Fabrication of Durable Ordered Ta2O5 Nanotube Arrays Decorated with Bi2S3 Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Colmenares, J.C.; Yi-Jun, X. Heterogeneous Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Lee, B.; Domen, K.; Kondo, J.N. Synthesis of Crystallized Mesoporous Tantalum Oxide and Its Photocatalytic Activity for Overall Water Splitting under Ultraviolet Light Irradiation. Chem. Mater. 2008, 20, 5361–5367. [Google Scholar] [CrossRef]
- Li, J.; Dai, W.; Wu, G.; Guan, N.; Li, L. Fabrication of Ta2O5 films on tantalum substrate for efficient photocatalysis. Catal. Commun. 2015, 65, 24–29. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Migowski, P.; Wender, H.; Eberhardt, D.; Weibel, D.E.; Sonaglio, F.C.; Zapata, M.J.M.; Dupont, J.; Feil, A.F.; Teixeira, S.R. Ta2O5 Nanotubes Obtained by Anodization: Effect of Thermal Treatment on the Photocatalytic Activity for Hydrogen Production. J. Phys. Chem. C 2012, 116, 14022–14030. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications. Mater. Sci. Eng. R Rep. 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Baltes, M.; Kyto, A.; Weckhuysen, B.M.; Schoonheydt, R.A.; Van Der Voort, P.; Vansant, E.F. Supported Tantalum Oxide and Supported Vanadia-tantala Mixed Oxides: Structural Characterization and Surface Properties. J. Phys. Chem. B 2001. [Google Scholar] [CrossRef]
- Azim, O.A.; Abdel-Aziz, M.M.; Yahia, I.S. Structure and optical analysis of Ta2O5 deposited on infrasil substrate. Appl. Surf. Sci. 2009, 255, 4829–4835. [Google Scholar] [CrossRef]
- Kesmez, Ö.; Akarsu, E.; Çamurlu, H.E.; Yavuz, E.; Akarsu, M.; Arpaç, E. Preparation and characterization of multilayer anti-reflective coatings via sol-gel process. Ceram. Int. 2018, 44, 3183–3188. [Google Scholar] [CrossRef]
- Ezhilvalavan, S.; Tseng, T.Y. Preparation and properties of tantalum pentoxide (Ta2O5) thin films for ultra large scale integrated circuits (ULSIs) application—A review. J. Mater. Sci. Mater. Electron. 1999, 10, 9–31. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Duan, J.; Shi, W.; Xu, L.; Mou, G.; Xin, Q.; Guan, J. Hierarchical nanostructures of fluorinated and naked Ta2O5 single crystalline nanorods: Hydrothermal preparation, formation mechanism and photocatalytic activity for H2 production. Chem. Commun. 2012, 48, 7301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, A.; Zhao, X.; Zhang, H. Structural and electrical properties of Ta2O5 thin films prepared by photo-induced CVD. Bull. Mater. Sci. 2011, 34, 443–446. [Google Scholar] [CrossRef]
- Lee, K.; Schmuki, P. Highly ordered nanoporous Ta2O5 formed by anodization of Ta at high temperatures in a glycerol/phosphate electrolyte. Electrochem. Commun. 2011, 13, 542–545. [Google Scholar] [CrossRef]
- Su, Z.; Grigorescu, S.; Wang, L.; Lee, K.; Schmuki, P. Fast fabrication of Ta2O5 nanotube arrays and their conversion to Ta 3 N 5 for efficient solar driven water splitting. Electrochem. Commun. 2015, 50, 15–19. [Google Scholar] [CrossRef]
- Kobylański, M.P.; Mazierski, P.; Malankowska, A.; Kozak, M.; Diak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. TiO2–CoxOy composite nanotube arrays via one step electrochemical anodization for visible light–induced photocatalytic reaction. Surf. Interfaces 2018, 12, 179–189. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.M.; Schmuki, P. High aspect ratio ordered nanoporous Ta2O5 films by anodization of Ta. Electrochem. Commun. 2008, 10, 428–432. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled Interconversion of Nanoarray of Ta Dimples and High Aspect Ratio Ta Oxide Nanotubes. Nano Lett. 2009, 9, 1350–1355. [Google Scholar] [CrossRef]
- Young, L. Anodic oxide films. Influence of the film present before anodization. Trans. Faraday Soc. 1957, 53, 841. [Google Scholar] [CrossRef]
- Horwood, C.A.; El-Sayed, H.A.; Birss, V.I. Precise electrochemical prediction of short tantalum oxide nanotube length. Electrochim. Acta 2014, 132, 91–97. [Google Scholar] [CrossRef]
- Grigorescu, S.; So, S.; Yoo, J.E.; Mazare, A.; Hahn, R.; Schmuki, P. Open top anodic Ta3N5 nanotubes for higher solar water splitting efficiency. Electrochim. Acta 2015, 182, 803–808. [Google Scholar] [CrossRef]
- Namur, R.S.; Reyes, K.M.; Marino, C.E.B. Growth and Electrochemical Stability of Compact Tantalum Oxides Obtained in Different Electrolytes for Biomedical Applications. Mater. Res. 2015, 18, 91–97. [Google Scholar] [CrossRef]
- Mazierski, P.; Nadolna, J.; Nowaczyk, G.; Lisowski, W.; Winiarski, M.J.; Klimczuk, T.; Kobylański, M.P.; Jurga, S.; Zaleska-Medynska, A. Highly Visible-Light-Photoactive Heterojunction Based on TiO2 Nanotubes Decorated by Pt Nanoparticles and Bi2S3 Quantum Dots. J. Phys. Chem. C 2017, 121, 17215–17225. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, J.; Tripathi, S.K. Synthesis of Bi2S3 quantum dots for sensitized solar cells by reverse SILAR. AIP Conf. Proc. 2016, 1728, 020423. [Google Scholar]
- Wang, Z.J.; Li, R.; Landfester, K.; Zhang, K.A.I. Porous conjugated polymer via metal-free synthesis for visible light-promoted oxidative hydroxylation of arylboronic acids. Polymer 2017, 126, 291–295. [Google Scholar] [CrossRef]
- Lv, P.; Fu, W.; Yang, H.; Sun, H.; Chen, Y.; Ma, J.; Zhou, X.; Tian, L.; Zhang, W.; Li, M.; et al. Simple synthesis method of Bi2S3/CdS quantum dots cosensitized TiO2 nanotubes array with enhanced photoelectrochemical and photocatalytic activity. CrystEngComm 2013, 15, 7548–7555. [Google Scholar] [CrossRef]
- Ramanery, F.P.; Mansur, A.A.P.; Mansur, H.S.; Carvalho, S.M.; Fonseca, M.C. Biocompatible Fluorescent Core-Shell Nanoconjugates Based on Chitosan/Bi2S3 Quantum Dots. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Syed Abuthahir, K.A.Z.; Jagannathan, R. Reverse-loop impedance profile in Bi2S3 quantum dots. Mater. Chem. Phys. 2010, 121, 184–192. [Google Scholar] [CrossRef]
- Narayanan, R.; Deepa, M.; Friebel, F.; Srivastava, A.K. A CdS/Bi2S3 bilayer and a poly (3,4- ethylenedioxythiophene)/S2− interface control quantum dot solar cell performance. Electrochim. Acta 2013, 105, 599–611. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołabiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018. [Google Scholar] [CrossRef]
- Kadam, S.R.; Panmand, R.P.; Sonawane, R.S.; Gosavi, S.W.; Kale, B.B. A stable Bi2S3 quantum dot–glass nanosystem: Size tuneable photocatalytic hydrogen production under solar light. RSC Adv. 2015, 5, 58485–58490. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kowalska, E.; Nadolna, J.; Wei, Z.; Endo, M.; Ohtani, B.; Zaleska-Medynska, A. Preparation of CdS and Bi2S3 quantum dots co-decorated perovskite-type KNbO3 ternary heterostructure with improved visible light photocatalytic activity and stability for phenol degradation. Dalton Trans. 2018, 47, 15232–15245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, F.; Chen, J.; Xiang, T.; Yin, X. Photocatalytic reduction of CO2 in isopropanol on Bi2S3 quantum dots/TiO2 nanosheets with exposed {001} facets. J. Nanosci. Nanotechnol. 2017, 17, 1863–1869. [Google Scholar] [CrossRef]
- Zumeta-Dubé, I.; Ruiz-Ruiz, V.F.; Díaz, D.; Rodil-Posadas, S.; Zeinert, A. TiO2 sensitization with Bi2S3 quantum dots: The inconvenience of sodium ions in the deposition procedure. J. Phys. Chem. C 2014, 118, 11495–11504. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Tu, H.; Jia, Q.; Gong, H.; Guan, J. Synchronous etching-epitaxial growth fabrication of facet-coupling NaTaO3/Ta2O5 heterostructured nanofibers for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 184, 309–319. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Naumkin, A.; Kraut-Vass, A.; Gaarenstroom, S.C.P. NIST X-Ray Photoelectron Spectroscopy Database 20 Version, 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 1 August 2019).
- Gonçalves, R.V.; Wojcieszak, R.; Uberman, P.M.; Teixeira, S.R.; Rossi, L.M. Insights into the active surface species formed on Ta2O5 nanotubes in the catalytic oxidation of CO. Phys. Chem. Chem. Phys. 2014, 16, 5755. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; White, R.G.; Watts, J.F.; Baker, M.A. XPS investigation of monatomic and cluster argon ion sputtering of tantalum pentoxide. Appl. Surf. Sci. 2017, 405, 79–87. [Google Scholar] [CrossRef]
- Yu, X.; Li, W.; Huang, J.; Li, Z.; Liu, J.; Hu, P. Superstructure Ta2O5 mesocrystals derived from (NH4)2Ta2O3F6 mesocrystals with efficient photocatalytic activity. Dalton Trans. 2018, 47, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim, N.H.A.; Basirun, W.J.; Sulaiman, E.B. Nanomechanical properties, wear resistance and in-vitro characterization of Ta2O5 nanotubes coating on biomedical grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Schönberg, N.; Overend, W.G.; Munthe-Kaas, A.; Sörensen, N.A. An X-Ray Investigation of the Tantalum-Oxygen System. Acta Chem. Scand 1954, 8, 240–245. [Google Scholar] [CrossRef]
- Brauer, G.; Zapp, K.H. Die Nitride des Tantals. ZAAC J. Inorg. Gen. Chem. 1954, 277, 129–139. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Gazda, M.; Zaleska, A. Ordered TiO2 nanotubes: The effect of preparation parameters on the photocatalytic activity in air purification process. Appl. Catal. B Environ. 2014, 144, 674–685. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, P.; Liu, Y.; Liu, X.; Li, F.; Ni, L.; Yan, Y.; Huo, P. Construction of amorphous Ta2O5/g-C3N4 nanosheet hybrids with superior visible-light photoactivities for organic dye degradation and mechanism insight. Sep. Purif. Technol. 2016, 170, 10–21. [Google Scholar] [CrossRef]







| Sample Label | Preparation Conditions During Anodic Oxidation (AO) | External Diameter (nm) | Internal Diameter (nm) | Thickness (nm) | Length (µm) | Ta2O5 NTs Adhesion to Ta Foil | Toluene Decomposition (%) | |
|---|---|---|---|---|---|---|---|---|
| 5 min of Irradiation | 15 min of Irradiation | |||||||
| NTs_10 V_10 min_no_cleaned_Air_450 °C_1 h | AO (U = 10 V, t = 10 min.), dried (T = 80 °C, t = 24 h), and annealed in air (T = 450 °C; t = 1 h) | 44 ± 3 | 27 ± 2 | 9 ± 1 | 1.00 ± 0.13 | High | 64.22 ± 3.27 | 90.51 ± 3.27 |
| NTs_15 V_10 min_ no_cleaned Air_450 °C_1 h | AO (U = 15 V, t = 10 min.), dried (T = 80 °C, t = 24 h), and annealed in air (T = 450 °C; t = 1 h) | 46 ± 6 | 24 ± 2 | 11 ± 2 | 3.18 ± 0.09 | High | 93.55 ± 2.38 | 97.83 ± 1.29 |
| NTs_20 V_10 min_ no_cleaned Air_450 °C_1 h | AO (U = 20 V, t = 10 min.), dried (T = 80 °C, t = 24 h), and annealed in air (T = 450 °C; t = 1 h) | 48 ± 3 | 28 ± 3 | 10 ± 1 | 6.00 ± 0.19 | Weak | 91.05 ± 3.35 | 96.82 ± 2.45 |
| NTs_10 V_10 min_N2_450 °C_1 h | AO (U = 10 V, t = 10 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h) | 45 ± 5 | 20 ± 3 | 11 ± 2 | 1.74 ± 0.02 | Weak | 68.38 ± 0.35 | 94.65 ± 1.91 |
| NTs_15 V_5 min_N2_450 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h) | 41 ± 4 | 19 ± 3 | 10 ± 2 | 1.27 ± 0.05 | High | 95.36 ± 1.22 | 98.73 ± 0.47 |
| NTs_15 V_5 min_N2_450 °C_3 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 3 h) | 48 ± 2 | 25 ± 4 | 10 ± 1 | 1.78 ± 0.11 | High | 91.03 ± 0.47 | 98.98 ± 0.06 |
| NTs_15 V_5 min_N2_600 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 600 °C; t = 1 h) | 39 ± 2 | 23 ± 3 | 10 ± 2 | 1.21 ± 0.03 | Very weak | Sample was unstable | |
| NTs_15 V_5 min_N2_750 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 750 °C; t = 1 h) | 40 ± 3 | 21 ± 2 | 10 ± 1 | 1.43 ± 0.02 | Very weak | Sample was unstable | |
| NTs_15 V_10 min_N2_450 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h) | 42 ± 3 | 21 ± 2 | 10 ± 2 | 3.31 ± 0.08 | High | 92.74 ± 1.09 | 96.81 ± 0.35 |
| NTs_15 V_5 min_Air_450 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in air (T = 450 °C; t = 1 h) | 49 ± 7 | 23 ± 4 | 10 ± 2 | 1.46 ± 0.20 | High | 91.86 ± 2.16 | 97.50 ± 2.40 |
| NTs_15 V_5 min_N H3_450 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in NH3 (T = 450 °C; t = 1 h) | 47 ± 5 | 19 ± 3 | 13 ± 1 | 2.19 ± 0.07 | High | 93.37 ± 0.42 | 98.90 ± 0.00 |
| NTs_15 V_5 min_ H2_450 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in H2 (T = 450 °C; t = 1 h) | 44 ± 6 | 21 ± 3 | 10 ± 1 | 1.68 ± 0.03 | High | 94.20 ± 3.43 | 98.09 ± 0.03 |
| NTs_15 V_5 min_N2_300 °C_1 h | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 300 °C; t = 1 h) | 47 ± 5 | 24 ± 3 | 11 ± 1 | 2.25 ± 0.06 | High | 94.08 ± 0.42 | 97.36 ± 0.25 |
| NTs_15 V_5 min_N2_450 °C_1 h_two_step | AO (I step: U = 15 V, t = 5 min.), removing of NTs layer, AO (II step, U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h) | 46 ± 5 | 24 ± 4 | 10 ± 1 | 1.15 ± 0.05 | Weak | 89.28 ± 0.14 | 96.53 ± 0.30 |
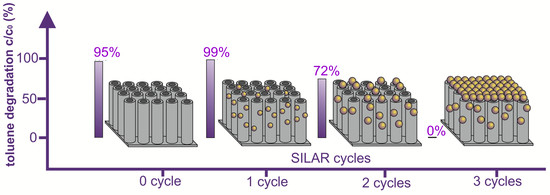
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 1x | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h), 1 cycle of SILAR | 50 ± 4 | 31 ± 3 | 10 ± 1 | 1.42 ± 0.03 | High | 99.17 ± 0.14 | 100 |
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 2x | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h), 2 cycles of SILAR | 41 ± 4 | 28 ± 3 | 8 ± 1 | 1.61 ± 0.11 | High | 71.56 ± 1.12 | 100 |
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 3x | AO (U = 15 V, t = 5 min.), ultrasonically cleaned (1 min.), dried (T = 80 °C, t = 24 h), and annealed in N2 (T = 450 °C; t = 1 h), 3 cycles of SILAR | Coated with Bi2S3 layer | 1.40 ± 0.05 | High | Inactive | |||
| Sample Label | Elemental Composition (at. %) | Bi 4f7/2 Fractions (%) | Ta 4f7/2 Fractions (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ta | O | C | S | Bi | Residue (F, N, Na) | Bi0 157.0 ± 0.2 eV | Bi2S3 158.0 ± 0.3 eV | Bi2O3 159.5 ± 0.3 eV | Ta2O5_Surf 27.0 ± 0.1 eV | Ta2O5 26.2 ± 0.2 eV | Ta1+ 22.1 ± 0.1 eV | Ta0 21.0 ± 0.3 eV | |
| NTs_15 V_5 min_N2_450 °C_1 h | 22.44 | 46.00 | 24.31 | 3.10 | - | 4.15 | 0 | 0 | 0 | 0 | 96.84 | 1.72 | 1.44 |
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 1x | 15.79 | 58.20 | 11.02 | 6.26 | 2.37 | 6.36 | 2.28 | 46.50 | 51.22 | 23.55 | 73.42 | 1.86 | 1.17 |
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 2x | 16.24 | 57.47 | 12.13 | 5.66 | 2.63 | 5.87 | 3.21 | 53.10 | 43.69 | 24.81 | 72.51 | 1.91 | 0.77 |
| NTs_15 V_5 min_N2_450 °C_1 h_QDs_SILAR 3x | 0.63 | 29.47 | 26.72 | 15.14 | 22.60 | 5.44 | 1.24 | 76.58 | 22.18 | 19.61 | 80.39 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baluk, M.A.; Kobylański, M.P.; Lisowski, W.; Trykowski, G.; Klimczuk, T.; Mazierski, P.; Zaleska-Medynska, A. Fabrication of Durable Ordered Ta2O5 Nanotube Arrays Decorated with Bi2S3 Quantum Dots. Nanomaterials 2019, 9, 1347. https://doi.org/10.3390/nano9101347
Baluk MA, Kobylański MP, Lisowski W, Trykowski G, Klimczuk T, Mazierski P, Zaleska-Medynska A. Fabrication of Durable Ordered Ta2O5 Nanotube Arrays Decorated with Bi2S3 Quantum Dots. Nanomaterials. 2019; 9(10):1347. https://doi.org/10.3390/nano9101347
Chicago/Turabian StyleBaluk, Mateusz A., Marek P. Kobylański, Wojciech Lisowski, Grzegorz Trykowski, Tomasz Klimczuk, Paweł Mazierski, and Adriana Zaleska-Medynska. 2019. "Fabrication of Durable Ordered Ta2O5 Nanotube Arrays Decorated with Bi2S3 Quantum Dots" Nanomaterials 9, no. 10: 1347. https://doi.org/10.3390/nano9101347
APA StyleBaluk, M. A., Kobylański, M. P., Lisowski, W., Trykowski, G., Klimczuk, T., Mazierski, P., & Zaleska-Medynska, A. (2019). Fabrication of Durable Ordered Ta2O5 Nanotube Arrays Decorated with Bi2S3 Quantum Dots. Nanomaterials, 9(10), 1347. https://doi.org/10.3390/nano9101347