Preparation and Enhanced Photocatalytic Properties of 3D Nanoarchitectural ZnO Hollow Spheres with Porous Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
2.4. Photocatalytic Activity Measurements
3. Results and Discussion
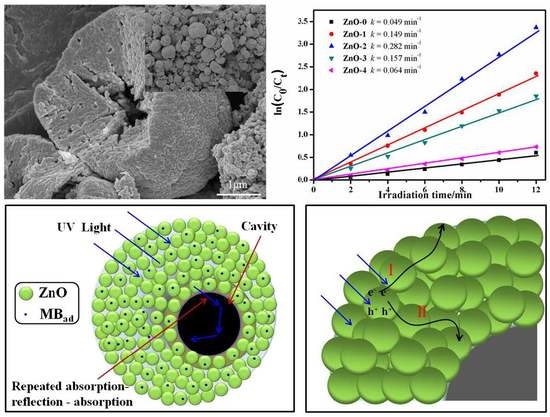
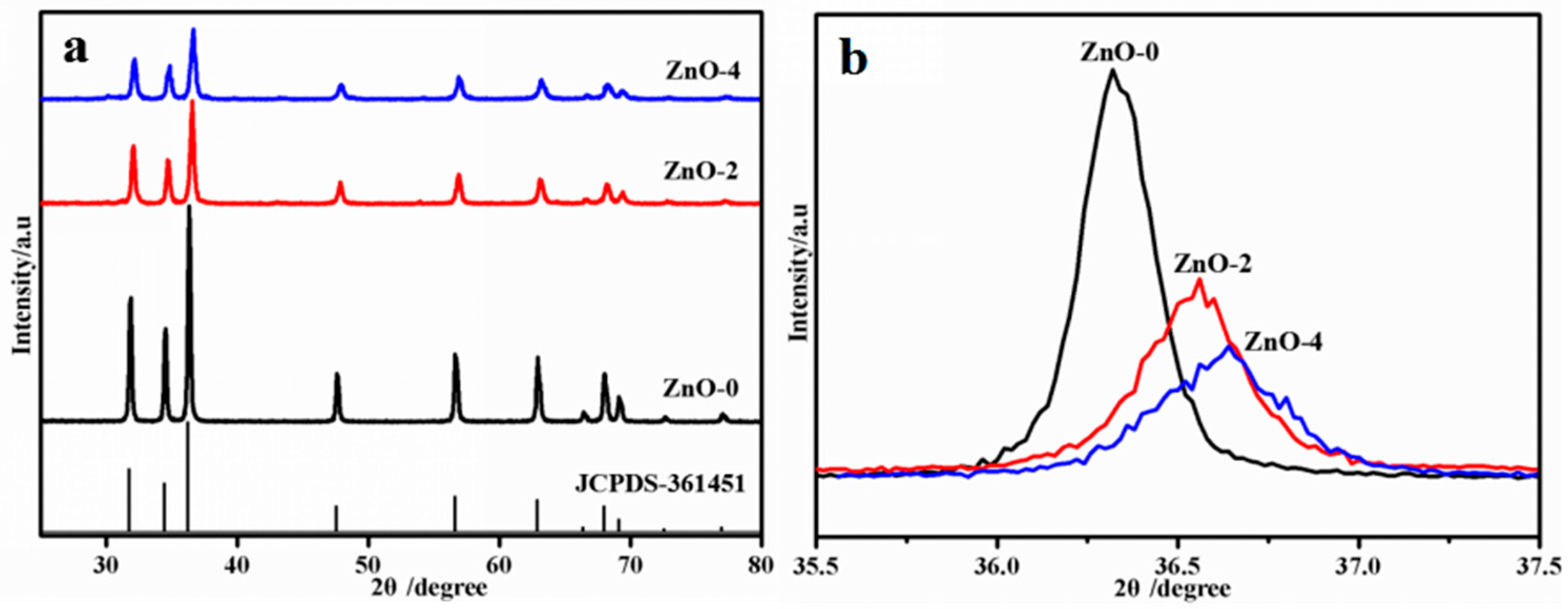
3.1. XRD Analysis
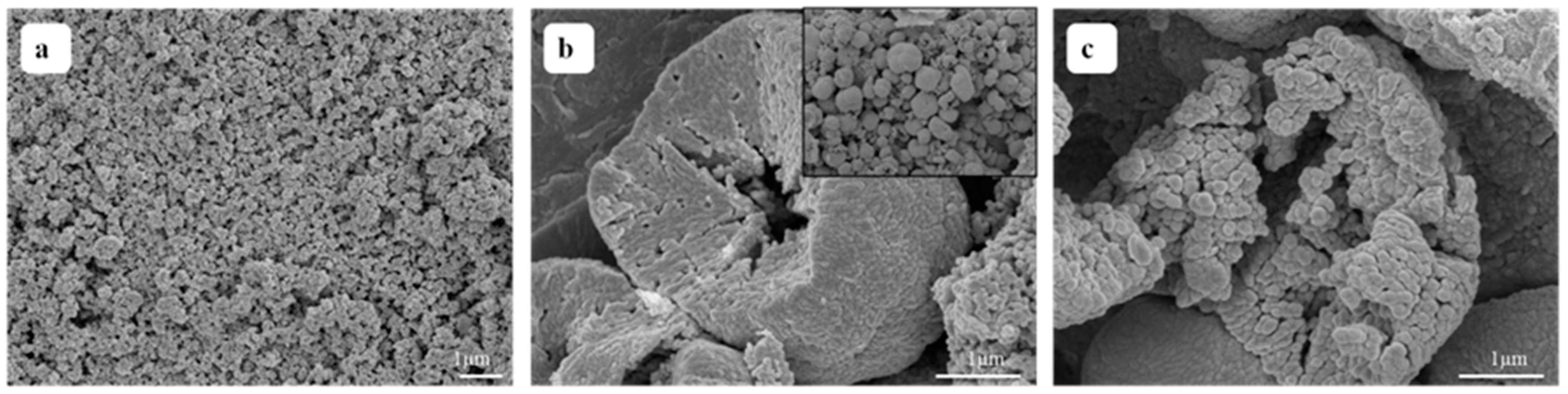
3.2. SEM Analysis
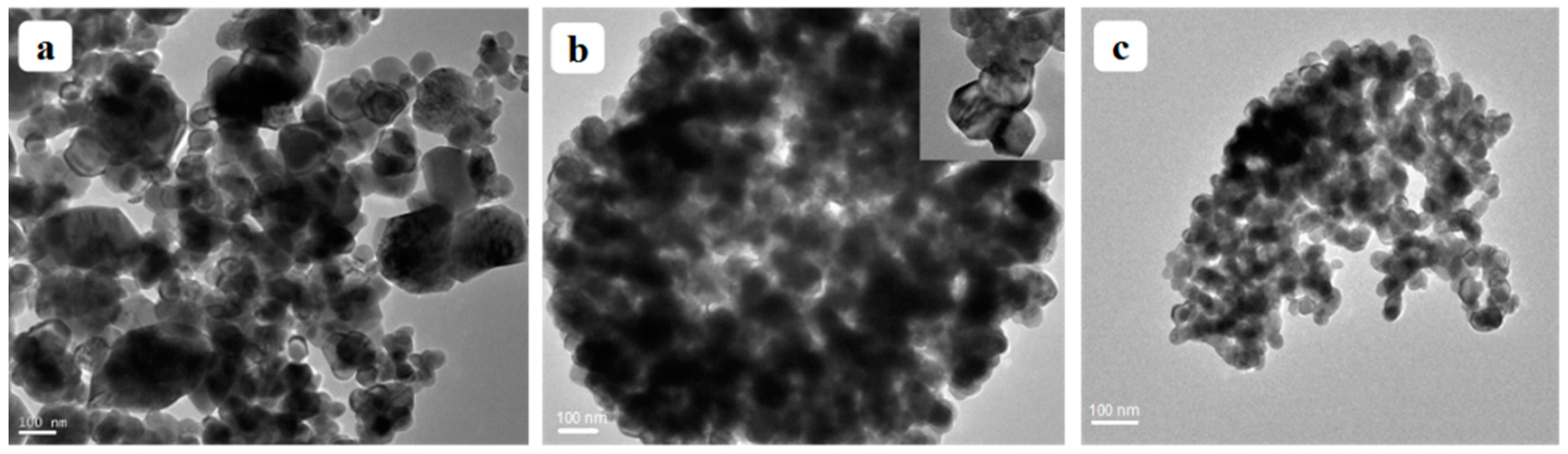
3.3. TEM Analysis
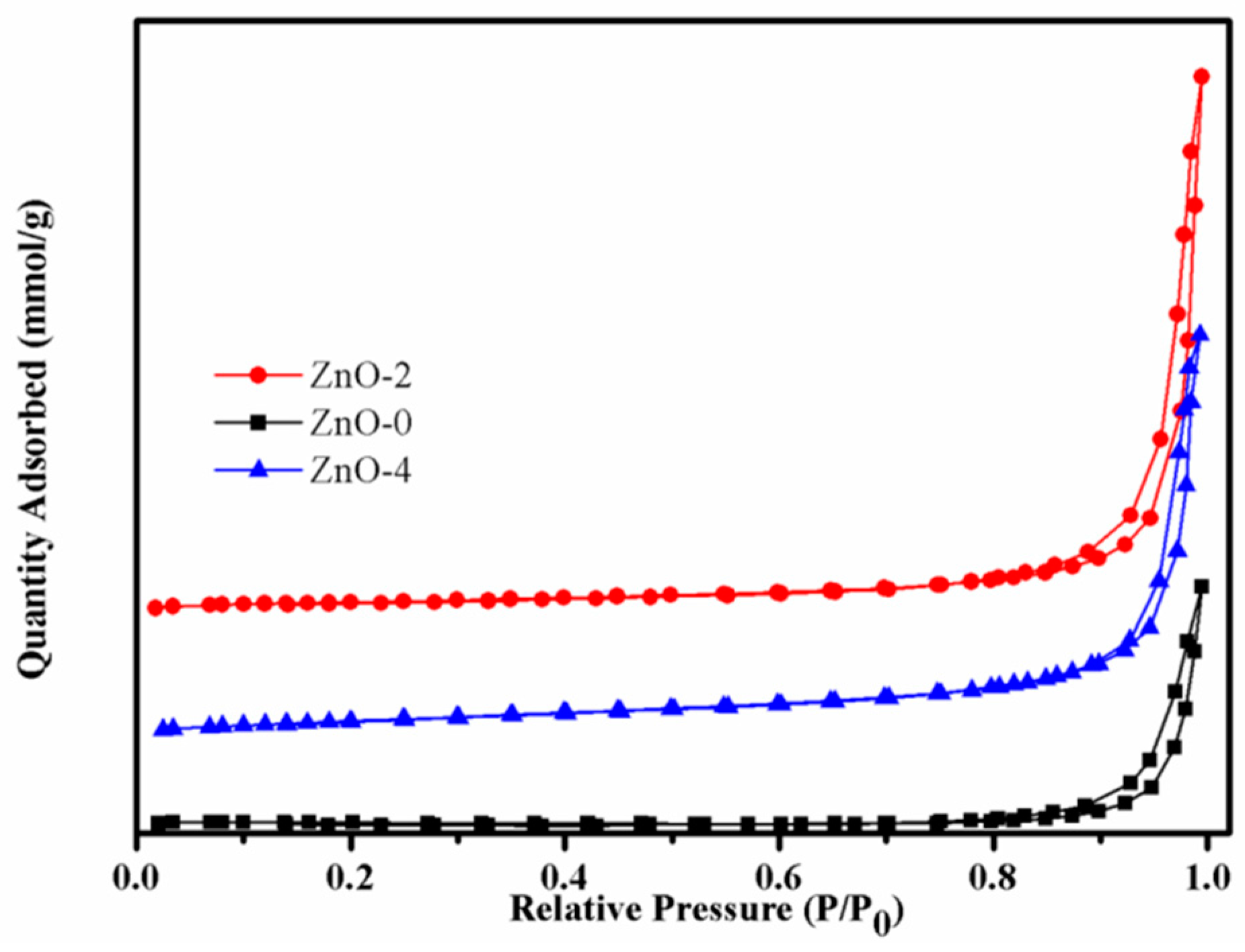
3.4. BET analysis
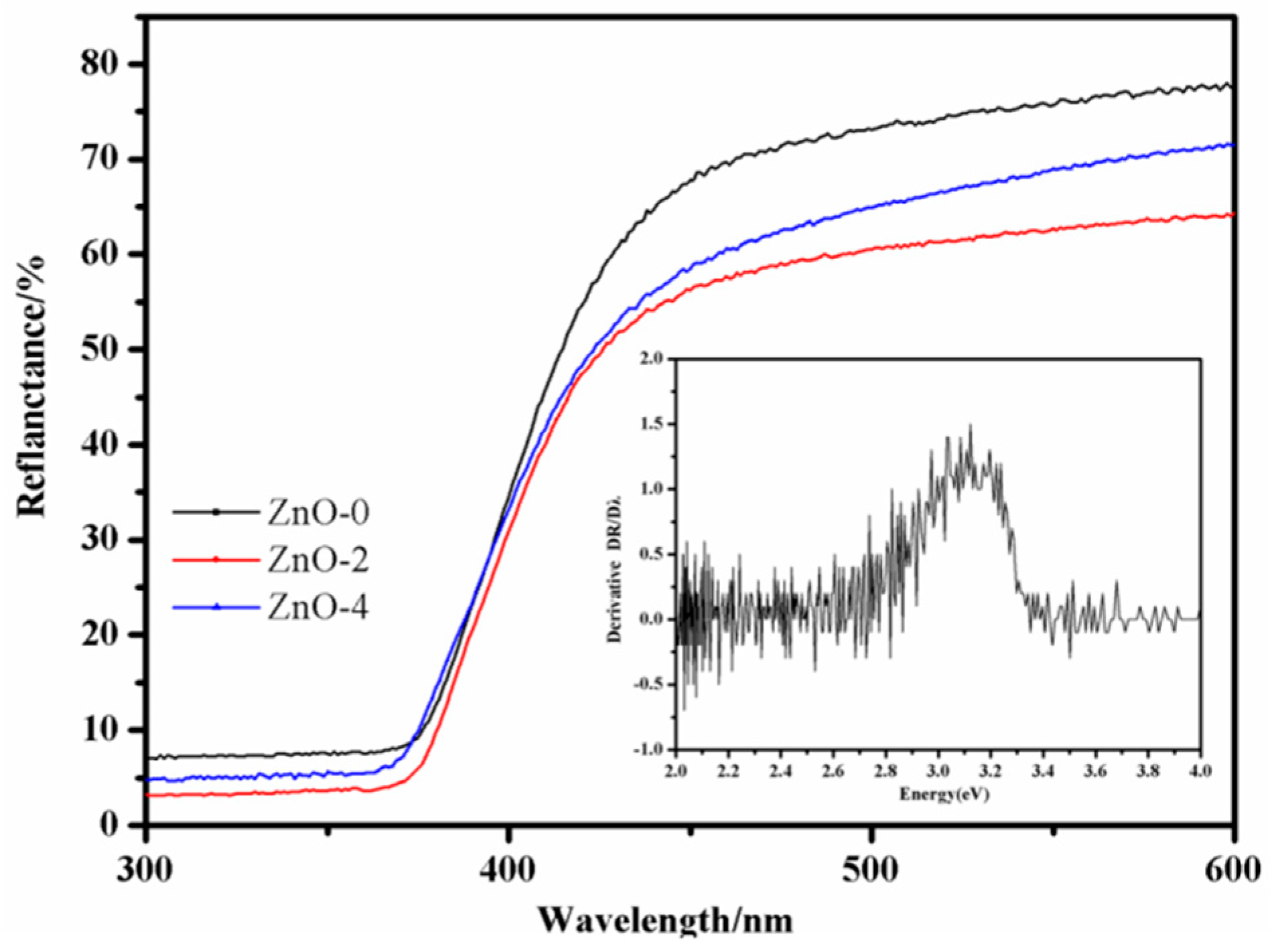
3.5. DRS Analysis
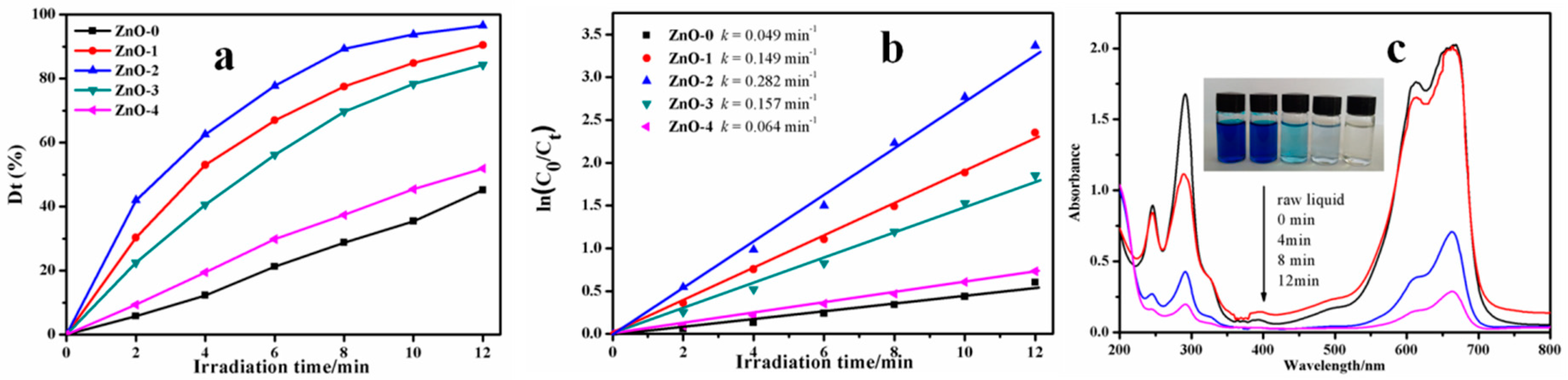
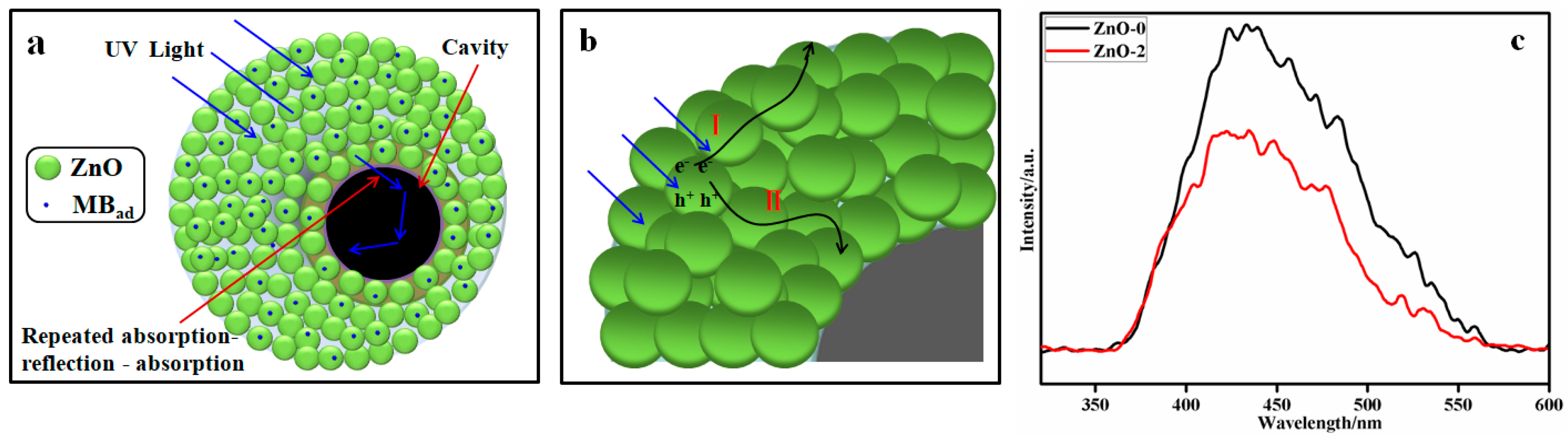
3.6. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Iervolino, G. Facile method to immobilize ZnO particles on glass spheres for the photocatalytic treatment of tannery wastewater. J. Colloid Interface Sci. 2018, 51, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Wang, M.; Ren, F.; Zhou, J.G.; Cai, G.X.; Li, C.; Hu, Y.F.; Wang, D.N.; Liu, Y.C.; Guo, L.J.; Shen, S.H. N doping to ZnO nanorods for photoelectrochemical water splitting under visible light: engineered impurity distribution and terraced band structure. Sci. Rep. 2015, 5, 12925. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Abdizadeh, H.; Golobostanfard, M.R. Formation of urchin-like ZnO nanostructures by sol-gel electrophoretic deposition for photocatalytic application. J. Alloys Compd. 2017, 725, 291–301. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Jin, J.; Liu, J.; Li, Y.; Wu, M.; Chen, L.H.; Wang, B.J.; Yang, X.Y.; Su, B.L. Probing effective photocorrosion inhibition and highly improved photocatalytic hydrogen production on monodisperse PANI@CdS core-shell nanospheres. Appl. Catal. B Environ. 2016, 188, 351–359. [Google Scholar] [CrossRef]
- Liang, X.Y.; Yang, X.Z.; Gao, G.J.; Li, C.F.; Li, Y.Y.; Zhang, W.D.; Chen, X.T.; Zhang, Y.B.; Zhang, B.B.; Lei, Y.Q.; et al. Performance and mechanism of CuO/CuZnAl hydrotalcites-ZnO for photocatalytic selective oxidation of gaseous methanol to methyl formate at ambient temperature. J. Catal. 2016, 339, 68–76. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, Y.M.; Zheng, Y.; Ding, K.; Nie, S.J.; Yang, P. Synthesis of butterfly-like ZnO nanostructures and study of their self-reducing ability toward Au3+ ions for enhanced photocatalytic efficiency. Phys. Chem. Chem. Phys. 2016, 18, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.S.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 2018, 448, 481–488. [Google Scholar] [CrossRef]
- Yu, W.L.; Zhang, J.F.; Peng, T.Y. New insight into the enhanced photocatalytic activity of N-, C- and S-doped ZnO photocatalysts. Appl. Catal. B Environ. 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.W.; Wang, T.; Ji, X.H. A facile approach for the fabrication of Au/ZnO-hollow-sphere-monolayer thin films and their photocatalytic properties. Appl. Surf. Sci. 2017, 412, 69–76. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Faisal, M.; Ibrahim, A.A.; Harraz, F.A.; Bouzid, H.; Al-Assiri, M.S.; Ismail, A.A. SnO2 doped ZnO nanostructures for highly efficient photocatalyst. J. Mol. Catal. A Chem. 2015, 397, 19–25. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sarı, F.; Gülce, H.D.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119–120, 197–206. [Google Scholar] [CrossRef]
- Chen, T.T.; Chang, I.C.; Yang, M.H.; Chiu, H.T.; Lee, C.Y. The exceptional photo-catalytic activity of ZnO/RGO composite via metal and oxygen vacancies. Appl. Catal. B Environ. 2013, 142–143, 442–449. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.Q.; Liu, W.F.; Yang, D.J. Facile preparation of well-combined lignin-based carbon/ZnO hybrid composite with excellent photocatalytic activity. Appl. Surf. Sci. 2017, 426, 206–216. [Google Scholar] [CrossRef]
- Lukas, S.; Judith, L.M. ZnO-nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Guo, Y.X.; Lin, S.W.; Li, X.; Liu, Y.P. Amino acids assisted hydrothermal synthesis of hierarchically structured ZnO with enhanced photocatalytic activities. Appl. Surf. Sci. 2016, 384, 83–91. [Google Scholar] [CrossRef]
- Su, B.T.; Dong, Y.Y.; Jin, Z.J.; Wang, Q.Z.; Lei, Z.Q. Enhanced photocatalytic performance of ZnO/rGO composite materials prepared via an improved two-steps method. Ceram. Int. 2016, 42, 7632–7638. [Google Scholar] [CrossRef]
- Dong, N.; He, F.Z.; Xin, J.L.; Wang, Q.Z.; Lei, Z.Q.; Su, B.T. A novel one-step hydrothermal method to prepare CoFe2O4/graphene-like carbons magnetic separable adsorbent. Mater. Res. Bull. 2016, 80, 186–190. [Google Scholar] [CrossRef]
- Gröttrup, J.; Schütt, F.; Smazna, D.; Lupan, O.; Adelung, R.; Mishra, Y.K. Porous ceramics based on hybrid inorganic tetrapodal networks for efficient photocatalysis and water purification. Ceram. Int. 2017, 43, 14915–14922. [Google Scholar] [CrossRef]
- Gröttrup, J.; Paulowicz, I.; Schuchardt, A.; Kaidas, V.; Kaps, S.; Lupan, O.; Adelung, R.; Mishra, Y.K. Three-dimensional flexible ceramics based on interconnected network of highly porous pureand metal alloyed ZnO tetrapods. Ceram. Int. 2016, 42, 8664–8676. [Google Scholar] [CrossRef]
- Xie, X.; Wang, X.; Tian, J.; Liu, J.; Cao, H.; Song, X.; Wei, N.; Cui, H. Facile synthesis and superior ethyl acetate sensing performance of Au decorated ZnO flower-like architectures. Ceram. Int. 2017, 43, 5053–5060. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Kuk, H.; Lee, D.H.; Hahn, Y.B.; Khang, G. Rapid methyl orange degradation using porous ZnO spheres photocatalyst. J. Photochem. Photobiol. B 2016, 161, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, H.J.; Yuan, S.; Jiao, Z.; Zhu, X.D. Preparation of flower-like ZnO architectures assembled with nanosheets for enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 462, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, H.H.; Peng, D.L.; Chen, T.; Dong, S.J.; Chang, Y. Synthesis and properties of Au/ZnO nanorods as a plasmonic photocatalyst. Physica E 2016, 78, 41–48. [Google Scholar] [CrossRef]
- Camaratta, R.; Messana, J.O.; Bergmann, C.P. Synthesis of ZnO through biomimetization of eggshell membranes using different precursors and its characterization. Ceram. Int. 2015, 41, 14826–14833. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wang, X.D.; Wang, Z.L. Controlled replication of butterfly wings for achieving tunable photonic properties. Nano Lett. 2006, 6, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Su, B.T.; Xin, J.L.; Li, J.J.; Dong, N.; Shao, K.R.; Wang, Q.Z.; Lei, Z.Q. Comparing the photocatalytic property of TiO2 nanoparticles and structured nanofiber materials. Micro Nano Lett. 2016, 11, 323–325. [Google Scholar] [CrossRef]
- Su, B.T.; Xin, J.L.; Li, J.J.; Zheng, T.; Wang, Q.Z.; Lei, Z.Q. The role of multi-level structure for the improved photocatalytic performance of TiO2 fiber nanomaterials. Appl. Phys. A Mater. 2016, 122, 32. [Google Scholar] [CrossRef]
- Zheng, T.; Tian, Z.; Su, B.T.; Lei, Z.Q. Facile method to prepare TiO2 hollow fiber materials via replication of cotton fiber. Ind. Eng. Chem. Res. 2012, 51, 1391–1395. [Google Scholar] [CrossRef]
- Su, B.T.; Wang, K.; Dong, N.; Mu, H.M.; Lei, Z.Q.; Tong, Y.C.; Bai, J. Biomorphic synthesis of long ZnO hollow fibers with porous walls. J. Mater. Process. Technol. 2009, 209, 4088–4092. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Yang, H.; Wang, T.; Wang, C. Green synthesis and antimicrobial activity of monodisperse silver nanoparticles synthesized using Ginkgo Biloba leaf extract. Phys. Lett. A 2016, 380, 3773–3777. [Google Scholar] [CrossRef]
- Yang, Z.W.; Xu, X.Q.; Liang, X.X.; Lei, C.; Cui, Y.H.; Wu, W.H.; Yang, Y.X.; Zhang, Z.; Lei, Z.Q. Construction of heterostructured MIL-125/Ag/g-C3N4 nanocomposite as an efficient bifunctional visible light photocatalyst for the organic oxidation and reduction reactions. Appl. Catal. B Environ. 2017, 205, 42–54. [Google Scholar] [CrossRef]
- Jin, Z.J.; Dong, Y.Y.; Dong, N.; Yang, Z.W.; Wang, Q.Z.; Lei, Z.Q.; Su, B.T. One-step synthesis of magnetic nanocomposite Fe3O4/C based on the waste chicken feathers by a green solvothermal method. Mater. Lett. 2017, 186, 322–325. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Gröttrup, J.; Mishra, A.K.; de Leeuw, N.H.; Carreira, J.F.C.; Rodrigues, J.; Ben Sedrine, N.; Correia, M.R.; Monteiro, T.; et al. Hybridization of zinc oxide tetrapods for selective gas sensing applications. ACS Appl. Mater. Interfaces 2017, 9, 4084–4099. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Z.; Ren, T.; Ding, H.; Yao, W.; Zong, R.; Zhu, Y. Influence of Defects on the Photocatalytic Activity of ZnO. J. Phys. Chem. C 2014, 118, 15300–15307. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today. 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, S.; Lim, S.K.; Park, H. Photocatalytic comparison of TiO2 nanoparticles and electrospun TiO2 nanofibers: Effects of mesoporosity and interparticle charge transfer. J. Phys. Chem. C. 2010, 114, 16475–16480. [Google Scholar] [CrossRef]
- Zhou, Y.; Shuai, L.; Jiang, X.Y.; Jiao, F.; Yu, J. Visible-light-driven photocatalytic properties of layered double hydroxide supported-Bi2O3 modified by Pd(II) for methylene blue. Adv. Powder Technol. 2015, 26, 439–447. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Han, L.; Han, Y.; Yang, Z.; Su, B.; Lei, Z. Preparation and Enhanced Photocatalytic Properties of 3D Nanoarchitectural ZnO Hollow Spheres with Porous Shells. Nanomaterials 2018, 8, 687. https://doi.org/10.3390/nano8090687
Li L, Han L, Han Y, Yang Z, Su B, Lei Z. Preparation and Enhanced Photocatalytic Properties of 3D Nanoarchitectural ZnO Hollow Spheres with Porous Shells. Nanomaterials. 2018; 8(9):687. https://doi.org/10.3390/nano8090687
Chicago/Turabian StyleLi, Lan, Lijuan Han, Yuqi Han, Zhiwang Yang, Bitao Su, and Ziqiang Lei. 2018. "Preparation and Enhanced Photocatalytic Properties of 3D Nanoarchitectural ZnO Hollow Spheres with Porous Shells" Nanomaterials 8, no. 9: 687. https://doi.org/10.3390/nano8090687
APA StyleLi, L., Han, L., Han, Y., Yang, Z., Su, B., & Lei, Z. (2018). Preparation and Enhanced Photocatalytic Properties of 3D Nanoarchitectural ZnO Hollow Spheres with Porous Shells. Nanomaterials, 8(9), 687. https://doi.org/10.3390/nano8090687