Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal
Abstract
1. Introduction
2. Materials and Methods
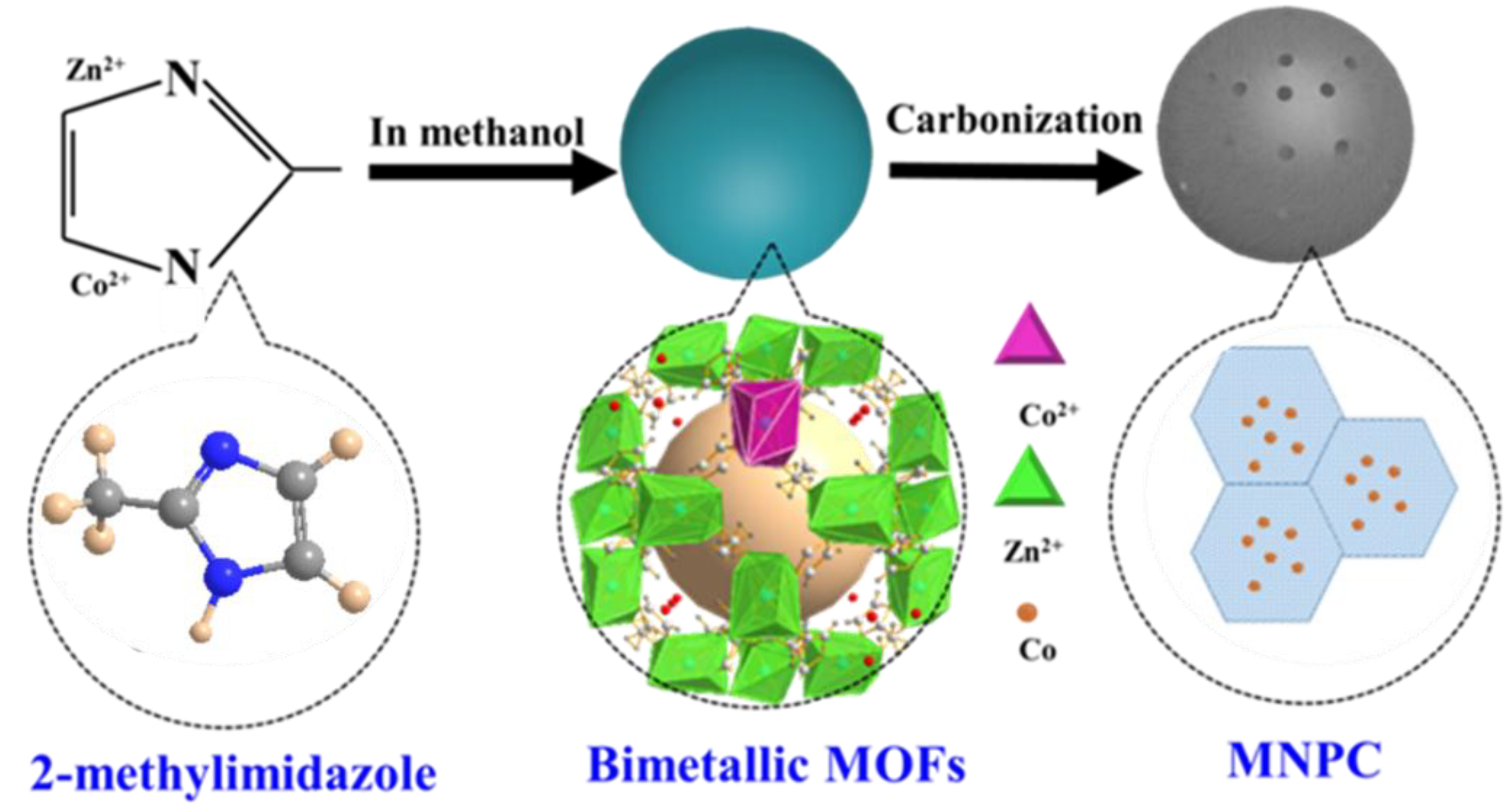
2.1. Synthesis of MNPC
2.2. Adsorption Performance of MNPC
3. Results
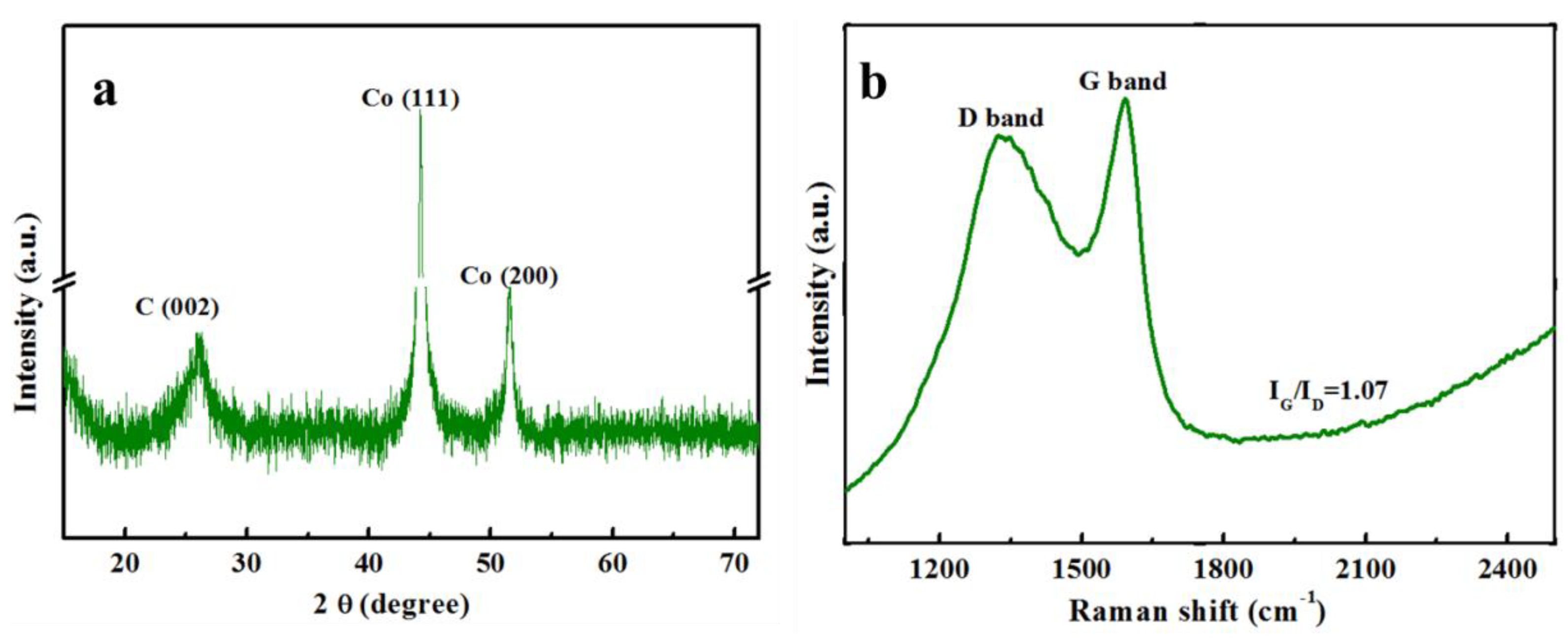
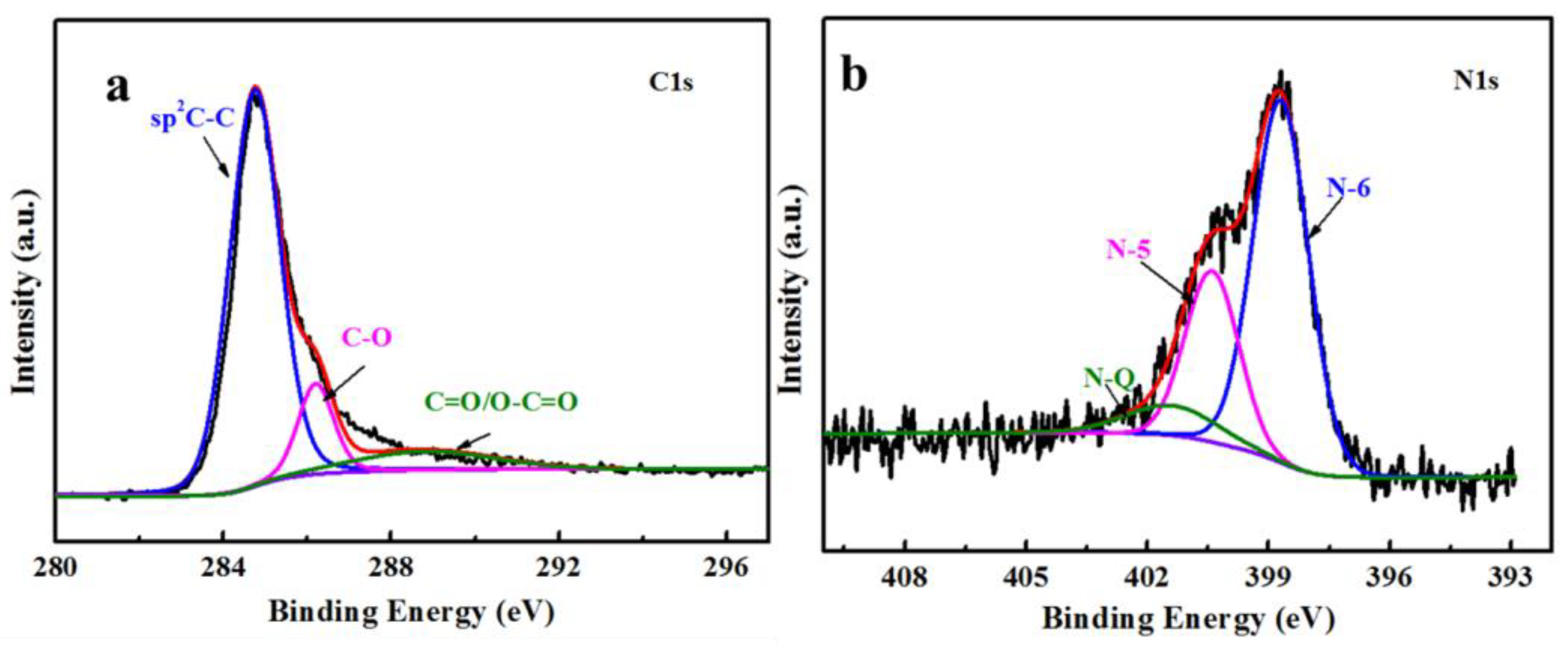
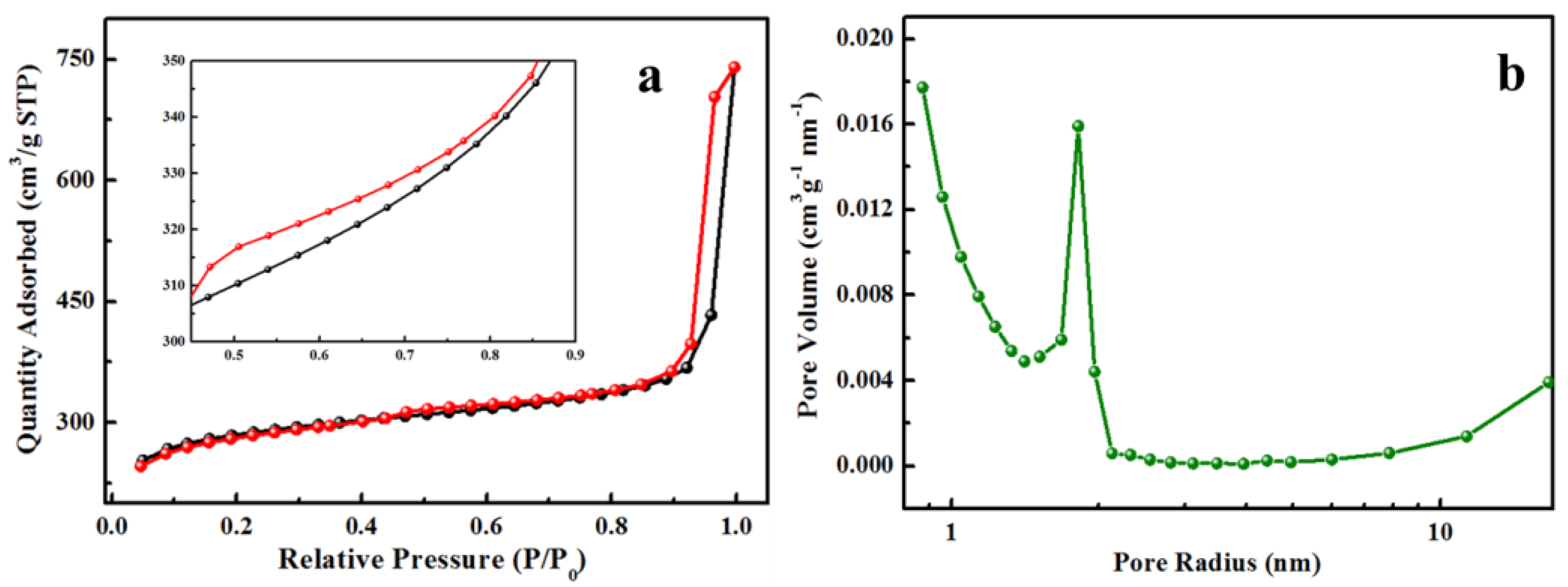
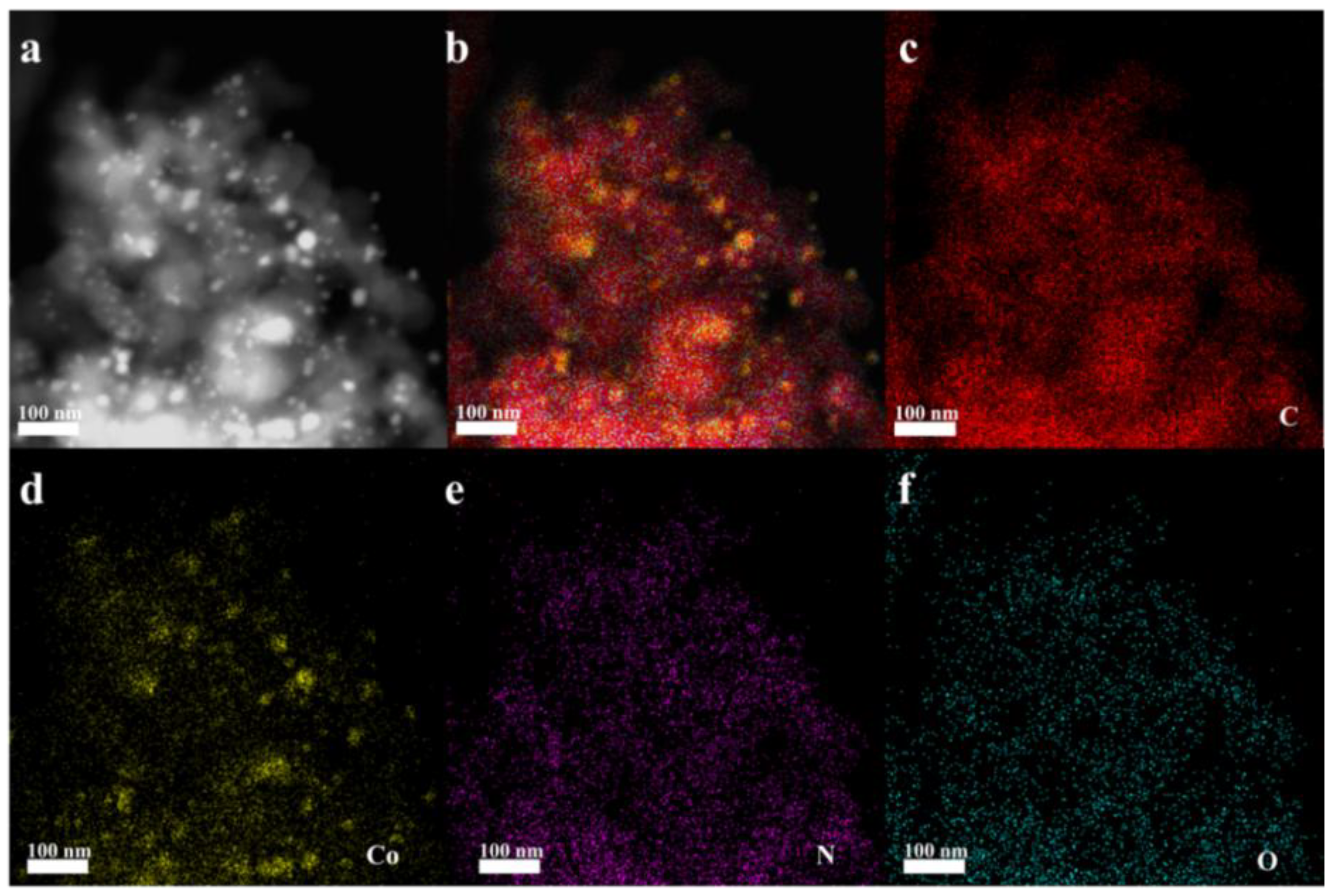
3.1. Characterization
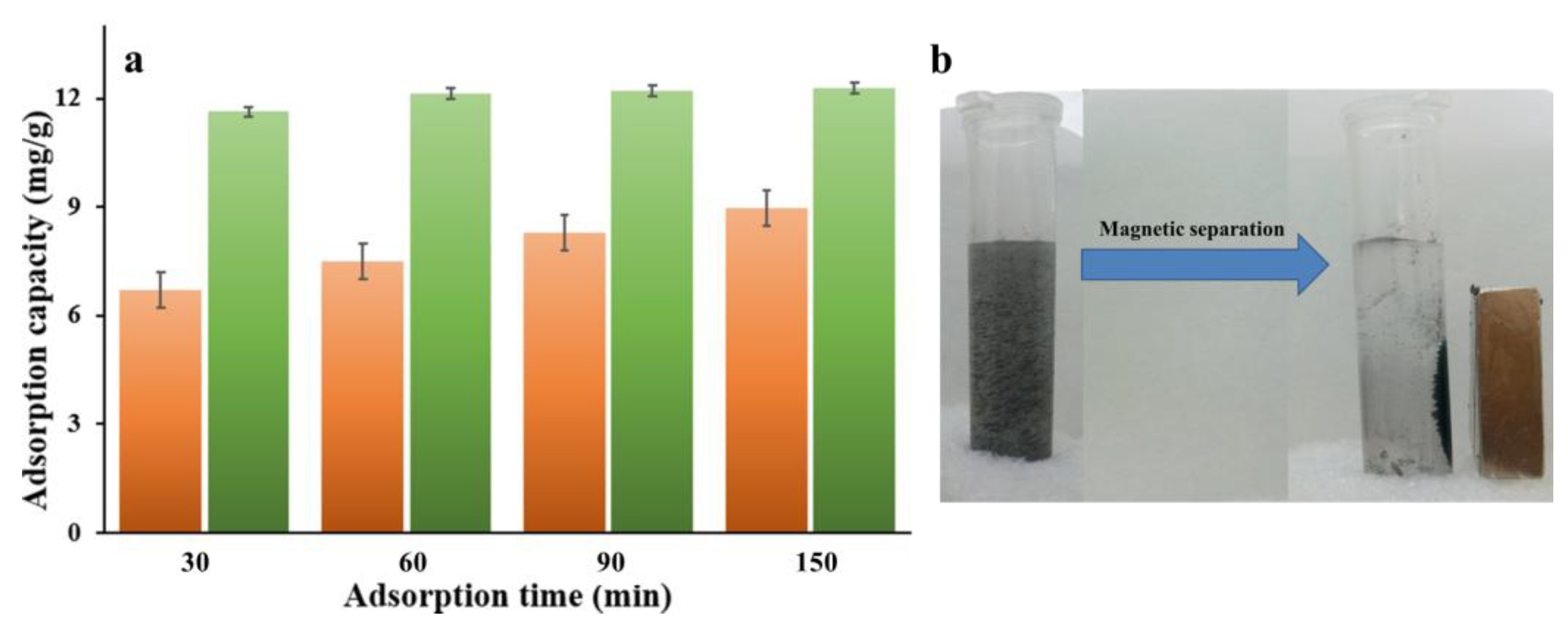
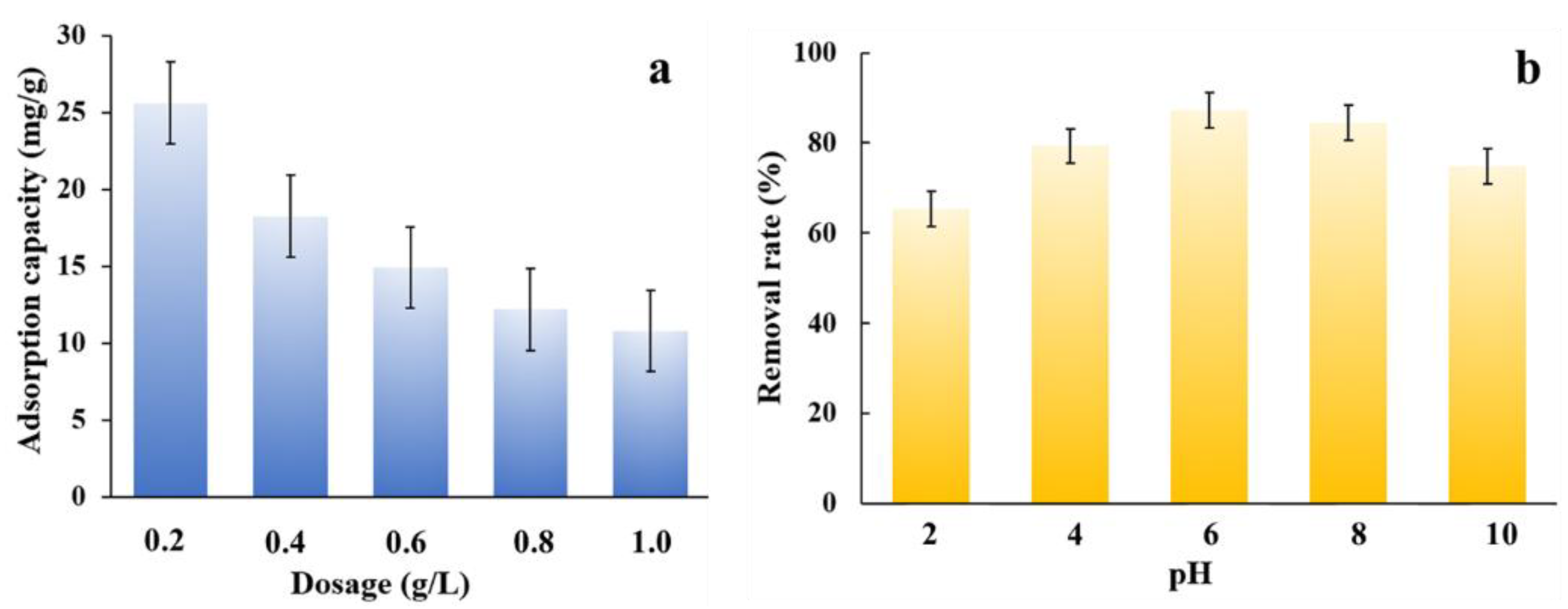
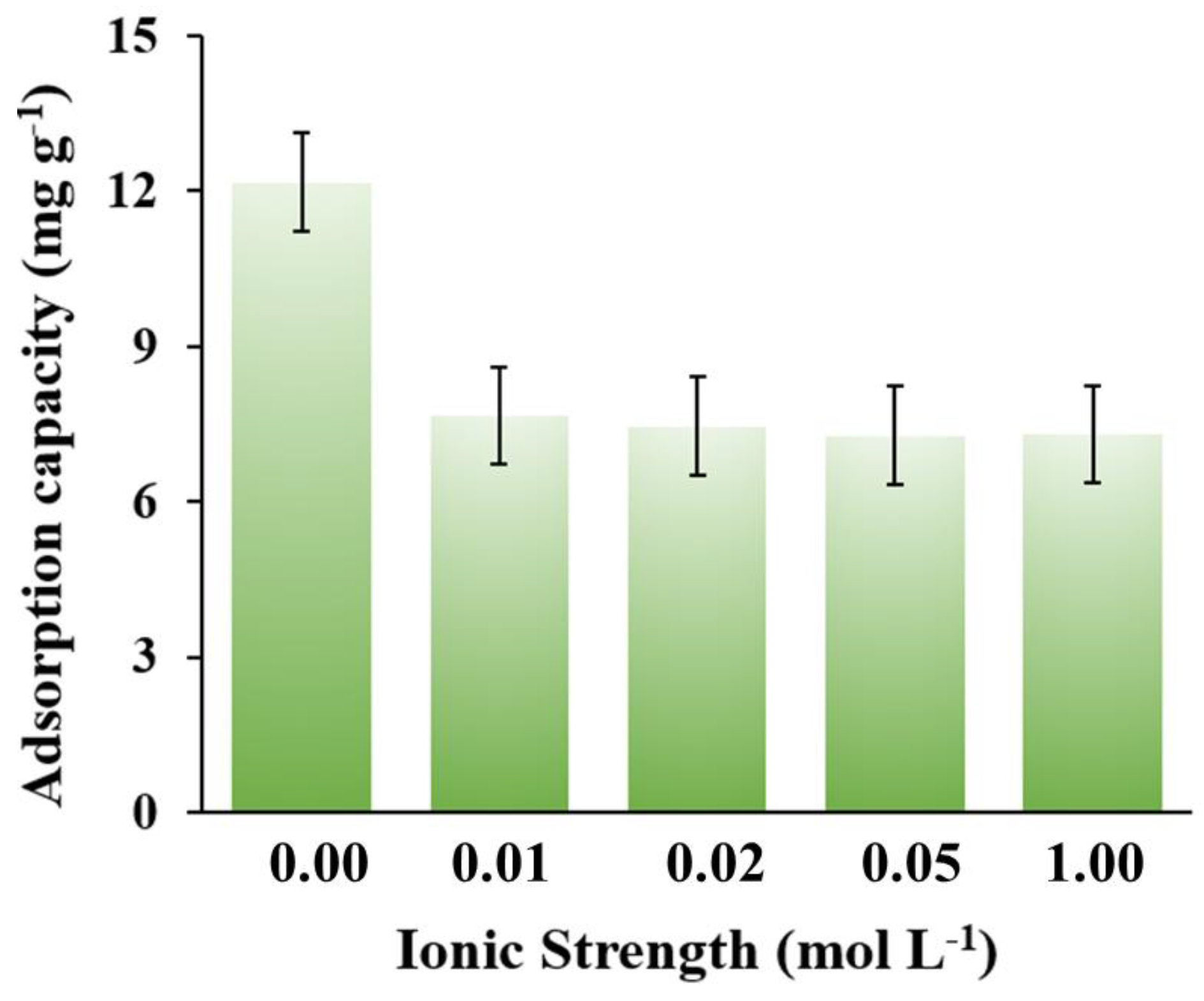
3.2. Adsorption Performance
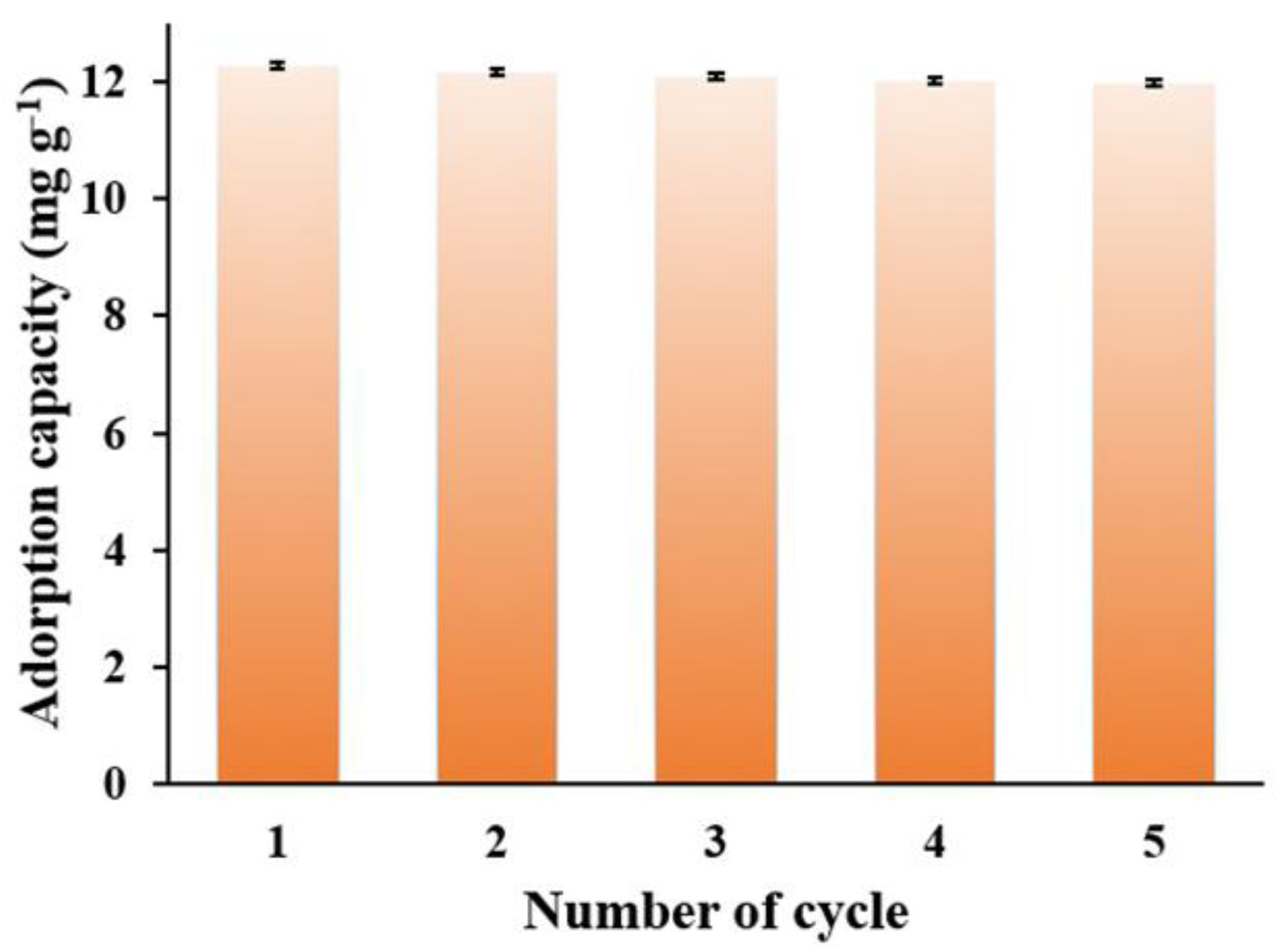
3.3. Recyclability
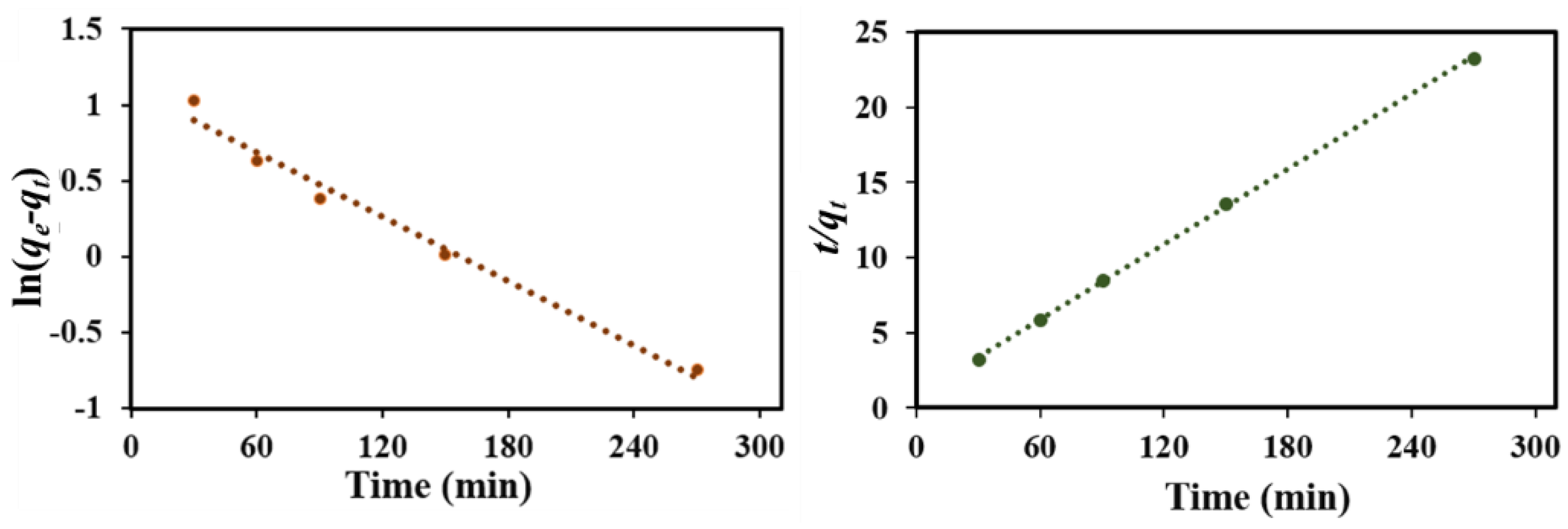
3.4. Adsorption Kinetics
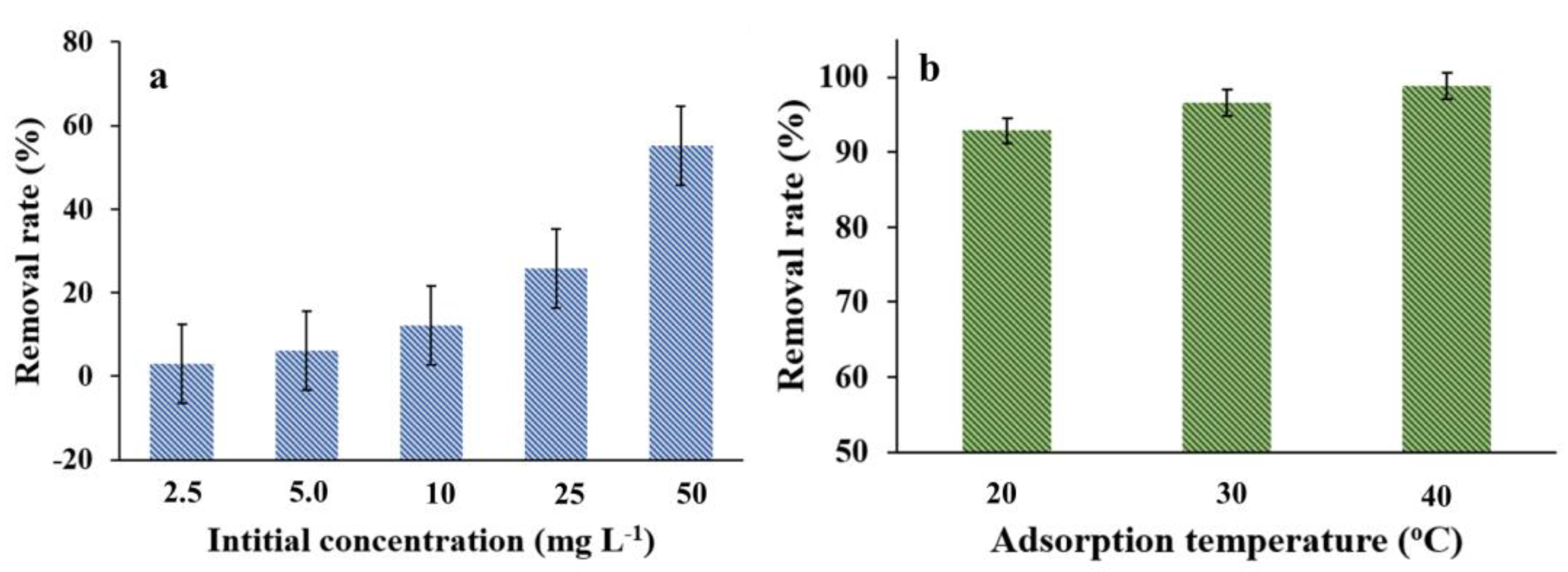
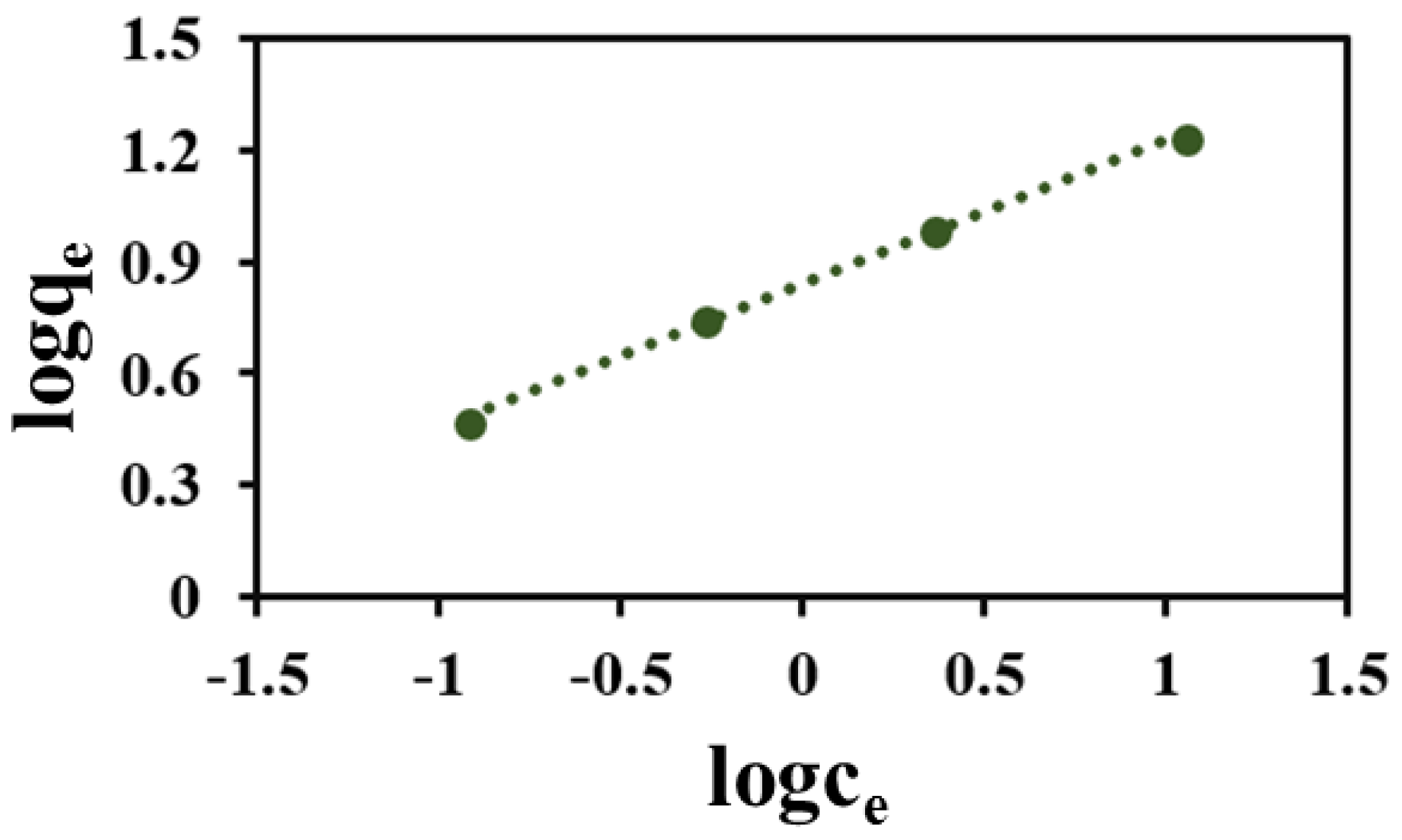
3.5. Adsorption Isotherm
3.6. Adsorption Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Jia, Y.; Khanal, S.K.; Lu, H.; Fang, H.; Zhao, Q. Understanding the role of extracellular polymeric substances (EPS) on ciprofloxacin (CIP) adsorption in aerobic sludge, anaerobic sludge and sulfate-reducing bacteria (SRB) sludge systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhao, N.; Quan, X.; Chen, S.; Yu, H. Enhanced adsorption of ionizable antibiotics on activated carbon fiber under electrochemical assistance in continuous-flow modes. Water Res. 2018, 134, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, C.; Che, H.; Hu, H.; Hu, W.; Liu, C.; Ai, J.; Dong, H. Decoration of mesoporous Co3 O4 nanospheres assembled by monocrystal nanodots on g-C3 N4 to construct Z-scheme system for improving photocatalytic performance. Appl. Surf. Sci. 2018, 440, 308–319. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.L.; Feng, D.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.R.; Zhou, H.C. Highly stable Zr(IV)-based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lu, Y.; Zhang, X.; Chen, J.; Xu, S.; Li, X.; Li, X.; Tan, F. Elucidating the electrostatic interaction of sulfonic acid functionalized SBA-15 for ciprofloxain adsorption. Appl. Surf. Sci. 2015, 349, 224–229. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H.; Wang, J. Kinetics, electron-donor-acceptor interactions, and site energy distribution analyses of norfloxacin adsorption on pretreated barley straw. Chem. Eng. J. 2017, 330, 1211–1221. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Zhang, T.; Qiu, F.; Dai, H. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: A batch and fixed-bed column study. Bioresour. Technol. 2018, 249, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Li, F.; Huang, X.; Tian, B.; Hao, H. Highly efficient and reusable montmorillonite/Fe3O4/humic acid nanocomposites for simultaneous removal of Cr(VI) and aniline. Nanomaterials 2018, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yu, X.D.; Pan, B.; Xing, B.S. Norfloxacin sorption and its thermodynamics on surface-modified carbon nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Turco, A.; Monteduro, A.G.; Mazzotta, E.; Maruccio, G.; Malitesta, C. An innovative porous nanocomposite material for the removal of phenolic compounds from aqueous solutions. Nanomaterials 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.W.; Zhou, H.L.; Zhou, D.D.; Lin, R.B.; Liao, P.Q.; He, C.T.; Zhang, W.X.; Chen, X.M.; Zhang, J.P. Mesoporous metal-organic frameworks with exceptionally high working capacities for adsorption heat transformation. Adv. Mater. 2018, 30, 1704350. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Comm. 2014, 5, 5261. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Yu, L.; Lou, X.W. A dual-metal-organic-frameworks derived electrocatalyst for oxygen reduction. Energy Environ. Sci. 2016, 9, 3092–3096. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Kim, T.; Im, J.H.; Kim, Y.S.; Lee, K.; Jung, H.; Park, C.R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 2012, 24, 464–470. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huang, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: from materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Likon, M.; Cernec, F.; Svegl, F.; Saarela, J.; Zimmie, T.F. Papermill industrial waste as a sustainable source for high efficiency absorbent production. Waste Manage. 2011, 31, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Z.; Wu, R.; Wang, Z.; Chen, X.; Chan, T.D. Magnetic porous carbon derived from a bimetallic metal-organic framework for magnetic solid-phase extraction of organochlorine pesticides from drinking and environmental water samples. J. Chromatogr. A 2017, 1479, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interface Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, D.; Mei, G.; Hong, X.; Wu, J.; Zheng, J.; Pang, J.; Yan, Z. Preparation of konjac glucomannan-based zeolitic imidazolate framework-8 composite aerogels with high adsorptive capacity of ciprofloxacin from water. Colloid. Surface. A 2018, 544, 187–195. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Y.; Zhang, X.; Meng, Z.; Cai, Y. Easy synthesis of surface-tunable carbon-encapsulated magnetic nanoparticles: adsorbents for selective isolation and preconcentration of organic pollutants. ACS Appl. Mater. Inter. 2012, 4, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-enabled in Situ solvent and reagent recycle for sustainable continuous-flow synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Schaepertoens, M.; Didaskalou, C.; Kim, J.F.; Livingston, A.G.; Szekely, G. Solvent recycle with imperfect membranes: A semi-continuous workaround for diafiltration. J. Memb. Sci. 2016, 514, 646–658. [Google Scholar] [CrossRef]
- Li, N.; Yang, S.; Chen, J.; Gao, J.; He, H.; Sun, C. Electro-adsorption of tetracycline from aqueous solution by carbonized pomelo peel and composite with aniline. Appl. Surf. Sci. 2016, 386, 460–466. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.H.; Jiang, H.L. From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.L.; Hu, M.; Ishihara, S.; Sukegawa, H.; Belik, A.A.; Imura, M.; Ariga, K.; Sakka, Y.; Yamauchi, Y. Direct synthesis of MOF-derived nanoporous carbon with magnetic Co nanoparticles toward efficient water treatment. Small 2014, 10, 2096–2107. [Google Scholar]
- Ling, L.L.; Liu, W.J.; Zhang, S.; Jiang, H. Magnesium Oxide Embedded nitrogen self-doped biochar composites: fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ. Sci. Technol. 2017, 51, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Yan, T.T.; Wang, H.; Chen, G.R.; Huang, L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. High capacity and high rate capability of nitrogen-doped porous hollow carbon spheres for capacitive deionization. Appl. Surf. Sci. 2016, 369, 460–469. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Wang, M.; Ge, X. Fibrous N-doped hierarchical porous carbon microspheres: Synthesis and adsorption performance. Chem. Eng. J. 2017, 323, 224–232. [Google Scholar] [CrossRef]
- Wang, H.; Shi, L.; Yan, T.; Zhang, J.; Zhong, Q.; Zhang, D. Design of graphene-coated hollow mesoporous carbon spheres as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2014, 2, 4739–4750. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Zhang, Z.; Lei, Z. Formation of carbon nanosheets via simultaneous activation and catalytic carbonization of macroporous anion-exchange resin for supercapacitors application. ACS Appl. Mater. Interfaces 2014, 6, 20795–20803. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, D.S.; Yan, T.T.; Wen, X.R.; Zhang, J.P.; Shi, L.Y.; Zhong, Q.D. Three-dimensional macroporous graphene architectures as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2013, 1, 11778–11789. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Han, X.; Wang, H.; Zhang, L. Efficient removal of methyl blue using nanoporous carbon from the waste biomass. Water Air Soil Poll. 2018, 229, 26. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, X.; Wang, Y.; Quan, G.; Han, X.; Yan, J. Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal. Nanomaterials 2018, 8, 664. https://doi.org/10.3390/nano8090664
Wang H, Zhang X, Wang Y, Quan G, Han X, Yan J. Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal. Nanomaterials. 2018; 8(9):664. https://doi.org/10.3390/nano8090664
Chicago/Turabian StyleWang, Hui, Xi Zhang, Yan Wang, Guixiang Quan, Xiangyun Han, and Jinlong Yan. 2018. "Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal" Nanomaterials 8, no. 9: 664. https://doi.org/10.3390/nano8090664
APA StyleWang, H., Zhang, X., Wang, Y., Quan, G., Han, X., & Yan, J. (2018). Facile Synthesis of Magnetic Nitrogen-Doped Porous Carbon from Bimetallic Metal–Organic Frameworks for Efficient Norfloxacin Removal. Nanomaterials, 8(9), 664. https://doi.org/10.3390/nano8090664




