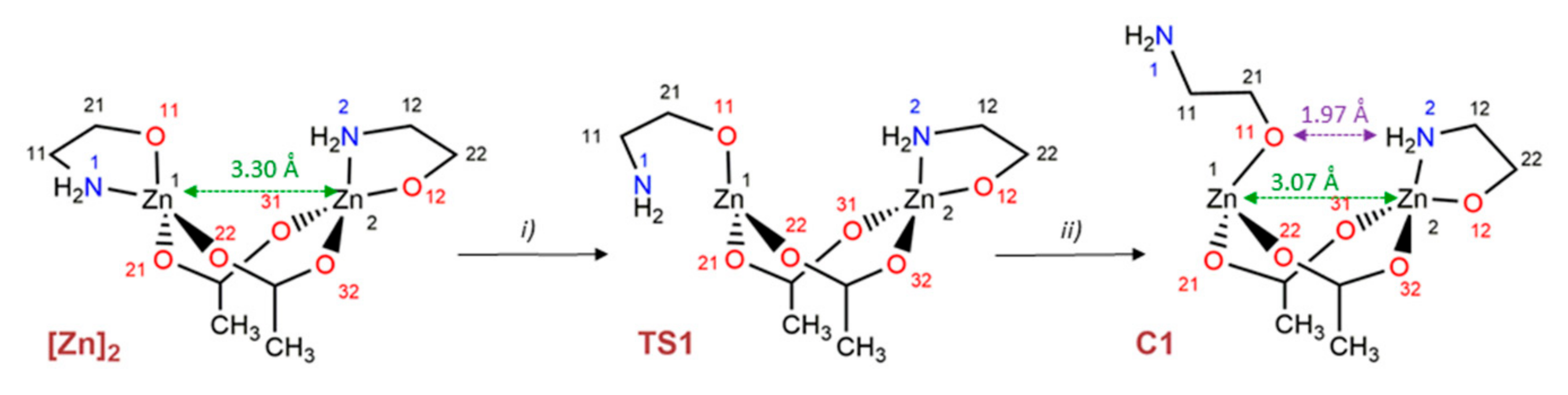
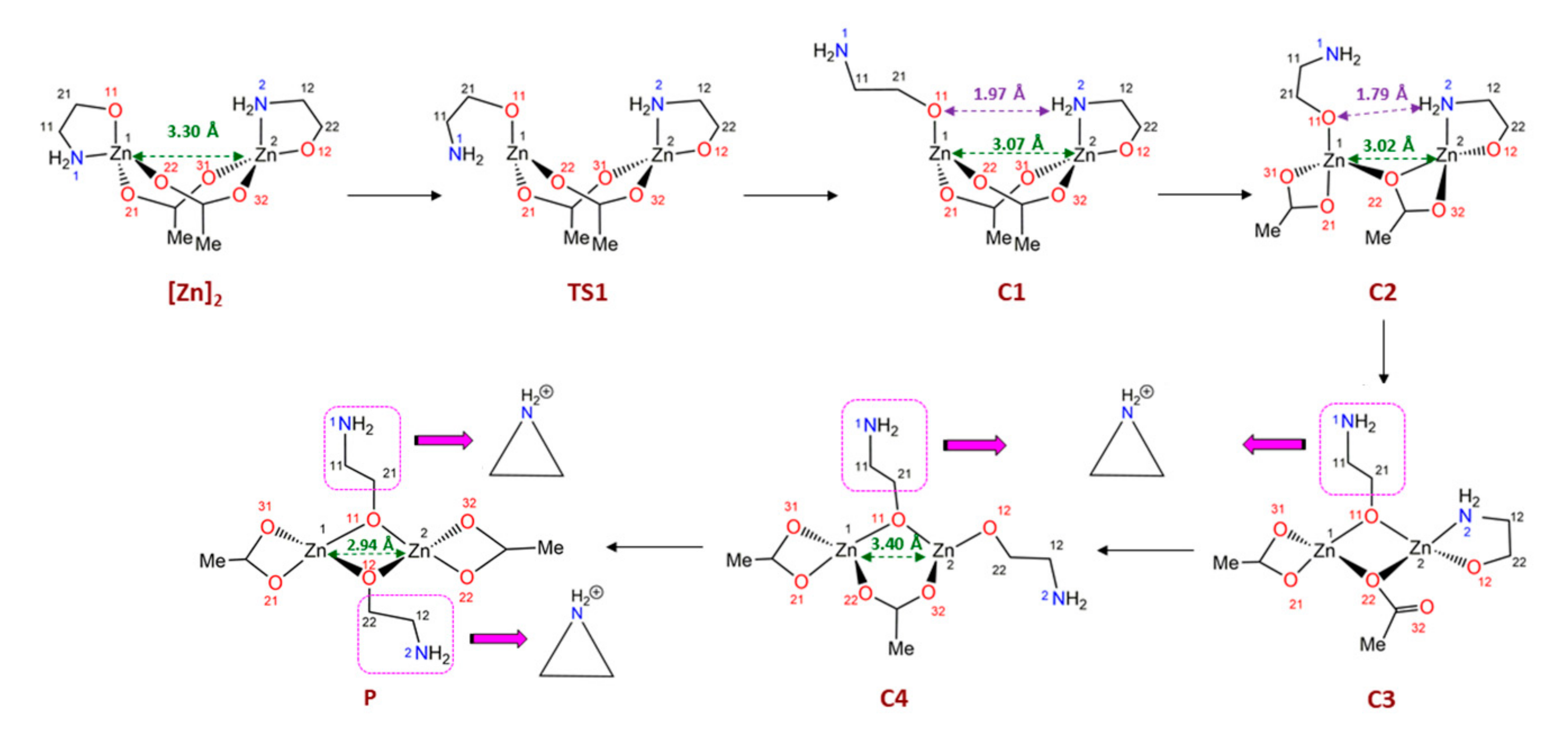
3.3. Metadynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y. ZnO Nanostructures: Fabrication and Applications; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Gómez-Núñez, A.; López, C.; Alonso-Gil, S.; Roura, P.; Vilà, A. Study of a sol-gel precursor and its evolution towards ZnO. Mater. Chem. Phys. 2015, 162, 645–651. [Google Scholar] [CrossRef][Green Version]
- Gómez-Núñez, A.; Roura, P.; López, C.; Vilà, A. Comparison of the thermal decomposition processes of several aminoalcohol-based ZnO inks with one containing ethanolamine. Appl. Surf. Sci. 2016, 381, 48–53. [Google Scholar] [CrossRef][Green Version]
- Foo, K.L.; Hashim, U.; Muhammad, K.; Voon, C.H. Sol–gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic application. Nanoscale Res. Lett. 2014, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Naeem, A. Sol-gel-derived doped ZnO thin films: Processing, properties and applications. In Recent Applications in Sol-Gel Synthesis; Chandra, U., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Prabakaran, E.; Pillay, K. Synthesis of N-doped ZnO nanoparticles with cabbage morphology as a catalyst for the efficient photocatalytic degradation of methylene blue under UV and visible light. RSC Adv. 2019, 9, 7509–7535. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, J.Q. One-step synthesis of nitrogen-doped ZnO nanocrystallites and their properties. Appl. Surf. Sci. 2009, 255, 5656–5661. [Google Scholar] [CrossRef]
- Sun, S.; Chang, X.; Li, X.; Li, Z. Synthesis of N-doped ZnO nanoparticles with improved photocatalytical activity. Ceram. Int. 2013, 39, 5197–5203. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram. Soc. 2015, 3, 305–310. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Nogueira, A.E.; Gonçalves, M.C.P.; Paris, E.C.; Ribeiro, C.; Poirier, G.Y.; Giraldi, T.R. Photoactivity of N-doped ZnO nanoparticles in oxidative and reductive reactions. Appl. Surf. Sci. 2018, 433, 879–886. [Google Scholar] [CrossRef]
- Macías-Sánchez, J.J.; Hinojosa-Reyes, L.; Caballero-Quintero, A.; de la Cruz, W.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-María, J.L. Synthesis of nitrogen-doped ZnO by sol–gel method: Characterization and its application on visible photocatalytic degradation of 2,4-D and picloram herbicides. Photochem. Photobiol. Sci. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Bu, I.Y.; Chen, S. Fully-solution processed ZnO nanowires/cupric heterojunction for low-cost photovoltaic applications. Optik 2017, 130, 427–432. [Google Scholar] [CrossRef]
- Gómez-Núñez, A.; Alonso-Gil, S.; López, C.; Roura, P.; Vilà, A. Role of ethanolamine on the stability of a sol-gel ZnO ink. J. Phys. Chem. C 2017, 121, 23839–23846. [Google Scholar] [CrossRef]
- Grossner, U.; Karazhanov, S.Z.H.; Ravindran, P. Computational materials science—Fundamentals and application to ZnO and GaN. In Wide Band Gap Materials and New Developments; Research Signpost: Trivandrum, India, 2006; pp. 165–186. [Google Scholar]
- Wu, P. Digital materials design: Computational methodologies as a discovery tool. MRS Bull. 2006, 31, 995–998. [Google Scholar] [CrossRef]
- Nagare, B.J.; Chavan, S.; Bambole, V. Study of electronic and optical properties of ZnO clusters using TD-DFT method. Mater. Res. Express 2017, 4, 106304. [Google Scholar] [CrossRef]
- Azzaz, Y.; Kacimi, S.; Zaoui, A.; Bouhafs, B. Electronic properties and stability of ZnO from computational study. Phys. B Condens. Matter 2008, 403, 3154–3158. [Google Scholar] [CrossRef]
- Viñes, F.; Illas, F. Electronic structure of stoichiometric and reduced ZnO from periodic relativistic all electron hybrid density functional calculations using numeric atom-centered orbitals. J. Comput. Chem. 2017, 38, 523–529. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Ruiz, J.; Ruiz, E.; Aravena, D.; Seco, J.M.; Colacio, E. Increasing the effective energy barrier promoted by the change of a counteranion in a Zn-Dy-Zn SMM: Slow relaxation via the second excited state. Chem. Commun. 2015, 51, 12353–12356. [Google Scholar] [CrossRef]
- Pandey, A.; Scherich, H.; Drabold, D.A. Density functional theory model of amorphous zinc oxide (a-ZnO) and a-X0.375Z0.625O (X = Al, Ga and In). J. Non Cryst. Solids 2017, 455, 98–101. [Google Scholar] [CrossRef][Green Version]
- Mei, Y.; Sherman, D.M.; Liu, W.; Etschmann, B.; Testemale, D.; Brugger, J. Zinc complexation in chloride-rich hydrothermal fluids (25–600 °C): A thermodynamic model derived from Ab initio molecular dynamics. Geochim. Cosmochim. Acta 2015, 150, 265–284. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, P. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Laio, A.; Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fan, X.; Fan, Y.; Wen, Y. Reactive molecular dynamics simulation of the pyrolysis and combustion of benzene: Ultrahigh temperature and oxygen-induced enhancement of initiation pathways and their effect on carbon black generation. RSC Adv. 2015, 5, 43695–43704. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Cheng, X.; Li, X. Reactive molecular dynamics simulation on thermal decomposition of n-heptane. Chin. J. Chem. Phys. 2013, 26, 211–219. [Google Scholar] [CrossRef]
- Chang, J.; Lian, P.; Wei, D.-Q.; Chen, X.-R.; Zhang, Q.-M.; Gong, Z.-Z. Thermal decomposition of the solid phase of nitromethane: Ab initio molecular dynamics simulations. Phys. Rev. Lett. 2010, 105, 188302. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Liu, J.; Wang, Z.; Gong, X.; Guo, L.; Song, W. Pyrolysis of Liulin coal simulated by GPU-based ReaxFF MD with cheminformatics analysis. Energy Fuels 2014, 28, 522–534. [Google Scholar] [CrossRef]
- Ramin, L.; Assadi, M.H.N.; Sahajwalla, V. High-density polyethylene degradation into low molecular weight gases at 1823 K: An atomistic simulation. J. Anal. Appl. Pyrolysis 2014, 110, 318–321. [Google Scholar] [CrossRef]
- Jee, C.; Guo, Z.; Stoliarov, S.; Nyden, M. Experimental and molecular dynamics studies of the thermal de-composition of a polyisobutylene binder. Acta Mater. 2006, 54, 4803–4813. [Google Scholar] [CrossRef]
- Mosey, N.J.; Woo, T.K. Finite temperature structure and dynamics of zinc dialkyldithiophosphate wear inhibitors: A density functional theory and ab initio molecular dynamics study. J. Phys. Chem. A 2003, 107, 5058–5070. [Google Scholar] [CrossRef]
- Alonso-Gil, S.; Males, A.; Fernandes, P.Z.; Williams, S.J.; Davies, G.J.; Rovira, C. Computational design of experiment unveils the conformational reaction coordinate of GH125 α-mannosidases. J. Am. Chem. Soc. 2017, 139, 1085–1088. [Google Scholar] [CrossRef]
- CPMD. Copyright MPI Für Festkörperforschung, Stuttgart 1997–2001. Available online: http://www.cpmd.org/ (accessed on 25 March 2017).
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 1985, 81, 511–519. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation. A Systematic Approach; CRC Press LLC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day Inc.: San Francisco, CA, USA, 1977. [Google Scholar]
- Van Werde, K.; Mondelars, D.; Vanhoyland, G.; Nelis, D.; van Bael, M.K.; Mullens, J.; van Poucke, L.C. Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J. Mater. Sci. 2002, 37, 81–88. [Google Scholar] [CrossRef]
- Mihaiu, S.; Szelágyi, I.M.; Atkinson, I.; Mocioiu, O.C.; Hunyadi, D.; Pandele-Cusu, J.; Toader, A.; Munteanu, C.; Boyadjiev, S.; Madarász, J.; et al. Thermal study on the synthesis of the doped ZnO to be used in TCO films. J. Therm. Anal. Calorim. 2016, 124, 71–80. [Google Scholar] [CrossRef][Green Version]
- Bouchoux, G.; Choret, N.; Berruyer-Penaud, F.; Flammang, R. Thermochemistry and unimolecular reactivity of protonated α,ω-aminoalcohols in the gas phase. Int. J. Mass Spectrom. 2002, 217, 195–230. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Przybyl, B.; Janczak, J. Structural characterisation and DFT calculations of three new complexes of zinc phthalocyanine with n-alkylamines. Dyes Pigment. 2014, 100, 247–254. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Mamleeva, E.; Yu, I.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. The role of nitrogen donors in zinc catalysts for lactide ring-opening polymerization. Inorg. Chem. 2016, 55, 9445–9453. [Google Scholar] [CrossRef]
- Boyle, T.J.; Bunge, S.D.; Andrews, N.L.; Matzen, L.E.; Sieg, K.; Rodriguez, M.A.; Headley, T.J. Precursor structural influences on the final ZnO nanoparticle morphology from a novel family of structurally characterized zinc alkoxy alkyl precursors. Chem. Mater. 2004, 16, 3279–3288. [Google Scholar] [CrossRef]
- Zelga, K.; Leszczynski, M.; Justyniak, I.; Kornowicz, A.; Cabaj, M.; Wheatley, A.E.H.; Lewinski, J. Synthesis, structure and unique reactivity of the ethylzinc derivative of a bicyclic guanidine. Dalton Trans. 2012, 41, 5934–5938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xi, X.; Ye, C.; Gong, T.; Yang, Z.; Cui, Y. Chiral metal–organic frameworks bearing free carboxylic acids for organocatalyst encapsulation. Angew. Chem. Int. Ed. 2014, 53, 13821–13825. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, C.A.; Redshaw, C.; Elsegood, M.R.J.; Wright, V.E.; Yoshizawa, A.; Yamato, T. Iron(III) and Zinc(II) calixarene complexes: Synthesis, structural studies, and use as procatalysts for ε-caprolactone polymerization. Chem. Asian J. 2010, 5, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Kingsley, A.J.; Kociok-Köhn, G.; Molloy, K.C.; Sudlow, A.L. Inorganic and organozinc fluorocarboxylates: Synthesis, structure and materials chemistry. Inorg. Chem. 2013, 52, 5515–5526. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, K.; Justyniak, I.; Bury, W.; Grzonka, J.; Kaszkur, Z.; Makolski, L.; Dutkiewicz, M.; Lewalska, A.; Krajewska, E.; Kubicki, D.; et al. Tert-Butyl(tert-butoxy)zinc hydroxides: Hybrid models for single-source precursors of ZnO nanocrystals. Chem. Eur. J. 2015, 21, 5488–5495. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Eckhardt, B.; Inoue, S.; Irran, E.; Driess, M. Facile and efficient reduction of ketones in the presence of zinc catalysts modified by phenol ligands. Chem. Asian J. 2010, 5, 2027–2035. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Katsoulakou, E.; Kaplanis, M.; Bekiari, V.; Terzis, A.; Raptopoulou, C.P.; Manessi-Zoupa, E.; Perlepes, S.P. A tetrahedron in a cube: A dodecanuclear ZnII benzoate cluster from the use of 2-pyridinealdoxime. Dalton Trans. 2010, 39, 4492–4494. [Google Scholar] [CrossRef]
- Dreher, M.A.; Krumm, M.; Lizandara-Pueyo, C.; Polarz, S. Chromium containing zinc oxide materials from organobimetallic precursors. Dalton Trans. 2010, 39, 2232–2238. [Google Scholar] [CrossRef]
- Lewinski, J.; Suwala, K.; Kubisiak, M.; Ochal, V.; Justyniak, I.; Lipkowski, J. Oxygenation of a Me2Zn/α-diimine system: A unique zinc methylperoxide cluster and evidence for its sequential decomposition pathways. Angew. Chem. Int. Ed. 2008, 47, 7888–7891. [Google Scholar] [CrossRef]
- Orlov, A.; Roy, A.; Lehmann, M.; Driess, M.; Polarz, S. Organometallics meet colloid chemistry: A case study in three phases based on molecular carbonyl precursors containing zinc and manganese. J. Am. Chem. Soc. 2007, 129, 371–375. [Google Scholar] [CrossRef][Green Version]
- Woodward, J.D.; Backov, R.V.; Abboud, K.A.; Talham, D.R. Monomers, chains, ladders, and two-dimensional sheets: Structural diversity in six new compounds of Zn(II) with 4,4′-bipyridine. Polyhedron 2006, 25, 2605–2615. [Google Scholar] [CrossRef]
- Phuengphai, P.; Youngme, S.; Kongsaeree, P.; Pakawatchai, C.; Chaichit, N.; Teat, S.J.; Gámez, P.; Reedijk, J. Drastic steric effects from, respectively, a hydrogen, a methyl and an ethyl group on the coordination network of a zinc(II)–4,4′-bipyridine–carboxylato ternary system. CrystEngComm 2009, 11, 1723–1732. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A.; Crochet, A.; Batten, S.R. Do perfluoroarene…arene and C–H…F interactions make a difference to the structures of 4,2′:6′,4″-terpyridine-based coordination polymers? CrystEngComm 2013, 15, 10068–10078. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhir, A.; Pradeep, C.P. Multifunctional Zn(II) complexes: Photophysical properties and catalytic transesterification toward biodiesel synthesis. Inorg. Chem. 2016, 55, 7492–7500. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Ballistreri, F.P.; Gangemi, C.M.A.; Toscano, R.M.; Tomaselli, G.A.; Pappalardo, A.; Sfrazzetto, G.T. Chiral Zn-salen complexes: A new class of fluorescent receptors for enantiodiscrimination of chiral amines. New J. Chem. 2017, 41, 911–915. [Google Scholar] [CrossRef]
- Karambelkar, V.V.; Krishnamurthy, D.; Stern, C.L.; Zakharov, L.N.; Rheingold, A.L.; Goldberg, D.P. A new bis(imidazolyl)(alkylthiolate) tripodal ligand and the spontaneous formation of a disulfide-linked, hydroxo-bridged dinuclear zinc complex. Chem. Commun. 2002, 23, 2772–2773. [Google Scholar] [CrossRef]
- Johnson, A.L.; Hollingsworth, N.; Kociok-Köhn, G.; Molloy, K.C. Organozinc aminoalcoholates: Synthesis, structure, and materials chemistry. Inorg. Chem. 2008, 47, 12040–12048. [Google Scholar] [CrossRef] [PubMed]
- Hsuan-Ying, C.; Hui-Yi, T.; Chu-Cieh, L. Ring-opening polymerization of lactides initiated by zinc alkoxides derived from NNO-tridentate ligands. Macromolecules 2008, 39, 3745–3752. [Google Scholar]
- Umayal, M.; Mugesh, G. Metallo-β-lactamase and phosphotriesterase activities of some zinc(II) complexes. Inorg. Chim. Acta 2011, 372, 353–361. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, C.Y.; Huang, B.H.; Lin, C.C.; Ko, B.T. Synthesis and structural determination of zinc complexes based on an anilido-aldimine ligand containing an O-donor pendant arm: Zinc alkoxide derivative as an efficient initiator for ring-opening polymerization of cyclic esters. Dalton Trans. 2013, 42, 10875–10884. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.U.; Barends, S.; Özalp-Yaman, S.; de Hoog, P.; Casellas, H.; Teat, S.J.; Massera, C.; Lutz, M.; Spek, A.L.; van Wezel, G.P.; et al. Unique ligand-based oxidative DNA cleavage by zinc(II) complexes of hpyramol and hpyrimol. Chem. Eur. J. 2007, 13, 5213–5222. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Núñez, A.; Alonso-Gil, S.; López, C.; Roura-Grabulosa, P.; Vilà, A. From Ethanolamine Precursor Towards ZnO—How N Is Released from the Experimental and Theoretical Points of View. Nanomaterials 2019, 9, 1415. https://doi.org/10.3390/nano9101415
Gómez-Núñez A, Alonso-Gil S, López C, Roura-Grabulosa P, Vilà A. From Ethanolamine Precursor Towards ZnO—How N Is Released from the Experimental and Theoretical Points of View. Nanomaterials. 2019; 9(10):1415. https://doi.org/10.3390/nano9101415
Chicago/Turabian StyleGómez-Núñez, Alberto, Santiago Alonso-Gil, Concepción López, Pere Roura-Grabulosa, and Anna Vilà. 2019. "From Ethanolamine Precursor Towards ZnO—How N Is Released from the Experimental and Theoretical Points of View" Nanomaterials 9, no. 10: 1415. https://doi.org/10.3390/nano9101415
APA StyleGómez-Núñez, A., Alonso-Gil, S., López, C., Roura-Grabulosa, P., & Vilà, A. (2019). From Ethanolamine Precursor Towards ZnO—How N Is Released from the Experimental and Theoretical Points of View. Nanomaterials, 9(10), 1415. https://doi.org/10.3390/nano9101415





