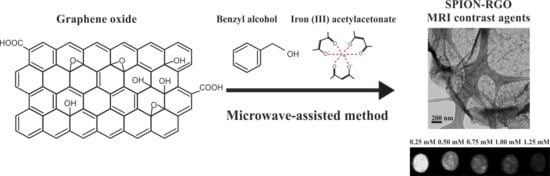
Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Graphene Oxide
2.3. Decoration of RGO with SPION
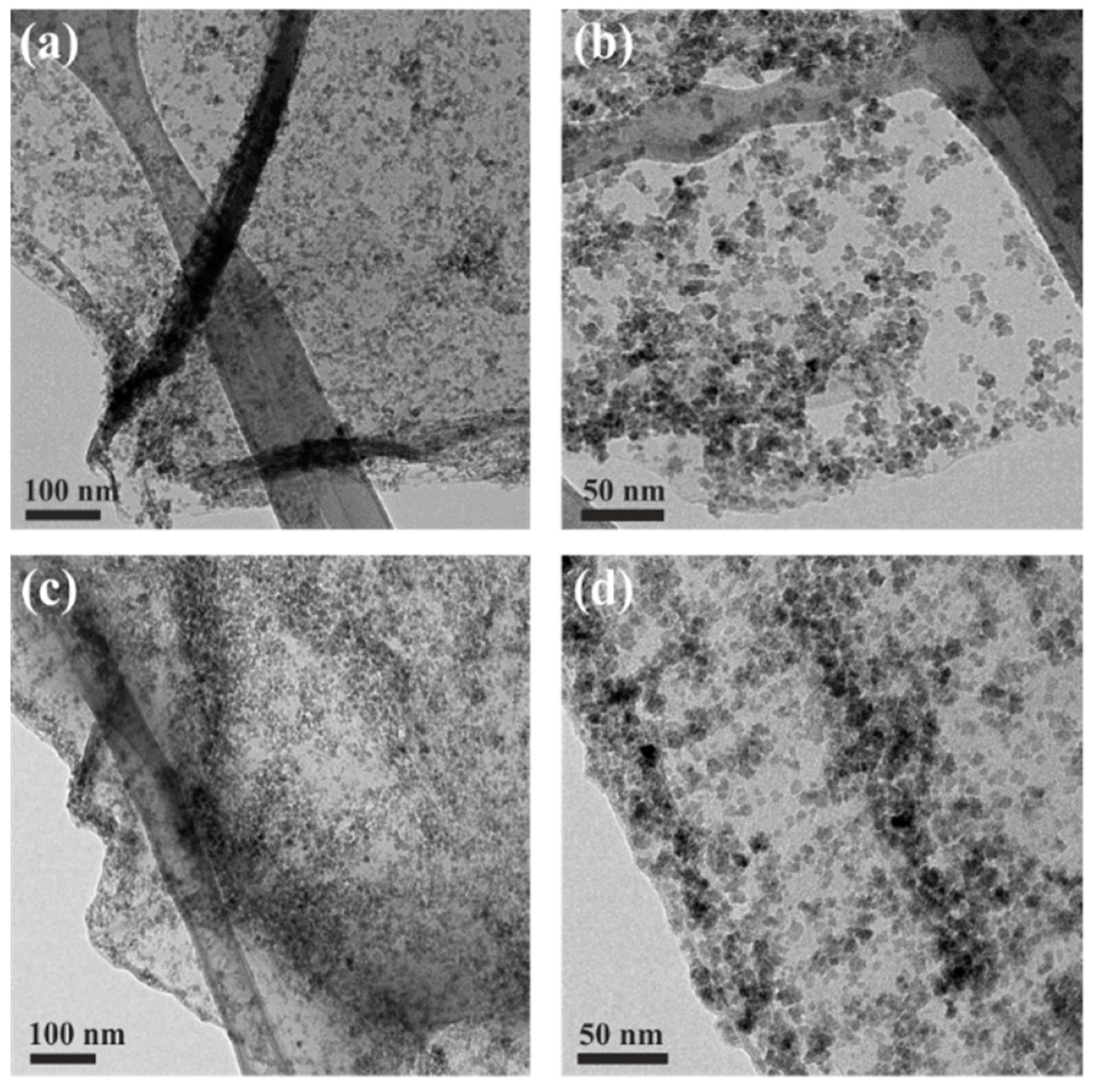
2.4. Characterization
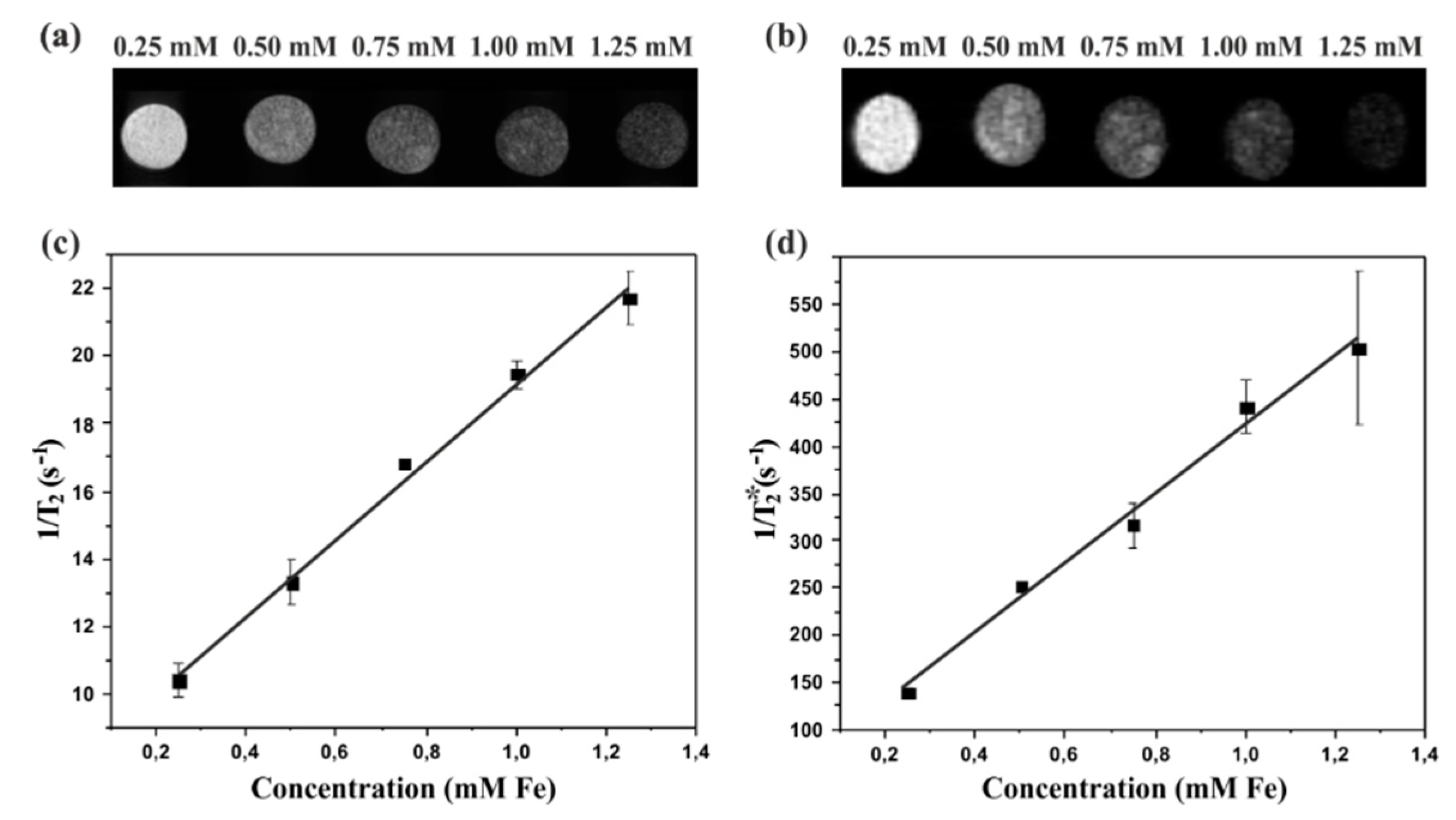
2.5. In Vitro MRI studies
2.6. In Vitro Stability Assay
2.7. In Vitro Modified Lactate Dehydrogenase (LDH) Assay
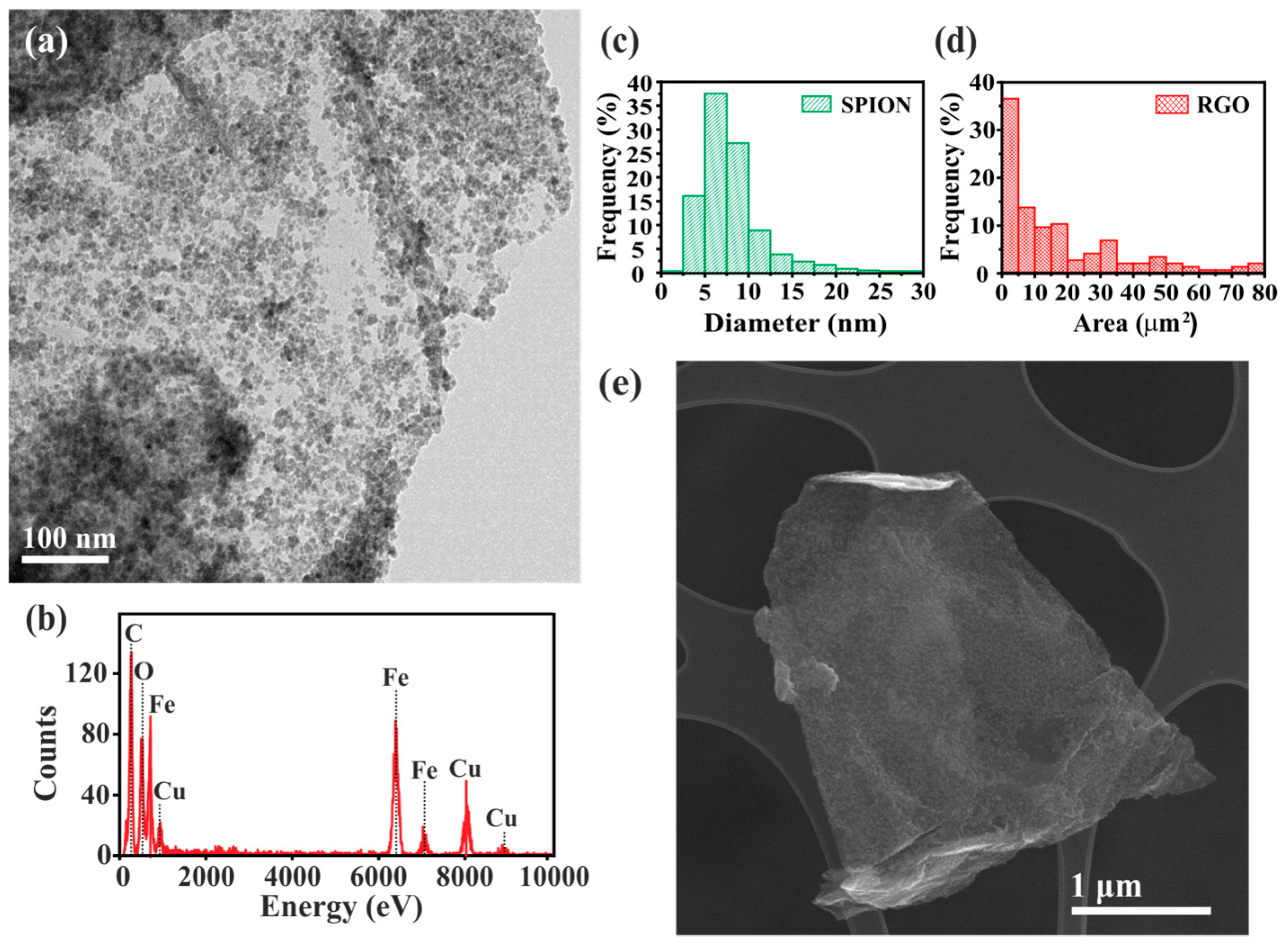
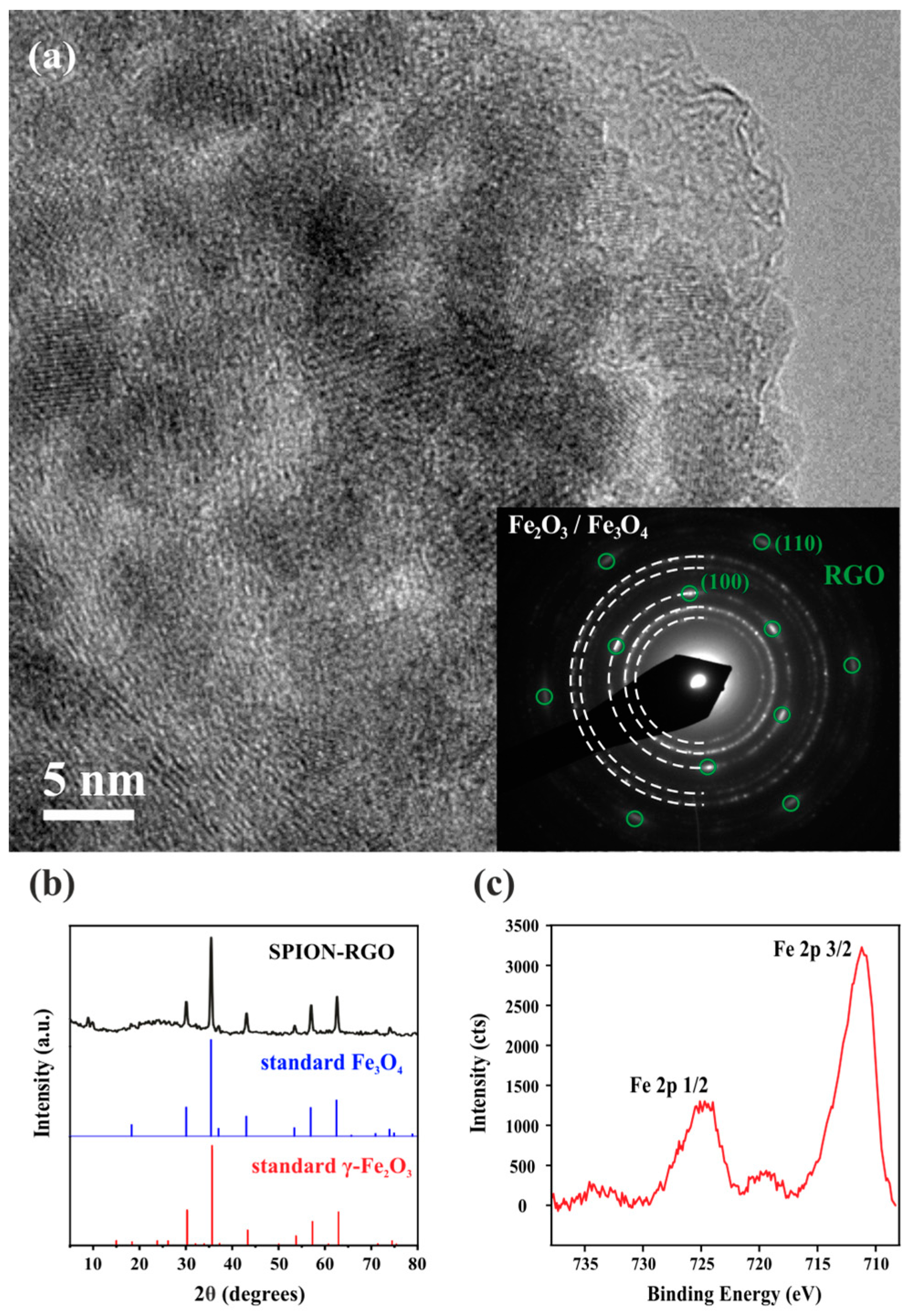
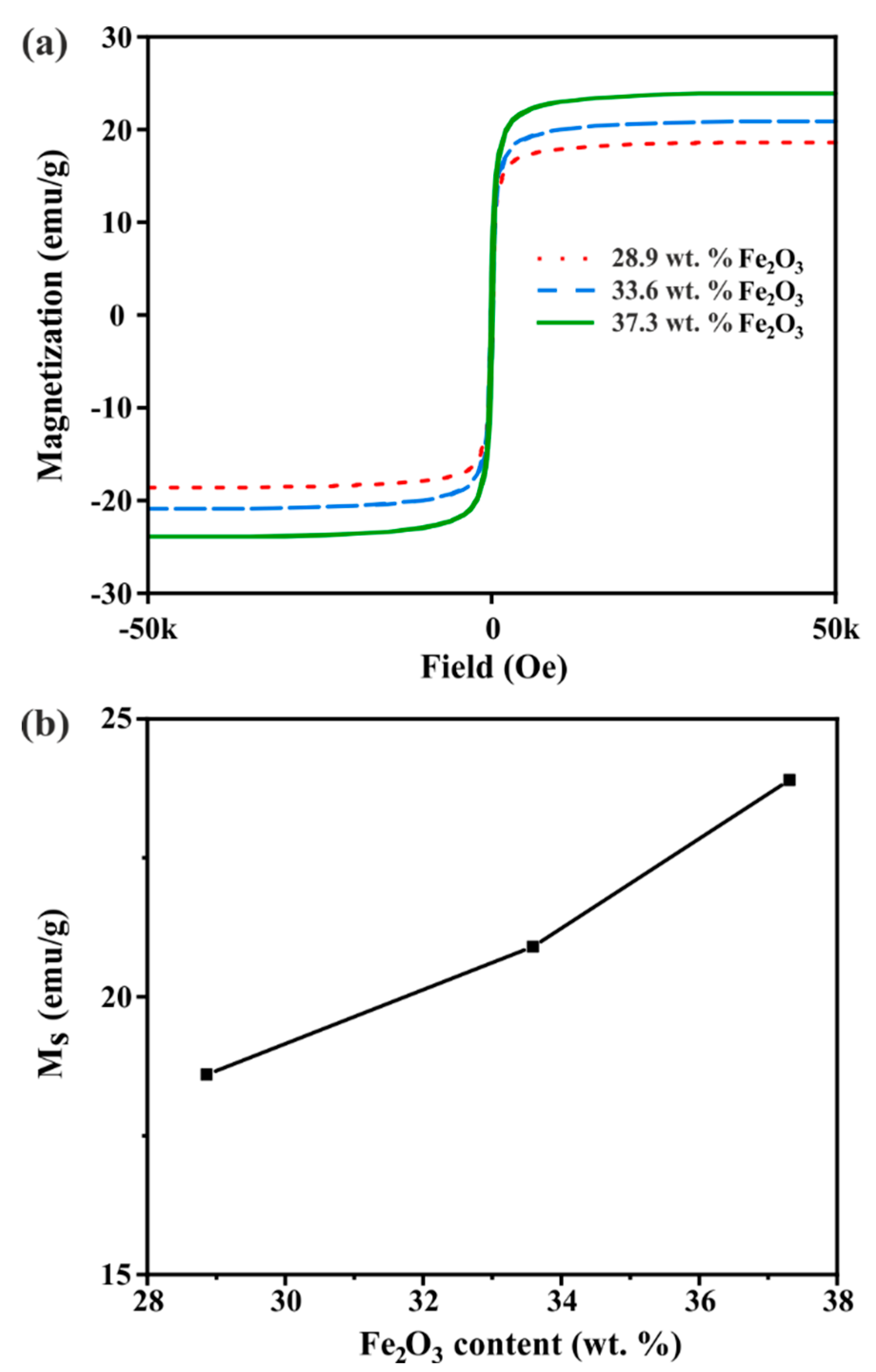
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fatima, H.; Kim, K.S. Iron-based magnetic nanoparticles for magnetic resonance imaging. Adv. Powder Technol. 2018, 29, 2678–2685. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Yim, H.; Seo, S.; Na, K. MRI Contrast Agent-Based Multifunctional Materials: Diagnosis and Therapy. J. Nanomater. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Peng, E.; Wang, F.; Xue, J.M. Nanostructured magnetic nanocomposites as MRI contrast agents. J. Mater. Chem. B 2015, 3, 2241–2276. [Google Scholar] [CrossRef]
- Zhang, Q.; Rajan, S.S.; Tyner, K.M.; Casey, B.J.; Dugard, C.K.; Jones, Y.; Paredes, A.M.; Clingman, C.S.; Howard, P.C.; Goering, P.L. Effects of iron oxide nanoparticles on biological responses and MR imaging properties in human mammary healthy and breast cancer epithelial cells. J. Biomed. Mater. Res. B 2016, 104, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C 2013, 33, 4485–4497. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef]
- Tao, C.; Zheng, Q.; An, L.; He, M.; Lin, J.; Tian, Q.; Yang, S. T1-Weight Magnetic Resonance Imaging Performances of Iron Oxide Nanoparticles Modified with a Natural Protein Macromolecule and an Artificial Macromolecule. Nanomaterials 2019, 9, 170. [Google Scholar] [CrossRef]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133. [Google Scholar] [CrossRef]
- Alazmi, A.; Singaravelu, V.; Batra, N.M.; Smajic, J.; Alyami, M.; Khashab, N.M.; Costa, P.M.F.J. Cobalt ferrite supported on reduced graphene oxide as a T2 contrast agent for magnetic resonance imaging. RSC Adv. 2019, 9, 6299–6309. [Google Scholar] [CrossRef]
- Tao, C.; Chen, Y.; Wang, D.; Cai, Y.; Zheng, Q.; An, L.; Lin, J.; Tian, Q.; Yang, S. Macromolecules with Different Charges, Lengths, and Coordination Groups for the Coprecipitation Synthesis of Magnetic Iron Oxide Nanoparticles as T1 MRI Contrast Agents. Nanomaterials 2019, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tang, Y.; Liu, T.; Chen, Q.; Zhou, X.; Wang, N.; Ma, M.; Cheng, Y.; Chen, H. Engineering graphene oxide with ultrasmall SPIONs and smart drug release for cancer theranostics. Chem. Commun. 2019, 55, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Soc. Nephrol. 2006, 17, 2359. [Google Scholar] [CrossRef]
- Wang, G.; Chen, G.; Wei, Z.; Dong, X.; Qi, M. Multifunctional Fe3O4/graphene oxide nanocomposites for magnetic resonance imaging and drug delivery. Mater. Chem. Phys. 2013, 141, 997–1004. [Google Scholar] [CrossRef]
- Srivastava, S.; Awasthi, R.; Tripathi, D.; Rai, M.K.; Agarwal, V.; Agrawal, V.; Gajbhiye, N.S.; Gupta, R.K. Magnetic-Nanoparticle-Doped Carbogenic Nanocomposite: An Effective Magnetic Resonance/Fluorescence Multimodal Imaging Probe. Small 2012, 8, 1099–1109. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.; Wu, B.; Wang, Z.; Cao, H.; Fu, C.; Jia, N. Magnetic Fe3O4-Reduced Graphene Oxide Nanocomposites-Based Electrochemical Biosensing. Nano Micro Lett. 2014, 6, 258–267. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Chi, X.; Bao, J.; Yang, L.; Zhao, W.; Chen, Z.; Wang, X.; Chen, X.; Gao, J. Engineered Iron-Oxide-Based Nanoparticles as Enhanced T1 Contrast Agents for Efficient Tumor Imaging. ACS Nano 2013, 7, 3287–3296. [Google Scholar] [CrossRef]
- Sherwood, J.; Lovas, K.; Rich, M.; Yin, Q.; Lackey, K.; Bolding, M.S.; Bao, Y. Shape-dependent cellular behaviors and relaxivity of iron oxide-based T1 MRI contrast agents. Nanoscale 2016, 8, 17506–17515. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, H.; Wang, M.; Huang, C.; Yang, D.; Jia, N. Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Mater. Sci. Eng. C 2017, 78, 817–825. [Google Scholar] [CrossRef]
- Zan, P.; Yang, C.; Sun, H.; Zhao, L.; Lv, Z.; He, Y. One-pot fabricating Fe3O4/graphene nanocomposite with excellent biocompatibility and non-toxicity as a negative MR contrast agent. Colloids Surf. B 2016, 145, 208–216. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of Aminodextran-Coated Fe3O4 Nanoparticles and Graphene Oxide for Cellular Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Song, X.; Gu, X.; Sun, H.; Fu, C.; Meng, F. Synthesis of Superparamagnetic Iron Oxide Nanoparticles Modified with MPEG-PEI via Photochemistry as New MRI Contrast Agent. J. Nanomater. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage Endocytosis of Superparamagnetic Iron Oxide Nanoparticles: Mechanisms and Comparison of Ferumoxides and Ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef]
- Nuria, A.; Alejandro, C.; Maurizio, P. Recent Advances of Graphene-based Hybrids with Magnetic Nanoparticles for Biomedical Applications. Curr. Med. Chem. 2017, 24, 529–536. [Google Scholar]
- Abbas, M.; Zhang, J.; Lin, K.; Chen, J. Fe3O4 nanocubes assembled on RGO nanosheets: Ultrasound induced in-situ and eco-friendly synthesis, characterization and their excellent catalytic performance for the production of liquid fuel in Fischer-tropsch synthesis. Ultrason. Sonochem. 2018, 42, 271–282. [Google Scholar] [CrossRef]
- Cabana, L.; Bourgognon, M.; Wang, J.T.W.; Protti, A.; Klippstein, R.; de Rosales, R.T.M.; Shah, A.M.; Fontcuberta, J.; Tobías-Rossell, E.; Sosabowski, J.K.; et al. The Shortening of MWNT-SPION Hybrids by Steam Treatment Improves Their Magnetic Resonance Imaging Properties In Vitro and In Vivo. Small 2016, 12, 2893–2905. [Google Scholar] [CrossRef]
- Cortijo-Campos, S.; Álvarez-Fraga, L.; Gonçalves, G.; Vila, M.; Álvarez, P.; Menéndez, R.; de Andrés, A.; Prieto, C. In-situ carboxylation of graphene by chemical vapor deposition growth for biosensing. Carbon 2019, 141, 719–727. [Google Scholar] [CrossRef]
- Verdanova, M.; Rezek, B.; Broz, A.; Ukraintsev, E.; Babchenko, O.; Artemenko, A.; Izak, T.; Kromka, A.; Kalbac, M.; Hubalek Kalbacova, M. Nanocarbon Allotropes—Graphene and Nanocrystalline Diamond—Promote Cell Proliferation. Small 2016, 12, 2499–2509. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Gonçalves, G.; Cicuéndez, M.; Mariz, I.; Silva, V.S.; Oliveira, H.; Campos, F.; Vieira, S.I.; Marques, P.A.A.P.; Maçôas, E.M.S.; et al. Biocompatible hybrids based on nanographene oxide covalently linked to glycolporphyrins: Synthesis, characterization and biological evaluation. Carbon 2018, 135, 202–214. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kalbac, M. Influence of the fetal bovine serum proteins on the growth of human osteoblast cells on graphene. J. Biomed. Mater. Res. A 2012, 100, 3001–3007. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef]
- Gu, H.; Tang, H.; Xiong, P.; Zhou, Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials 2019, 9, 130. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef]
- Shen, J.M.; Huang, G.; Zhou, X.; Zou, J.; Yang, Y.; Chen, Y.F.; Men, S.K. Safety evaluation of graphene oxide-based magnetic nanocomposites as MRI contrast agents and drug delivery vehicles. RSC Adv. 2014, 4, 50464–50477. [Google Scholar] [CrossRef]
- Yan, N.; Qin, L.; Li, J.; Zhao, F.; Feng, H. Atomic layer deposition of iron oxide on reduced graphene oxide and its catalytic activity in the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2018, 451, 155–161. [Google Scholar] [CrossRef]
- Singh, V.K.; Patra, M.K.; Manoth, M.; Gowd, G.S.; Vadera, S.R.; Kumar, N. In situ synthesis of graphene oxide and its composites with iron oxide. New Carbon Mater. 2009, 24, 147–152. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Cong, H.P.; He, J.J.; Lu, Y.; Yu, S.H. Water-Soluble Magnetic-Functionalized Reduced Graphene Oxide Sheets: In situ Synthesis and Magnetic Resonance Imaging Applications. Small 2010, 6, 169–173. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Venkatesha, N.; Poojar, P.; Qurishi, Y.; Geethanath, S.; Srivastava, C. Graphene oxide-Fe3O4 nanoparticle composite with high transverse proton relaxivity value for magnetic resonance imaging. J. Appl. Phys. 2015, 117, 154702. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Li, C. One-pot preparation of graphene/Fe3O4 composites by a solvothermal reaction. New J. Chem. 2010, 34, 2950–2955. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2018, 8, 048201. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Abdelsayed, V.; Khder, A.E.R.S.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Lin, Y.; Baggett, D.W.; Kim, J.W.; Siochi, E.J.; Connell, J.W. Instantaneous Formation of Metal and Metal Oxide Nanoparticles on Carbon Nanotubes and Graphene via Solvent-Free Microwave Heating. ACS Appl. Mater. Interfaces 2011, 3, 1652–1664. [Google Scholar] [CrossRef]
- Baek, S.; Yu, S.H.; Park, S.K.; Pucci, A.; Marichy, C.; Lee, D.C.; Sung, Y.E.; Piao, Y.; Pinna, N. A one-pot microwave-assisted non-aqueous sol–gel approach to metal oxide/graphene nanocomposites for Li-ion batteries. RSC Adv. 2011, 1, 1687–1690. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Chu, H.; Niu, L.; Sun, Z.; Pan, L.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured Reduced Graphene Oxide/Fe2O3 Composite as a High-Performance Anode Material for Lithium Ion Batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Microwave assisted fabrication of a nanostructured reduced graphene oxide (rGO)/Fe2O3 composite as a promising next generation energy storage material. RSC Adv. 2017, 7, 309–317. [Google Scholar] [CrossRef]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Kim, J.H. Differential Cytotoxicity of Different Sizes of Graphene Oxide Nanoparticles in Leydig (TM3) and Sertoli (TM4) Cells. Nanomaterials 2019, 9, 139. [Google Scholar] [CrossRef]
- Chen, W.; Wen, X.; Zhen, G.; Zheng, X. Assembly of Fe3O4 nanoparticles on PEG-functionalized graphene oxide for efficient magnetic imaging and drug delivery. RSC Adv. 2015, 5, 69307–69311. [Google Scholar] [CrossRef]
- Sandoval, S.; Kumar, N.; Oro-Solé, J.; Sundaresan, A.; Rao, C.N.R.; Fuertes, A.; Tobias, G. Tuning the nature of nitrogen atoms in N-containing reduced graphene oxide. Carbon 2016, 96, 594–602. [Google Scholar] [CrossRef]
- Carenza, E.; Barceló, V.; Morancho, A.; Montaner, J.; Rosell, A.; Roig, A. Rapid synthesis of water-dispersible superparamagnetic iron oxide nanoparticles by a microwave-assisted route for safe labeling of endothelial progenitor cells. Acta Biomater. 2014, 10, 3775–3785. [Google Scholar] [CrossRef]
- Jasuja, K.; Linn, J.; Melton, S.; Berry, V. Microwave-Reduced Uncapped Metal Nanoparticles on Graphene: Tuning Catalytic, Electrical, and Raman Properties. J. Phys. Chem. Lett. 2010, 1, 1853–1860. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Muñoz-Tabares, J.A.; Armandi, M.; Chiodoni, A.; Pirri, C.F. One-Pot Microwave-Assisted Synthesis of Reduced Graphene Oxide/Iron Oxide Nanocomposite Catalyst for the Oxygen Reduction Reaction. ChemistrySelect 2016, 1, 3640–3646. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Ballesteros, B.; Tobias, G.; Ward, M.A.H.; Green, M.L.H. Quantitative Assessment of the Amount of Material Encapsulated in Filled Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 2653–2656. [Google Scholar] [CrossRef]
- Hernández-Rivera, M.; Kumar, I.; Cho, S.Y.; Cheong, B.Y.; Pulikkathara, M.X.; Moghaddam, S.E.; Whitmire, K.H.; Wilson, L.J. High-Performance Hybrid Bismuth–Carbon Nanotube Based Contrast Agent for X-ray CT Imaging. ACS Appl. Mater. Interfaces 2017, 9, 5709–5716. [Google Scholar] [CrossRef]
- Sasikala, A.R.K.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35. [Google Scholar]
- Wang, J.T.W.; Cabana, L.; Bourgognon, M.; Kafa, H.; Protti, A.; Venner, K.; Shah, A.M.; Sosabowski, J.K.; Mather, S.J.; Roig, A.; et al. Magnetically Decorated Multiwalled Carbon Nanotubes as Dual MRI and SPECT Contrast Agents. Adv. Funct. Mater. 2014, 24, 1880–1894. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Müller, K.H.; Li, S.; Porter, A.E.; Eddaoudi, A.; Prato, M.; Bianco, A.; Kostarelos, K. Cellular Uptake and Cytotoxic Impact of Chemically Functionalized and Polymer-Coated Carbon Nanotubes. Small 2011, 7, 3230–3238. [Google Scholar] [CrossRef]
- Nkansah, M.K.; Thakral, D.; Shapiro, E.M. Magnetic poly(lactide-co-glycolide) and cellulose particles for MRI-based cell tracking. Magn. Reson. Med. 2011, 65, 1776–1785. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Shapiro, E.M. Biodegradable, polymer encapsulated, metal oxide particles for MRI-based cell tracking. Magn. Reson. Med. 2015, 73, 376–389. [Google Scholar] [CrossRef]
- Lee, P.W.; Hsu, S.H.; Wang, J.J.; Tsai, J.S.; Lin, K.J.; Wey, S.P.; Chen, F.R.; Lai, C.H.; Yen, T.C.; Sung, H.W. The characteristics, biodistribution, magnetic resonance imaging and biodegradability of superparamagnetic core–shell nanoparticles. Biomaterials 2010, 31, 1316–1324. [Google Scholar] [CrossRef]
- Prashant, C.; Dipak, M.; Yang, C.T.; Chuang, K.H.; Jun, D.; Feng, S.S. Superparamagnetic iron oxide—Loaded poly (lactic acid)-d-α-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials 2010, 31, 5588–5597. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine: NBM 2016, 12, 333–351. [Google Scholar] [CrossRef]


| Step | T (°C) | Time (Min) | Max. Power (W) | Max. Pressure (Bar) |
|---|---|---|---|---|
| 1 | 60 | 5 | 300 | 12 |
| 2 | 180 | 10 | 300 | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llenas, M.; Sandoval, S.; Costa, P.M.; Oró-Solé, J.; Lope-Piedrafita, S.; Ballesteros, B.; Al-Jamal, K.T.; Tobias, G. Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI). Nanomaterials 2019, 9, 1364. https://doi.org/10.3390/nano9101364
Llenas M, Sandoval S, Costa PM, Oró-Solé J, Lope-Piedrafita S, Ballesteros B, Al-Jamal KT, Tobias G. Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI). Nanomaterials. 2019; 9(10):1364. https://doi.org/10.3390/nano9101364
Chicago/Turabian StyleLlenas, Marina, Stefania Sandoval, Pedro M. Costa, Judith Oró-Solé, Silvia Lope-Piedrafita, Belén Ballesteros, Khuloud T. Al-Jamal, and Gerard Tobias. 2019. "Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI)" Nanomaterials 9, no. 10: 1364. https://doi.org/10.3390/nano9101364
APA StyleLlenas, M., Sandoval, S., Costa, P. M., Oró-Solé, J., Lope-Piedrafita, S., Ballesteros, B., Al-Jamal, K. T., & Tobias, G. (2019). Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI). Nanomaterials, 9(10), 1364. https://doi.org/10.3390/nano9101364