Ranolazine-Functionalized Copper Nanoparticles as a Colorimetric Sensor for Trace Level Detection of As3+
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Instrumentation
2.3. Preparation of Rano-Cu NPs
2.4. Procedure for Colorimetric Detection of As3+ by Rano-Cu NPs
2.5. Detection of As3+ Ion in Environmental Water Samples
3. Results and Discussion
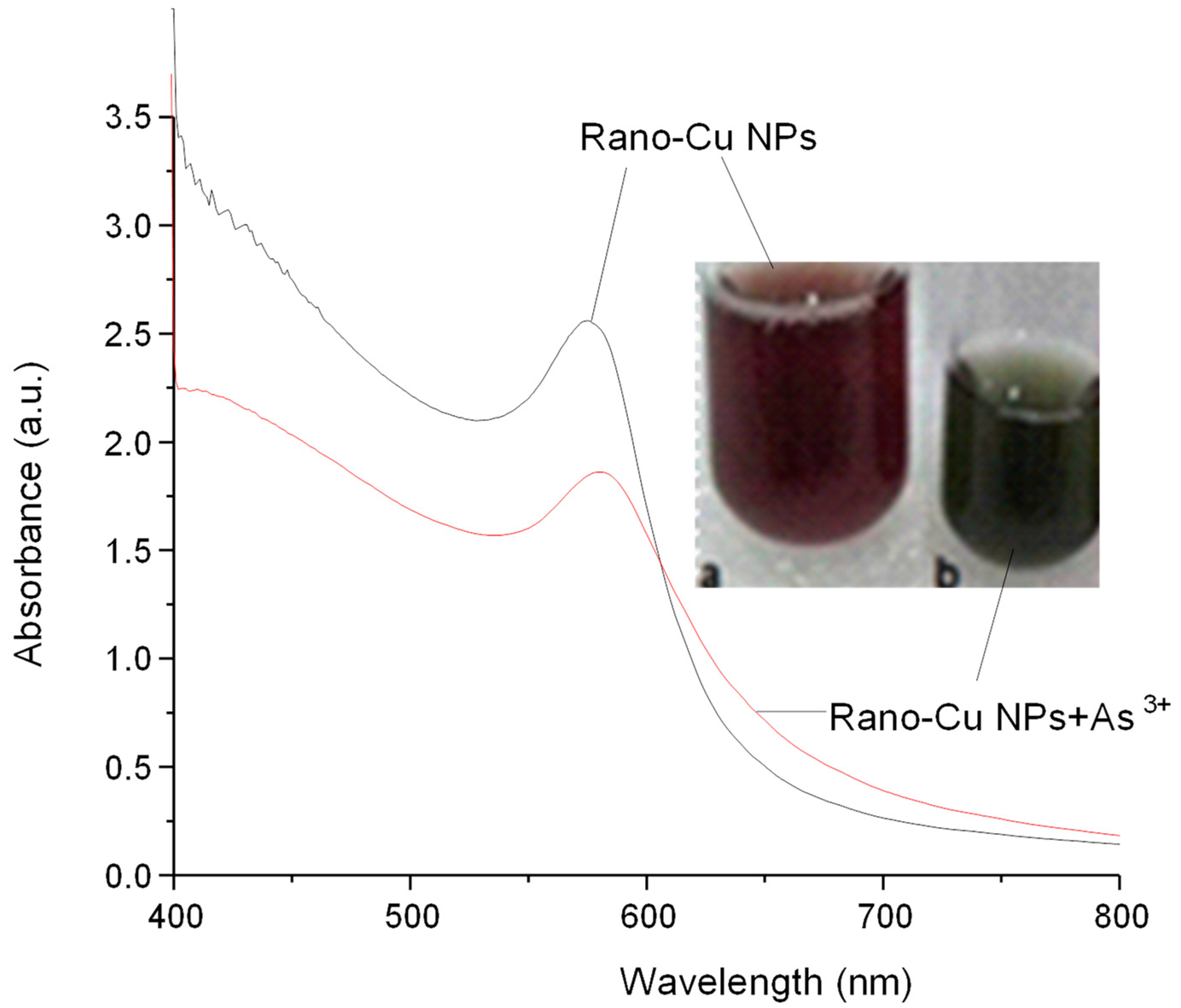
3.1. Optical Characterization
3.2. FTIR Studies
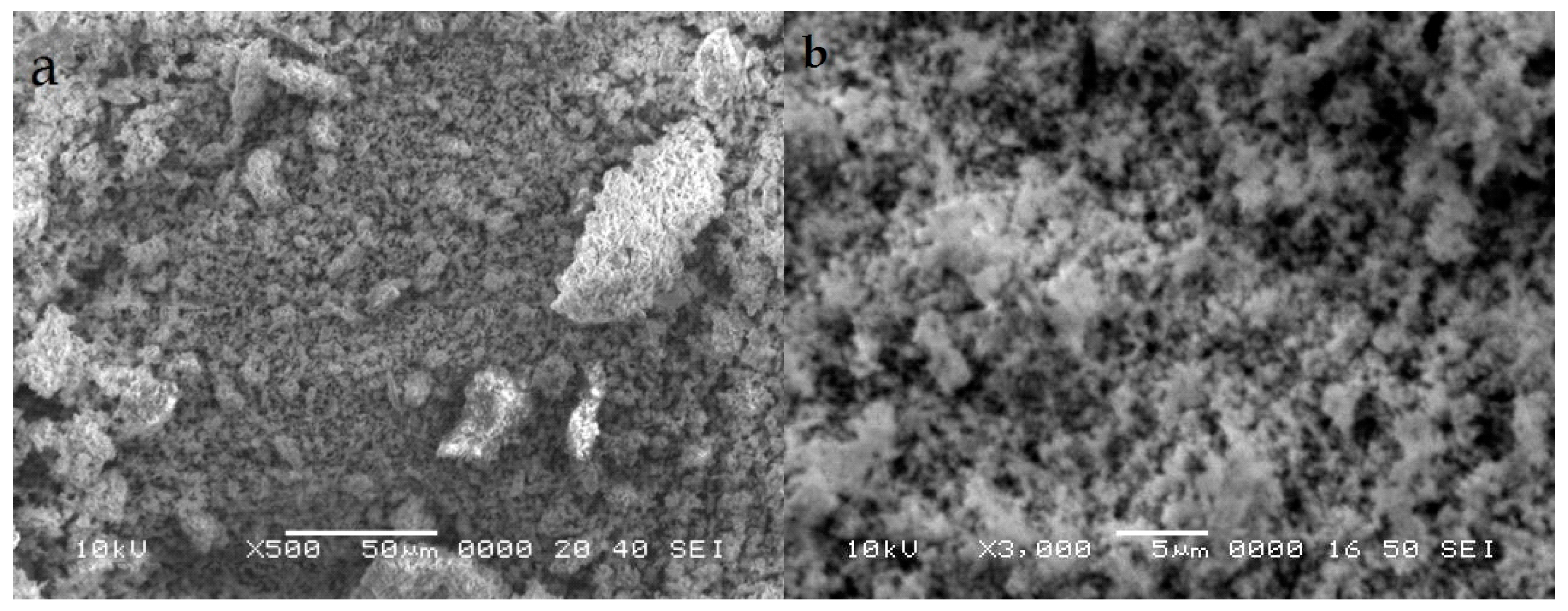
3.3. Scanning Electron Microscopy (SEM) Analysis
3.4. Powder X-ray Diffraction (PXRD) Studies
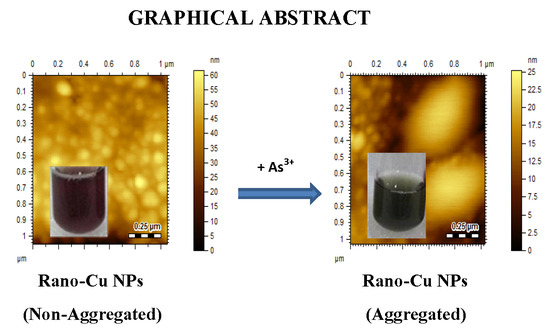
3.5. Colorimetric Assay for As3+ Ions
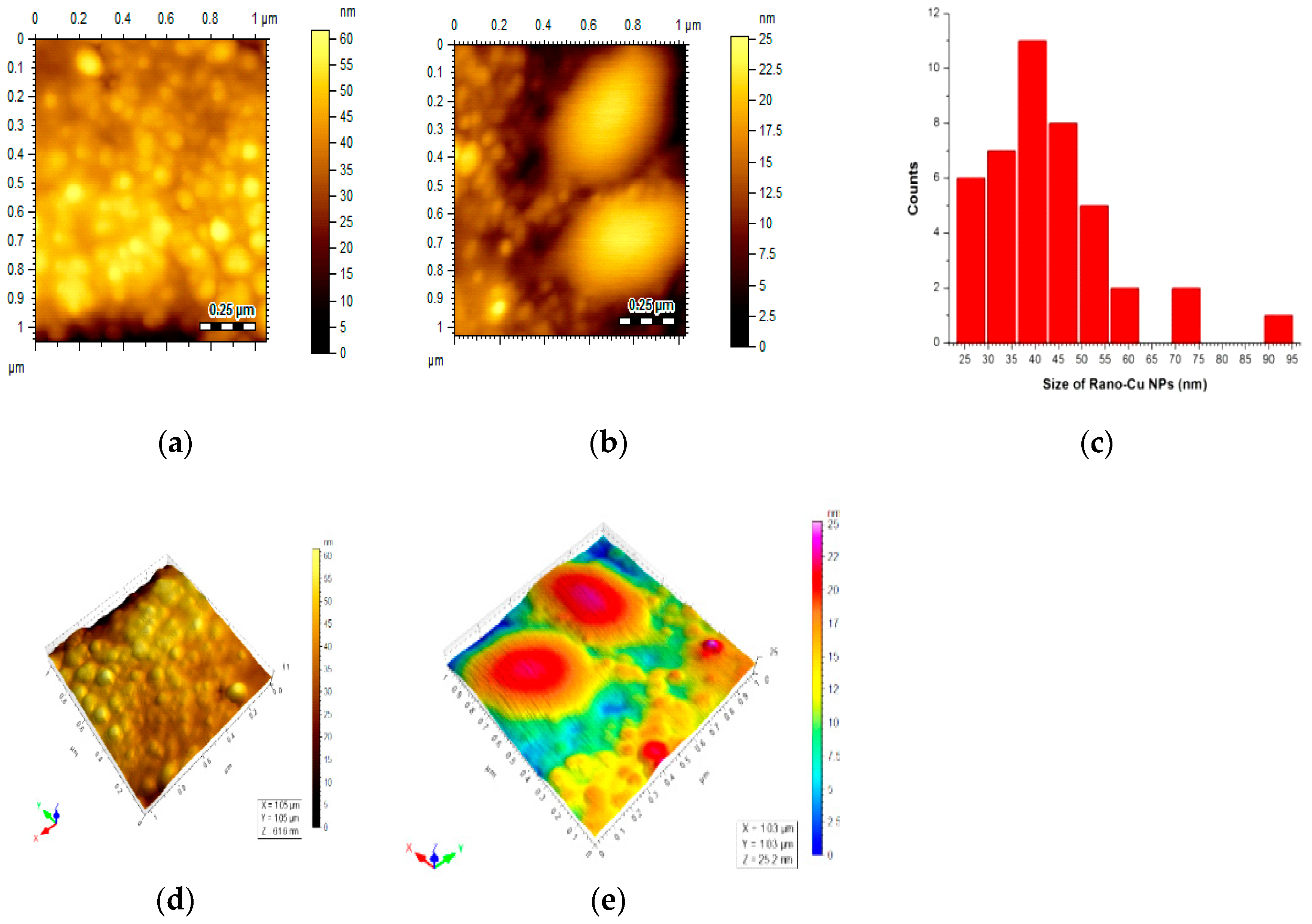
3.6. AFM Studies
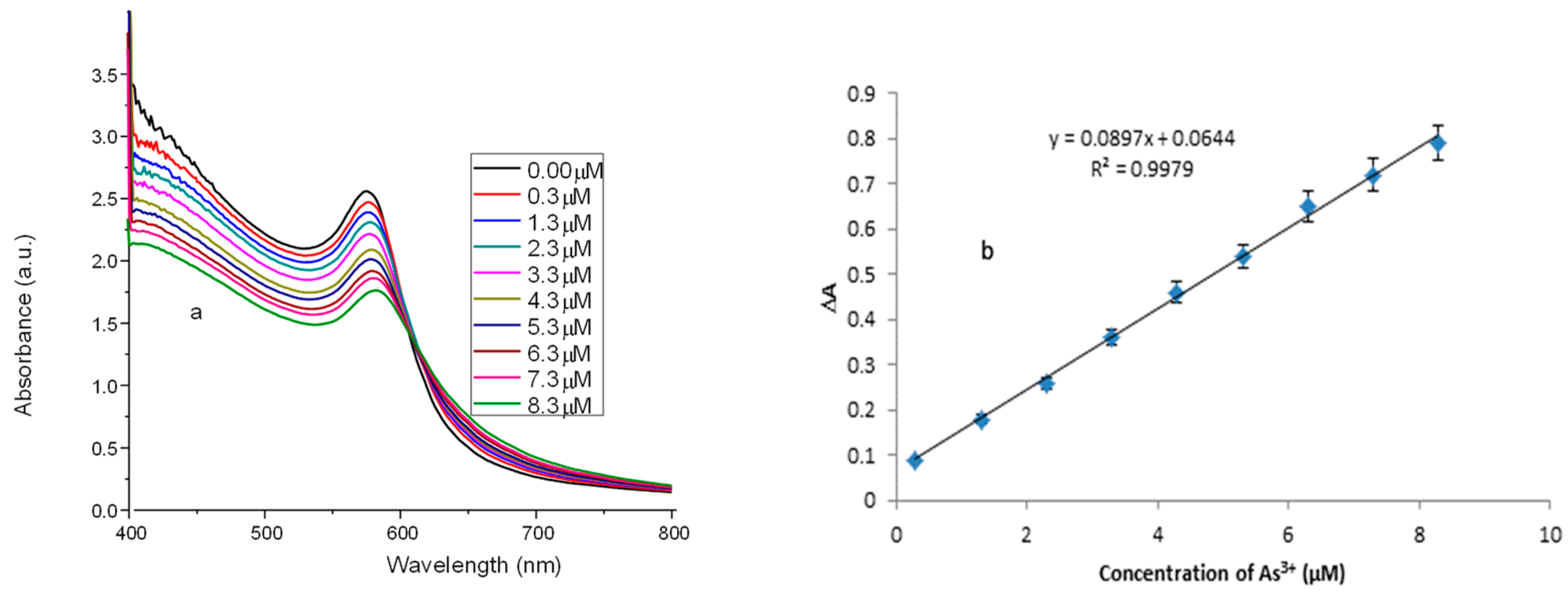
3.7. Analytical Performance of the Cu NPs for Detecting As3+
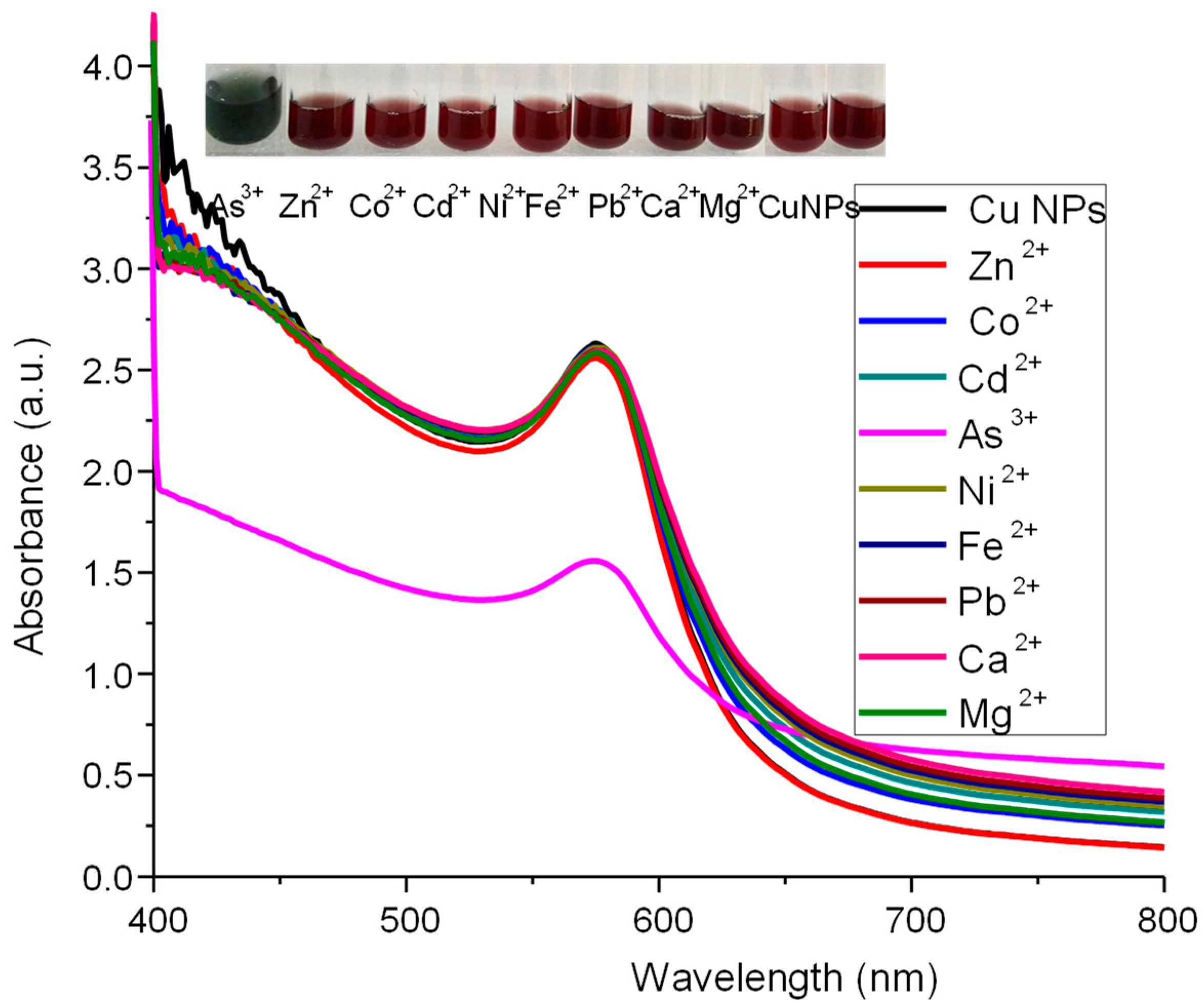
3.8. Selectivity of Developed Sensor
3.9. Application of Rano-Cu NPs for Detecting As3+ in Groundwater Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Kalluri, J.R.; Arbneshi, T.; Khan, S.A.; Neely, A.; Candice, P.; Varisli, B.; Washington, M.; McAfee, S.; Robinson, B.; Banerjee, S. Use of gold nanoparticles in a simple colorimetric and ultrasensitive dynamic light scattering assay: Selective detection of arsenic in groundwater. Angew. Chem. 2009, 121, 9848–9851. [Google Scholar] [CrossRef]
- Xie, X.; Stueben, D.; Berner, Z. The application of microelectrodes for the measurements of trace metals in water. Anal. Lett. 2005, 38, 2281–2300. [Google Scholar] [CrossRef]
- Vaishanav, S.K.; Korram, J.; Pradhan, P.; Chandraker, K.; Nagwanshi, R.; Ghosh, K.K.; Satnami, M.L. Green luminescent CdTe quantum dot based fluorescence nano-sensor for sensitive detection of arsenic (III). J. Fluor. 2017, 27, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Compton, R.G. Direct electrodeposition of gold nanoparticles onto indium tin oxide film coated glass: Application to the detection of arsenic (III). Anal. Sci. 2006, 22, 567–570. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of arsenic (III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992, 97, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultra trace arsenic, mercury, and copper. Anal. Chem. 2008, 80, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Sounderajan, S.; Udas, A.; Venkataramani, B. Characterization of arsenic (V) and arsenic (III) in water samples using ammonium molybdate and estimation by graphite furnace atomic absorption spectroscopy. J. Hazard. Mater. 2007, 149, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Pantuzzo, F.L.; Silva, J.C.J.; Ciminelli, V.S. A fast and accurate microwave-assisted digestion method for arsenic determination in complex mining residues by flame atomic absorption spectrometry. J. Hazard. Mater. 2009, 168, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Ferrua, N.; Salonia, J.; Olsina, R.; Martinez, L. On-line arsenic co-precipitation on ethyl vinyl acetate turning-packed mini-column followed by hydride generation-ICP OES determination. J. Hazard. Mater. 2007, 143, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Dufailly, V.; Noël, L.; Guérin, T. Optimization and critical evaluation of a collision cell technology ICP-MS system for the determination of arsenic in foodstuffs of animal origin. Anal. Chim. Acta 2008, 611, 134–142. [Google Scholar] [CrossRef]
- Al-Assaf, K.H.; Tyson, J.F.; Uden, P.C. Determination of four arsenic species in soil by sequential extraction and high performance liquid chromatography with post-column hydride generation and inductively coupled plasma optical emission spectrometry detection. J. Anal. At. Spectrosc. 2009, 24, 376–384. [Google Scholar] [CrossRef]
- Nath, P.; Arun, R.K.; Chanda, N. A paper based microfluidic device for the detection of arsenic using a gold nanosensor. RSC Adv. 2014, 4, 59558–59561. [Google Scholar] [CrossRef]
- Ghorbani, H.R. Chemical synthesis of copper nanoparticles. Orient. J. Chem. 2014, 30, 803–806. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Hussain, I.; Graham, S.; Wang, Z.; Tan, B.; Sherrington, D.C.; Rannard, S.P.; Cooper, A.I.; Brust, M. Size-controlled synthesis of near-monodisperse gold nanoparticles in the 1–4 nm range using polymeric stabilizers. J. Am. Chem. Soc. 2005, 127, 16398–16399. [Google Scholar] [CrossRef]
- Jian-Guang, Y.; Yuang-Lin, Z.; Okamoto, T.; Ichino, R.; Okido, M. A new method for preparing hydrophobic nano-copper powders. J. Mat. Sci. 2007, 42, 7638–7642. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Sengan, M.; Kumar, S.; Veerappan, A. Highly selective colorimetric cysteine sensor based on the formation of cysteine layer on copper nanoparticles. Sens. Actuators B Chem. 2016, 233, 431–437. [Google Scholar]
- El-Nour, K.M.A.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kong, C.; Deng, D.; Song, X.; Ding, B.; Yang, Z. Nanoparticle-aggregated octahedral copper hierarchical nanostructures. CrystEngComm 2011, 13, 63–66. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Noguez, C. Surface plasmons on metal nanoparticles: The influence of shape and physical environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165–169. [Google Scholar] [CrossRef]
- Al-Mamun, M.A.; Kusumoto, Y.; Muruganandham, M. Simple new synthesis of copper nanoparticles in water/acetonitrile mixed solvent and their characterization. Mater. Lett. 2009, 63, 2007–2009. [Google Scholar] [CrossRef]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- De, S.; Mandal, S. Surfactant-assisted shape control of copper nanostructures. Colloid Surf. A Physicochem. Eng. Asp. 2013, 421, 72–83. [Google Scholar] [CrossRef]
- Hatamie, A.; Zargar, B.; Jalali, A. Copper nanoparticles: A new colorimetric probe for quick, naked-eye detection of sulfide ions in water samples. Talanta 2014, 121, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ramajo, L.; Parra, R.; Reboredo, M.; Castro, M. Preparation of amine coated silver nanoparticles using triethylenetetramine. J. Chem. Sci. 2009, 121, 83–87. [Google Scholar] [CrossRef]
- Hussain, M.; Nafady, A.; Sherazi, S.T.H.; Shah, M.R.; Alsalme, A.; Kalhoro, M.S.; Mahesar, S.A.; Siddiqui, S. Cefuroxime derived copper nanoparticles and their application as a colorimetric sensor for trace level detection of picric acid. RSC Adv. 2016, 6, 82882–82889. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Yang, H.; Zhang, Y.; Yan, X.-P. Preparation, characterization and evaluation of water-soluble L-cysteine-capped-CdS nanoparticles as fluorescence probe for detection of Hg (II) in aqueous solution. Anal. Chim. Acta 2006, 559, 234–239. [Google Scholar] [CrossRef]
- Wang, L.N.; Li, M.Y.; Zeng, Q.X. Synthesis of copper nanoparticles by chemical reduction method and its influential factors. Adv. Mater. Res. 2013, 627, 804–808. [Google Scholar] [CrossRef]
- Gu, X.; Nguyen, T.; Oudina, M.; Martin, D.; Kidah, B.; Jasmin, J.; Rezig, A.; Sung, L.; Byrd, E.; Martin, J.W. Microstructure and morphology of amine-cured epoxy coatings before and after outdoor exposures—An AFM study. JCT Res. 2005, 2, 547–556. [Google Scholar] [CrossRef]
- Bothra, S.; Solanki, J.N.; Sahoo, S.K. Functionalized silver nanoparticles as chemosensor for pH, Hg2+ and Fe3+ in aqueous medium. Sens. Actuators B Chem. 2013, 188, 937–943. [Google Scholar] [CrossRef]
- Vasimalai, N.; Sheeba, G.; John, S.A. Ultrasensitive fluorescence-quenched chemosensor for Hg (II) in aqueous solution based on mercaptothiadiazole capped silver nanoparticles. J. Hazard. Mater. 2012, 213, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Umar, A. Fabrication and characterization of highly sensitive and selective arsenic sensor based on ultra-thin graphene oxide nanosheets. Sens. Actuators B Chem. 2016, 227, 29–34. [Google Scholar]
- Liao, V.H.C.; Ou, K.L. Development and testing of a green fluorescent protein-based bacterial biosensor for measuring bioavailable arsenic in contaminated groundwater samples. Environ. Toxicol. Chem. 2005, 24, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, Y.; Hou, X. A potential visual fluorescence probe for ultratrace arsenic (III) detection by using glutathione-capped CdTe quantum dots. Talanta 2011, 84, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Wang, F.; He, L.; Zhi, W.; Zhou, P. Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic (III) in aqueous solution. Chem. Commun. 2012, 48, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Du, B.; Pan, L.; Liu, Q.; Yang, K.; Wang, W.; Zhao, H.; Wu, L.; He, Y. Colorimetric aggregation assay for arsenic (III) using gold nanoparticles. Microchim. Acta 2017, 184, 1185–1190. [Google Scholar] [CrossRef]


| Methods | Linear Range (M) | Limit of Detection (M) | Need for Addition of Surface Modification | Reference |
|---|---|---|---|---|
| Electrochemical method, graphene oxide | 6.67 × 10−5 to 6.67 × 10−4 | 6.7 × 10−6 | Yes | [38] |
| Bioassay, GFP-bb | 1.0 × 10−6 to 2.5 × 10−5 | 4.0 × 10−7 | Yes | [39] |
| Bioassay, GOx/Co3O4/GCE | 6.67 × 10−6 to 1.4 × 10−4 | 5 × 10−8 | Yes | [40] |
| Fluorescence | 5.00 × 10−6 to 2.50 × 10−4 | 2.00 × 10−8 | Yes | [41] |
| Colorimetric assay, Au NPs | 2.67 × 10−8 to 2.67 × 10−7 | 3.34 × 10−8 | Yes | [2] |
| Colorimetric assay, Au NPs | 6.67 × 10−8 to 4.00 × 10−5 | 7.07 × 10−8 | Yes | [42] |
| Colorimetric assay, Au NPs | 5.34 × 10−8 to 1.33 × 10−6 | 2.4 × 10−8 | No | [43] |
| Colorimetric assay, Rano-Cu NPs | 3.0 × 10−7 to 8.3 × 10−6 | 1.6 × 10−8 | No | Present work |
| Sample No. | As3+ Added (µM) | As3+ Recovered (μM) | Recovery (%) |
|---|---|---|---|
| 1 | 4.8 | 4.92 ± 0.12 | 102.5 |
| 2 | 5.6 | 5.5 ± 0.13 | 97.69 |
| 3 | 6.6 | 6.70 ± 0.10 | 101.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laghari, G.N.; Nafady, A.; Al-Saeedi, S.I.; Sirajuddin; Sherazi, S.T.H.; Nisar, J.; Shah, M.R.; Abro, M.I.; Arain, M.; Bhargava, S.K. Ranolazine-Functionalized Copper Nanoparticles as a Colorimetric Sensor for Trace Level Detection of As3+. Nanomaterials 2019, 9, 83. https://doi.org/10.3390/nano9010083
Laghari GN, Nafady A, Al-Saeedi SI, Sirajuddin, Sherazi STH, Nisar J, Shah MR, Abro MI, Arain M, Bhargava SK. Ranolazine-Functionalized Copper Nanoparticles as a Colorimetric Sensor for Trace Level Detection of As3+. Nanomaterials. 2019; 9(1):83. https://doi.org/10.3390/nano9010083
Chicago/Turabian StyleLaghari, Gul Naz, Ayman Nafady, Sameerah I. Al-Saeedi, Sirajuddin, Syed Tufail H. Sherazi, Jan Nisar, Muhammad Raza Shah, Mohammad I. Abro, Munazza Arain, and Suresh K. Bhargava. 2019. "Ranolazine-Functionalized Copper Nanoparticles as a Colorimetric Sensor for Trace Level Detection of As3+" Nanomaterials 9, no. 1: 83. https://doi.org/10.3390/nano9010083
APA StyleLaghari, G. N., Nafady, A., Al-Saeedi, S. I., Sirajuddin, Sherazi, S. T. H., Nisar, J., Shah, M. R., Abro, M. I., Arain, M., & Bhargava, S. K. (2019). Ranolazine-Functionalized Copper Nanoparticles as a Colorimetric Sensor for Trace Level Detection of As3+. Nanomaterials, 9(1), 83. https://doi.org/10.3390/nano9010083