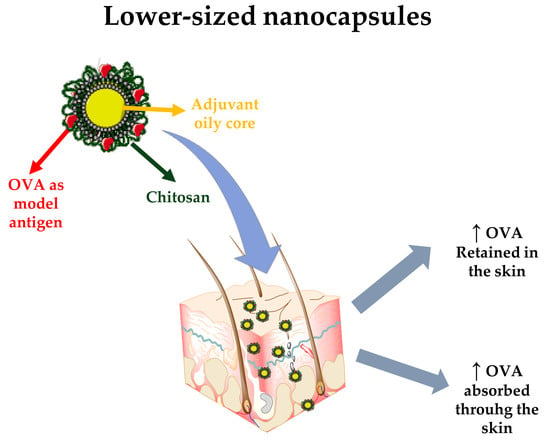
Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Development of Nanosystems
2.3. Physicochemical Characterization of the Nanosystems
2.4. Stability of Nanocapsules upon Physiological/RPMI and Storage Conditions
2.5. Ovalbumin Association to the Nanocapsules
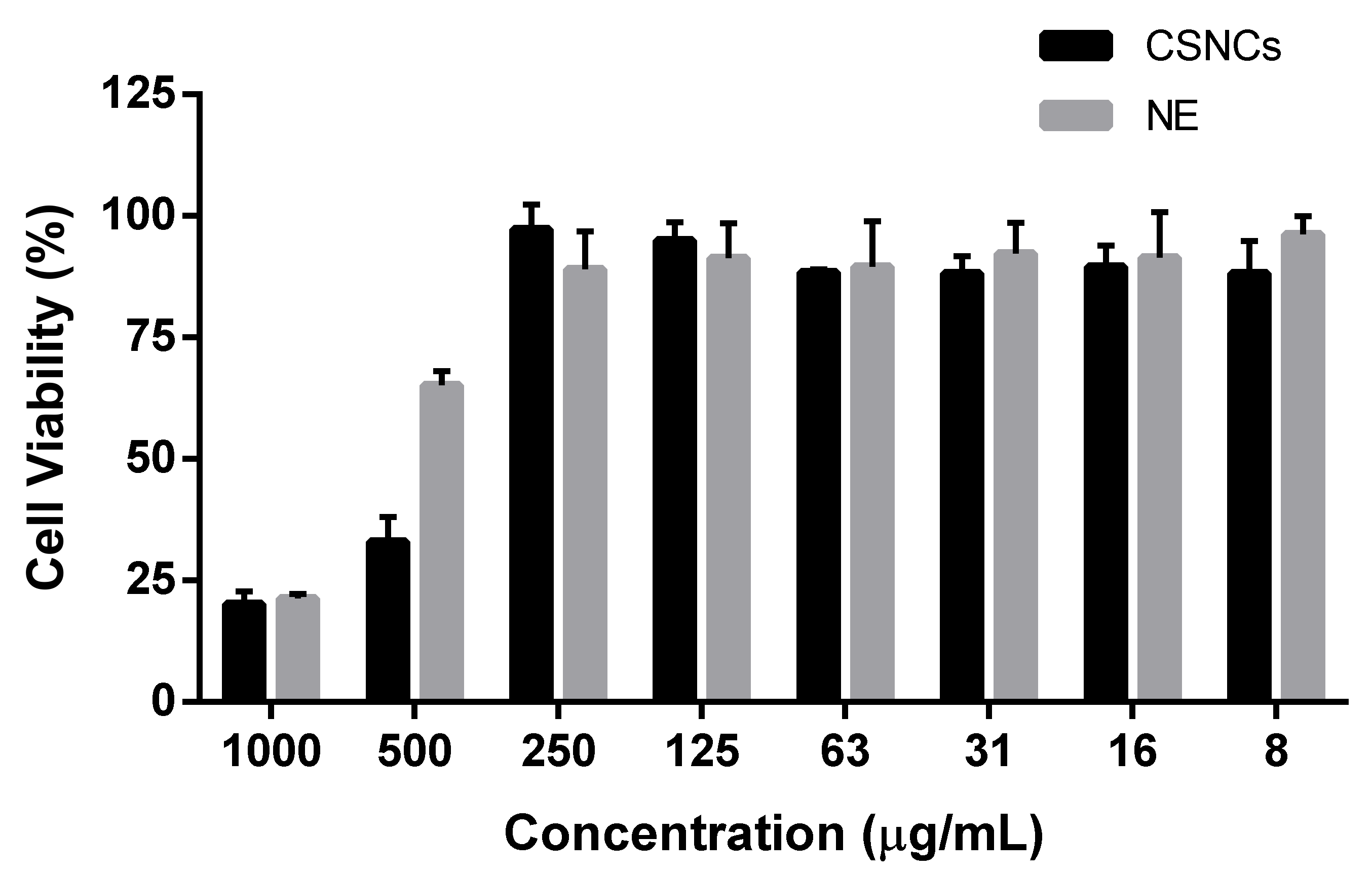
2.6. Study of the Cell Viability of the Nanosystems
- λ1 = 570 nm, λ2 = 600 nm
- (εOX) λ2 = Molar Extinction coefficient for alamarBlue® at 600 nm: 117,216
- (εOX) λ1 = Molar Extinction coefficient for alamarBlue® at 570 nm: 80,586
- Aλ1 = Observed absorbance reading for test well
- Aλ2 = Observed absorbance reading for test well
- A°λ1 = Observed absorbance reading for negative control well
- A°λ2 = Observed absorbance reading for negative control well
2.7. Study of Complement Activation by Chitosan Nanocapsules
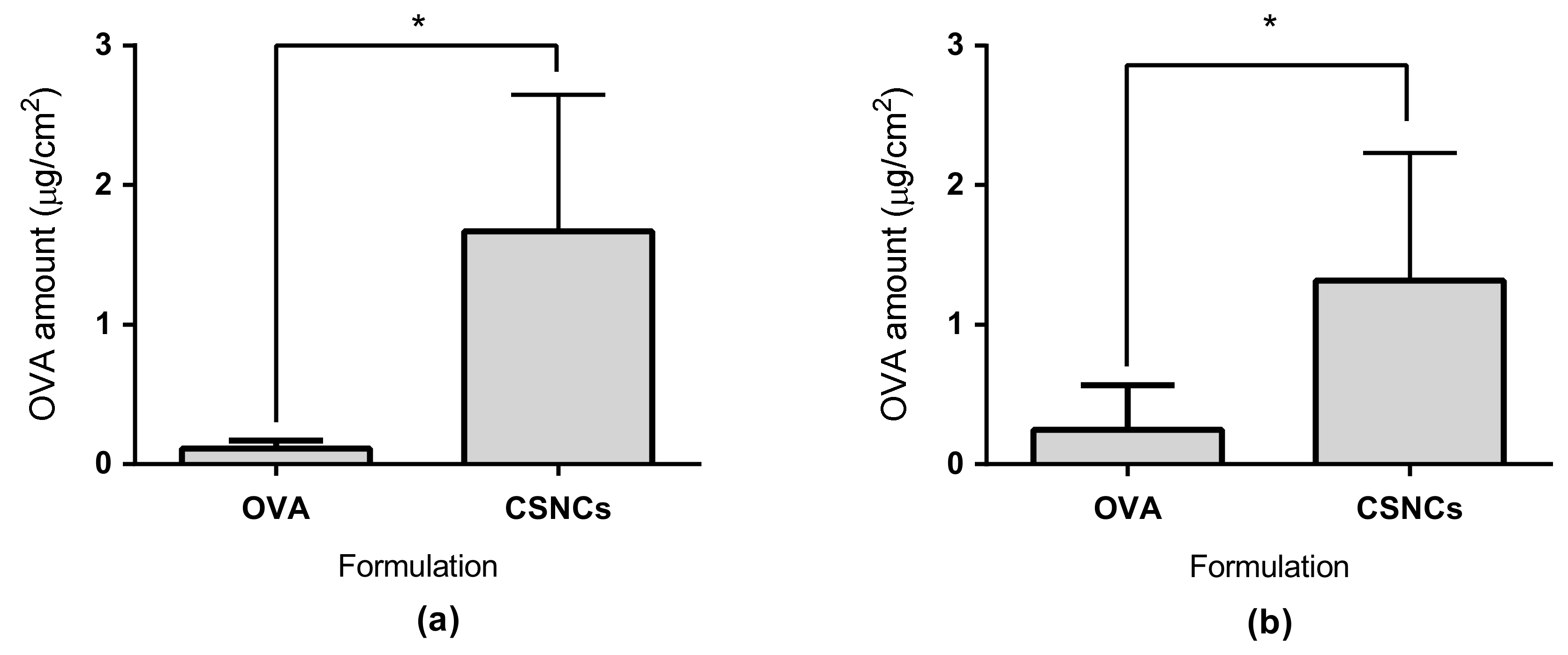
2.8. Transdermal Studies
2.9. Data Analysis
3. Results and Discussion
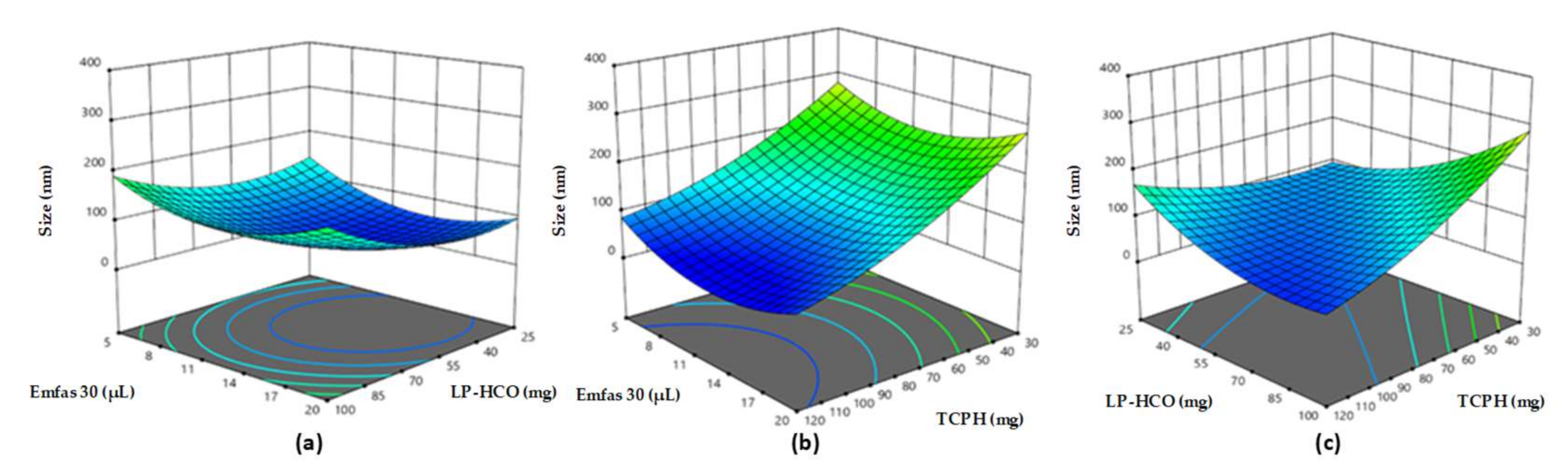
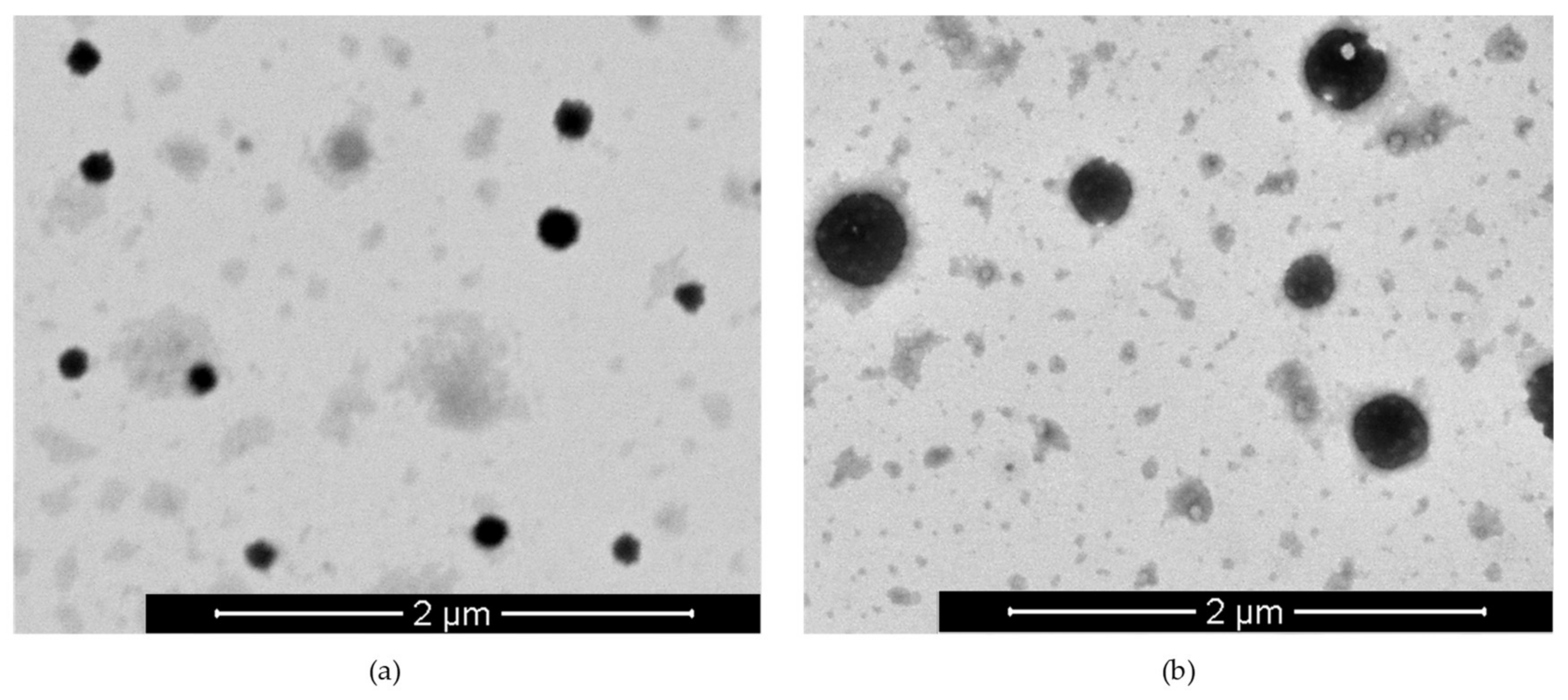
3.1. Preparation and Characterization of Small-Sized Chitosan Nanocapsules
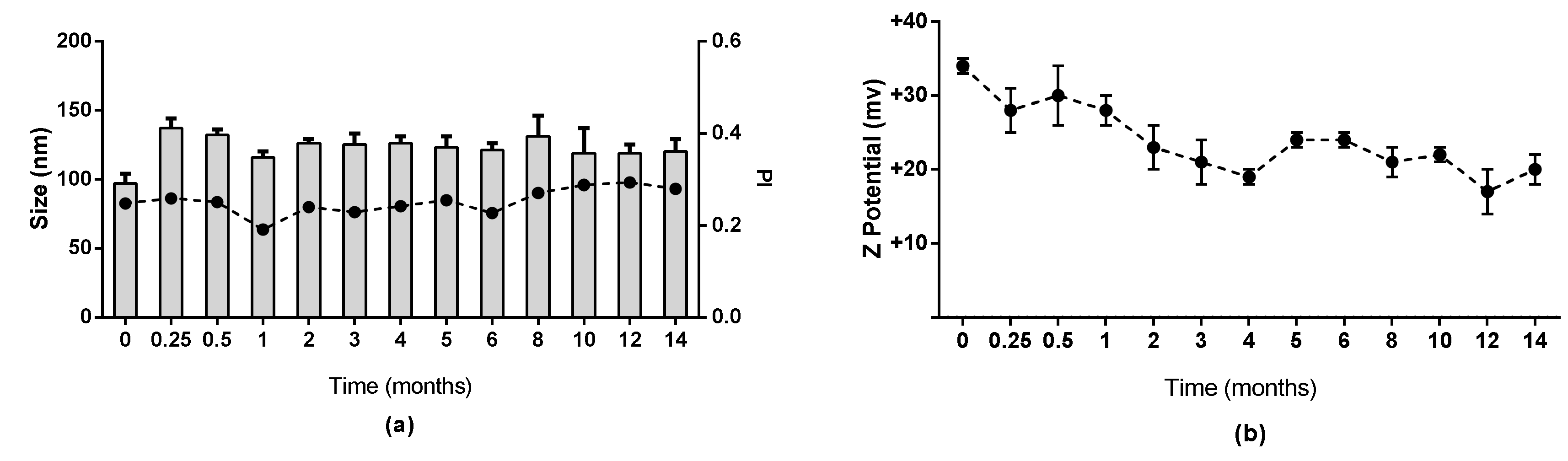
3.2. Stability of Chitosan Nanocapsules
3.3. Preparation and Characterization of Ovalbumin-Loaded Chitosan Nanocapsules
3.4. Cytotoxicity and Immune System Interaction of Chitosan Nanocapsules
3.5. Ex Vivo Transdermal Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schulze, K.; Ebensen, T.; Riese, P.; Prochnow, B.; Lehr, C.M.; Guzmán, C.A. New horizons in the development of novel needle-free immunization strategies to increase vaccination efficacy. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Stadler, M., Dersch, P., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 207–234. [Google Scholar]
- Chen, X.; Fernando, G.J.P.; Crichton, M.L.; Flaim, C.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Primiero, C.A.; Ansaldo, A.B.; Frazer, I.H.; et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J. Control. Release 2011, 152, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pisani, E. The cost of unsafe injections. Bull. World Health Organ. 1999, 77, 808–811. [Google Scholar] [PubMed]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Delivery Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.D.; Sadhna, D.; Nagpaal, D.; Chawla, L. Needle free injection technology: A complete insight. Int. J. Pharm. Investig. 2015, 5, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016, 38, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Okada, N.; Nakagawa, S. Transcutaneous vaccines – current and emerging strategies. Expert Opin. Drug Deliv. 2013, 10, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal delivery of vaccines–Recent progress and critical issues. Biomed. Pharmacother. 2016, 83, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.; Cary, J.H.; Maibach, H.I. Stratum corneum substantivity: Drug development implications. Arch. Dermatol. Res. 2018, 310, 537–549. [Google Scholar] [CrossRef]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kaurav, M.; Minz, S.; Sahu, K.; Kumar, M.; Madan, J.; Pandey, R.S. Nanoparticulate mediated transcutaneous immunization: Myth or reality. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1063–1081. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.V.; Cordeiro, A.S.; Csaba, N.; De la Fuente, M.; Alonso, M. Nanovaccine: Nanocarriers for antigen delivery. Biol. Aujourd'hui 2012, 206, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.; Peleteiro, M.; Gonzalez-Aramundiz, J.V.; Diaz-Freitas, B.; Martinez-Pulgarin, S.; Neissa, J.I.; Escribano, J.M.; Sanchez, A.; Gonzalez-Fernandez, A.; Alonso, M.J. Highly versatile immunostimulating nanocapsules for specific immune potentiation. Nanomedicne 2014, 9, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.V.; Lozano, M.V.; Sousa-Herves, A.; Fernandez-Megia, E.; Csaba, N. Polypeptides and polyaminoacids in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Raţă, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(n-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Griffiths, G. Layer-by-layer nanocoating of live bacille-calmette-guerin mycobacteria with poly(i:C) and chitosan enhances pro-inflammatory activation and bactericidal capacity in murine macrophages. Biomaterials 2016, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Chaves, P.S.; D'Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.R.; Pohlmann, A.R.; Button, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.O.; Issah, S.; Kola-Mustapha, A.T. Ex vivo skin permeation and retention studies on chitosan–ibuprofen–gellan ternary nanogel prepared by in situ ionic gelation technique—A tool for controlled transdermal delivery of ibuprofen. Int. J. Pharm. 2015, 490, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Dibyangana, S.D.; Varsha, M.J.; Vilasrao, J.K. Chitosan: A propitious biopolymer for drug delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar]
- Yao, G.; Quan, G.; Lin, S.; Peng, T.; Wang, Q.; Ran, H.; Chen, H.; Zhang, Q.; Wang, L.; Pan, X.; et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: In vitro and in vivo characterization. Int. J. Pharm. 2017, 534, 378–386. [Google Scholar] [CrossRef] [PubMed]
- González-Aramundiz, J.V.; Presas, E.; Dalmau-Mena, I.; Martínez-Pulgarín, S.; Alonso, C.; Escribano, J.M.; Alonso, M.J.; Csaba, N.S. Rational design of protamine nanocapsules as antigen delivery carriers. J. Control. Release 2017, 245, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Neun, B.W.; Dobrovolskaia, M.A. Qualitative analysis of total complement activation by nanoparticles. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Springer: Basel, Switzerland, 2011; Volume 697, pp. 237–245. [Google Scholar]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.; Fowler, S. Quantification of proteins on western blots using ECL. In The Protein Protocols Handbook; Walker, J., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 429–437. [Google Scholar]
- Musyanovych, A.; Landfester, K. Polymer micro- and nanocapsules as biological carriers with multifunctional properties. Macromol. Biosci. 2014, 14, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.; Diaz-Freitas, B.; Peleteiro, M.; Sanchez, A.; Pascual, D.W.; Gonzalez-Fernandez, A.; Alonso, M.J. A polymer/oil based nanovaccine as a single-dose immunization approach. PLoS ONE 2013, 8, e62500. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Cho, K.H.; Cui, L.; Park, I.-K.; Choi, Y.J.; Cho, C.-S. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int. J. Biol. Macromol. 2018, 110, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Mitkus, R.J. A first-generation physiologically based pharmacokinetic (pbpk) model of alpha-tocopherol in human influenza vaccine adjuvant. Regul. Toxicol. Pharmacol. 2015, 71, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Abellan-Pose, R.; Teijeiro-Valiño, C.; Santander-Ortega, M.J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M.; Csaba, N.; Alonso, M.J. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: Effect of the particle size. Int. J. Pharm. 2016, 509, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Poorani, G.; Uppuluri, S.; Uppuluri, K.B. Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems. J. Microencapsul. 2016, 33, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Wang, Q.; Jones, C.H.; Yu, Y.; Zhang, H.; Law, W.C.; Lai, C.K.; Zeng, Q.; Prasad, P.N.; Pfeifer, B.A.; et al. Synthesis of ph-responsive chitosan nanocapsules for the controlled delivery of doxorubicin. Langmuir 2014, 30, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Goethals, E.C.; Elbaz, A.; Lopata, A.L.; Bhargava, S.K.; Bansal, V. Decoupling the effects of the size, wall thickness, and porosity of curcumin-loaded chitosan nanocapsules on their anticancer efficacy: Size is the winner. Langmuir 2013, 29, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.V.; Devi, P.R.; Harish, S.; Hemananthan, E. Synthesis and characterisation of peg modified chitosan nanocapsules loaded with thymoquinone. IET Nanobiotechnol. 2017, 11, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release 2017, 256, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yanase, N.; Hiroko, T.; Hata, K.; Yagyu, S.; Seki, T.; Harada, M.; Kato, Y.; Mizuguchi, J. Ova-bound nanoparticles induce ova-specific igg1, igg2a, and igg2b responses with low ige synthesis. Vaccine 2014, 32, 5918–5924. [Google Scholar] [CrossRef] [PubMed]
- Mönkäre, J.; Pontier, M.; van Kampen, E.E.M.; Du, G.; Leone, M.; Romeijn, S.; Nejadnik, M.R.; O’Mahony, C.; Slütter, B.; Jiskoot, W.; et al. Development of plga nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. 2018, 129, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, S.; Lu, L.; Song, X.; Li, P.; Wang, F. Curdlan sulfate–o-linked quaternized chitosan nanoparticles: Potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int. J. Nanomedicine 2018, 13, 2377–2394. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.; Moravec, R.; Niles, A.; Benink, H.; Worzella, T.; Minor, L. Cell Viability Assay. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 24 August 2018).
- Chen, L.; Simpson, J.D.; Fuchs, A.V.; Rolfe, B.E.; Thurecht, K.J. Effects of surface charge of hyperbranched polymers on cytotoxicity, dynamic cellular uptake and localization, hemotoxicity, and pharmacokinetics in mice. Mol. Pharm. 2017, 14, 4485–4497. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwet, B. The complement system: An evolution in progress. F1000Research 2016, 5, 2840. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material properties in complement activation. Adv. Drug Delivery. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Mkrtichyan, M.; Ghochikyan, A.; Movsesyan, N.; Karapetyan, A.; Begoyan, G.; Yu, J.M.; Glenn, G.M.; Ross, T.M.; Agadjanyan, M.G.; Cribbs, D.H. Immunostimulant adjuvant patch enhances humoral and cellular immune responses to DNA immunization. DNA Cell Biol. 2008, 27, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Güven, E.; Duus, K.; Laursen, I.; Højrup, P.; Houen, G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS ONE 2013, 8, e74445. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, M.; Presas, E.; González-Aramundiz, J.V.; Sánchez-Correa, B.; Simón-Vázquez, R.; Csaba, N.; Alonso, M.J.; González-Fernández, Á. Polymeric nanocapsules for vaccine delivery: Influence of the polymeric shell on the interaction with the immune system. Front. Immunol. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W. Electrical, magnetic, photomechanical and cavitational waves to overcome skin barrier for transdermal drug delivery. J. Control. Release 2014, 193, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [PubMed]
- Khademi, F.; Taheri, R.A.; Yousefi Avarvand, A.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Schäfer-Korting, M. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Dasgupta, A.; Mitragotri, S. Transdermal immunomodulation: Principles, advances and perspectives. Adv. Drug Delivery. Rev. 2018, 127, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Shin, M.C.; Yang, V.C. Transcutaneous antigen delivery system. BMB Rep. 2013, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]

| Formulation | TCPH (mg) | LP-HCO (mg) | Size (nm) | PI | ζPotential (mV) |
|---|---|---|---|---|---|
| NE 1 | 60 | 50 | 80 ± 2 | 0.175 | −27 ± 2 |
| CSNCs (two-step) | 145 ± 20 | 0.381 | +32 ± 3 | ||
| CSNCs (one step) | 97 ± 7 | 0.248 | +36 ± 2 | ||
| NE 2 | 120 | 50 | 130 ± 3 | 0.129 | −27 ± 8 |
| CSNCs (two-step) | 235 ± 31 | 0.257 | +38 ± 3 | ||
| CSNCs (one step) | 192 ± 17 | 0.302 | +38 ± 1 | ||
| NE 3 | 120 | 100 | 167 ± 3 | 0.141 | −25 ± 2 |
| CSNCs (two-step) | 384 ± 25 | 0.241 | +34 ± 8 | ||
| CSNCs (one step) | 194 ± 12 | 0.280 | +32 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussio, J.I.; Molina-Perea, C.; González-Aramundiz, J.V. Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery. Nanomaterials 2018, 8, 659. https://doi.org/10.3390/nano8090659
Bussio JI, Molina-Perea C, González-Aramundiz JV. Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery. Nanomaterials. 2018; 8(9):659. https://doi.org/10.3390/nano8090659
Chicago/Turabian StyleBussio, Juan I., Carla Molina-Perea, and José Vicente González-Aramundiz. 2018. "Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery" Nanomaterials 8, no. 9: 659. https://doi.org/10.3390/nano8090659
APA StyleBussio, J. I., Molina-Perea, C., & González-Aramundiz, J. V. (2018). Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery. Nanomaterials, 8(9), 659. https://doi.org/10.3390/nano8090659